Accelerated Weathering and Soil Burial Effect on Biodegradability, Colour and Textureof Coir/Pineapple Leaf Fibres/PLA Biocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fibre Treatment
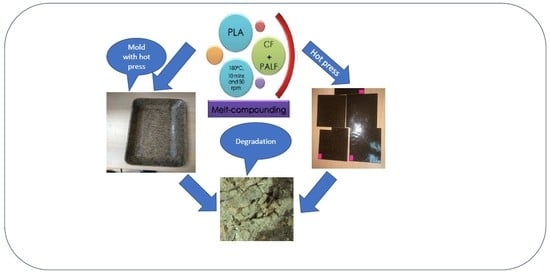
2.3. Composites Fabrication
2.4. Accelerated Weathering Test
2.4.1. Weight Change
2.4.2. Colorimetric Colour Analysis
2.4.3. Surface Texture Measurement
2.5. Soil Burial Test
3. Results
3.1. Accelerated Weathering: Weight Change
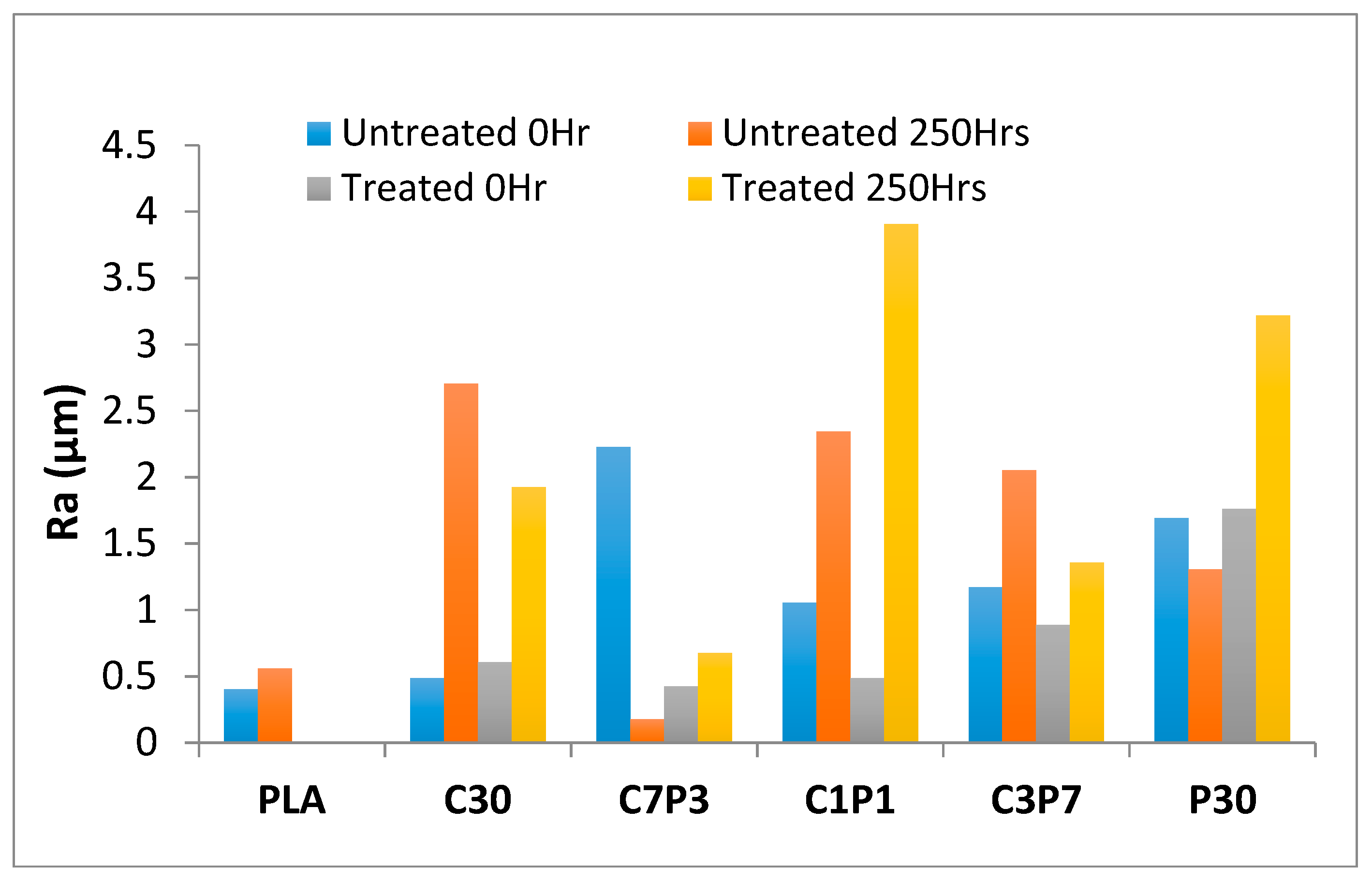
3.2. Accelerated Weathering: Surface Roughness
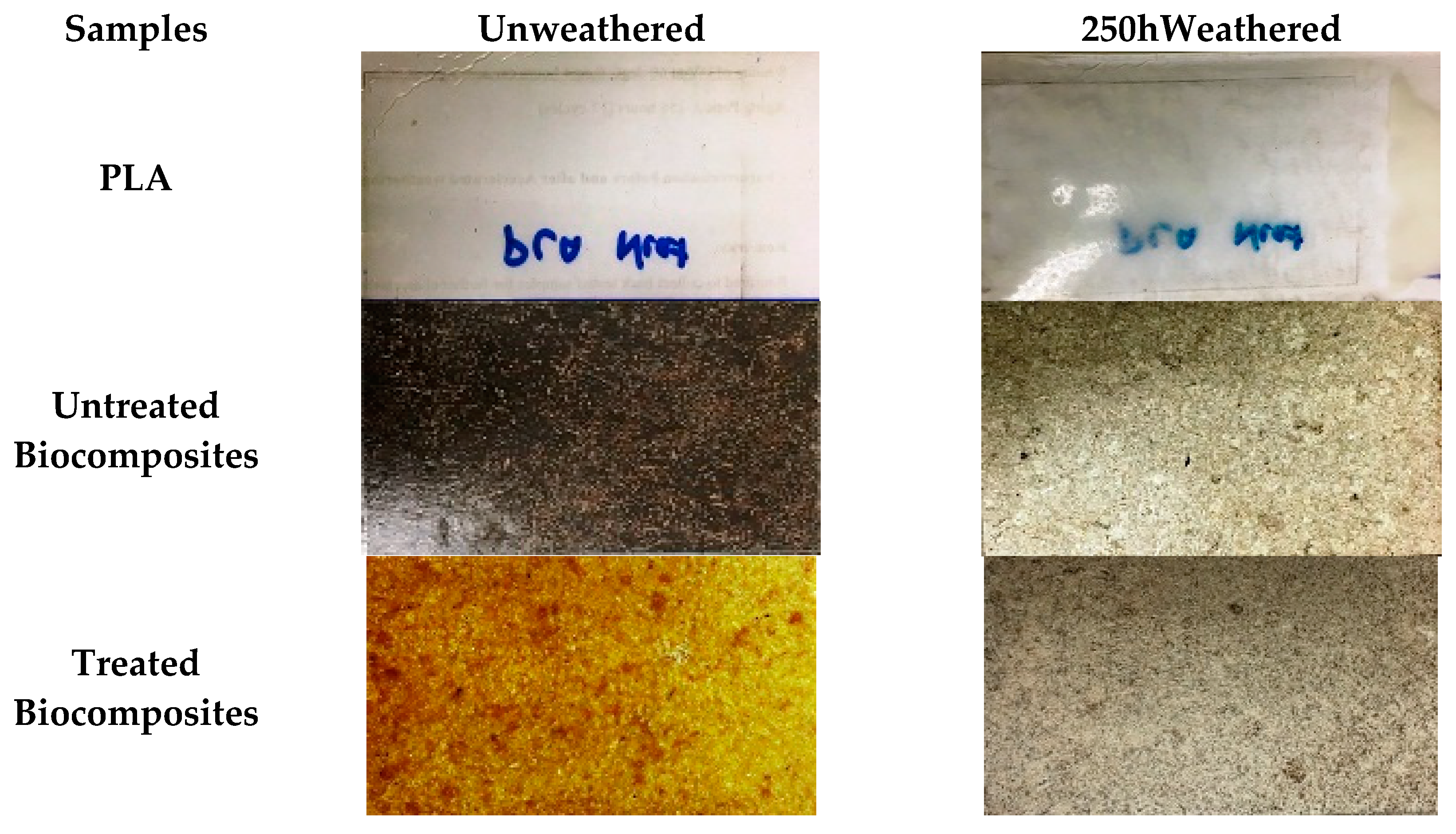
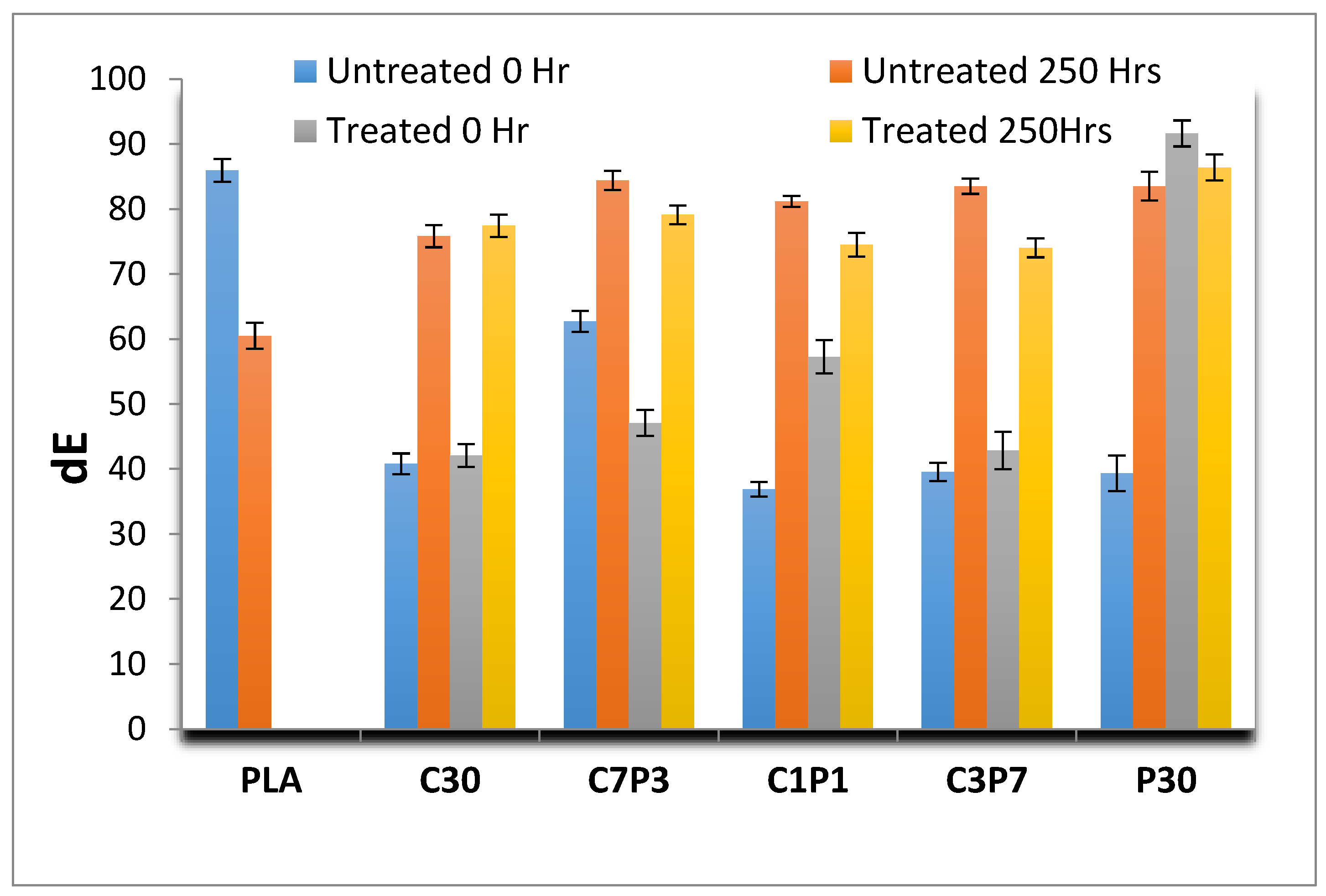
3.3. Accelerated Weathering: Colour Change
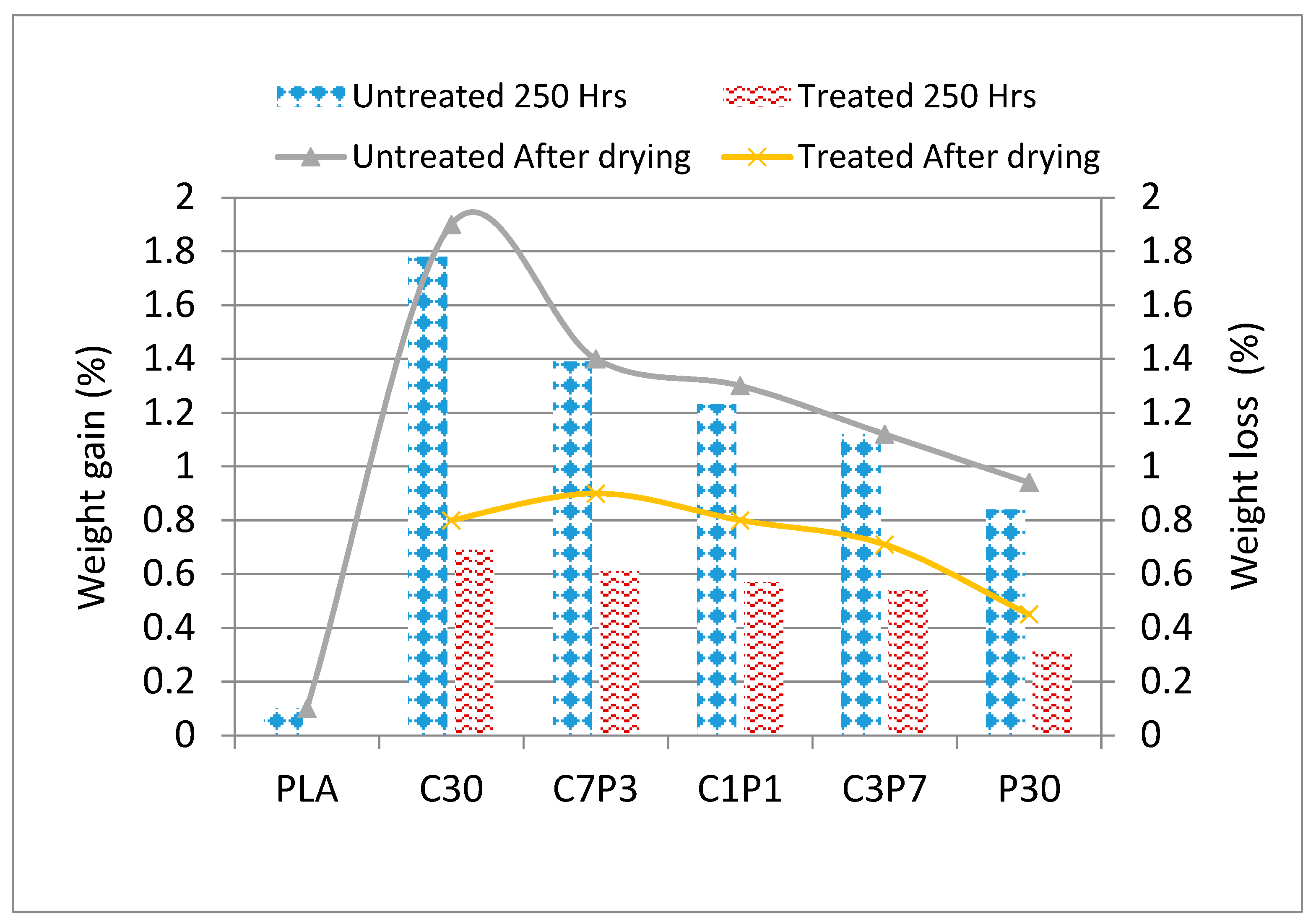
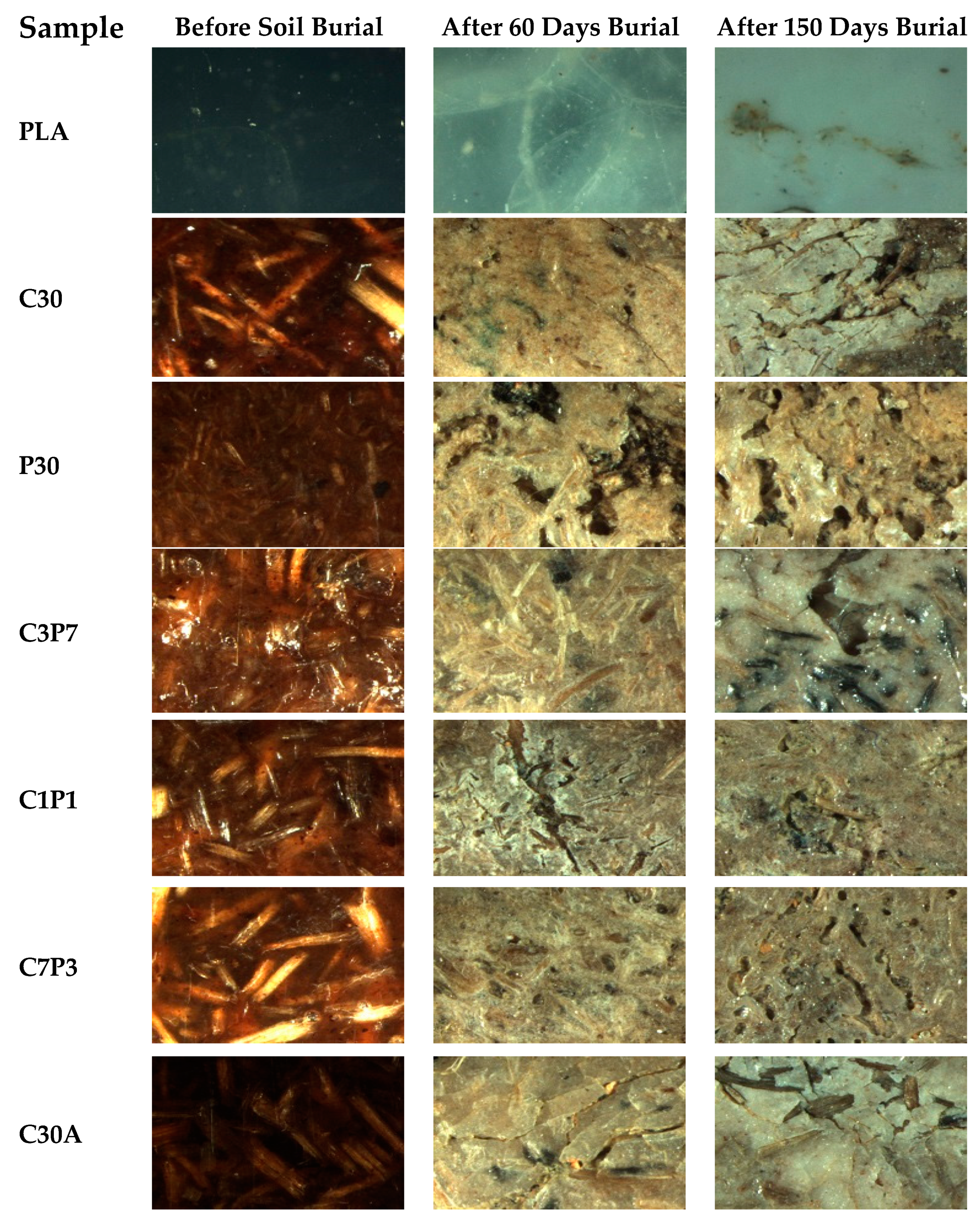
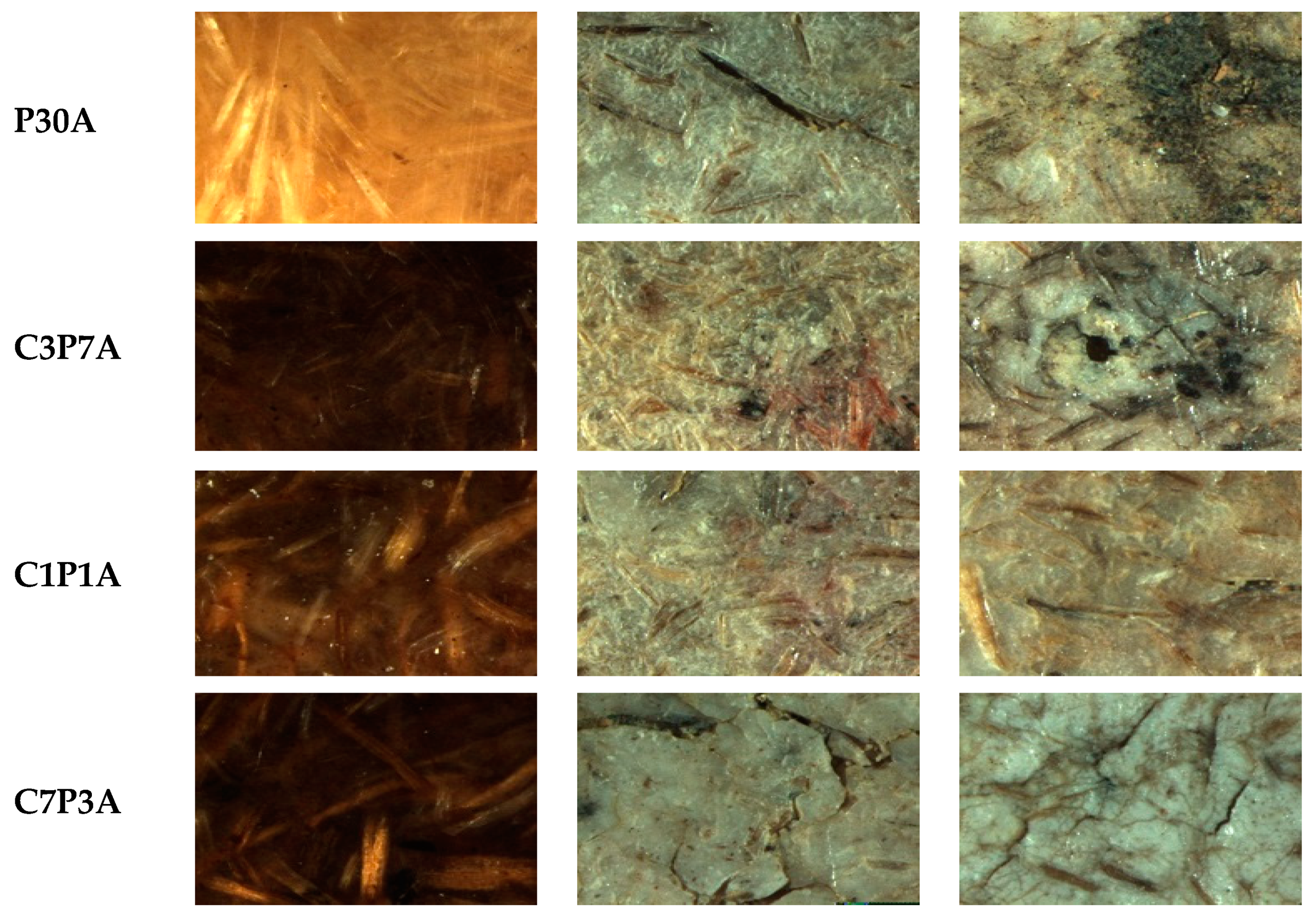
3.4. Soil Burial: Weight Loss
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Torres, F.; Rodriguez, S.; Saavedra, A. Green Composite Materials from Biopolymers Reinforced with Agroforestry Waste. J. Polym. Environ. 2019, 27, 2651–2673. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.; Asim, M.; Saba, N. Natural fiber reinforced polylactic acid composites: A review. Polym. Compos. 2019, 40, 446–463. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.; Hoque, M.E. A review on pineapple leaves fibre and its composites. Int. J. Polym. Sci. 2015, 2015, 950567. [Google Scholar] [CrossRef] [Green Version]
- Gheith, M.H.; Aziz, M.A.; Ghori, W.; Saba, N.; Asim, M.; Jawaid, M. Flexural, thermal and dynamic mechanical properties of date palm fibres reinforced epoxy composites. J. Mater. Res. Technol. 2019, 8, 853–860. [Google Scholar] [CrossRef]
- Nasir, M.; Khali, D.; Jawaid, M.; Tahir, P.; Siakeng, R.; Asim, M.; Khan, T. Recent development in binderless fiber-board fabrication from agricultural residues: A review. Constr. Build. Mater. 2019, 211, 502–516. [Google Scholar] [CrossRef]
- Madurwar, M.V.; Ralegaonkar, R.V.; Mandavgane, S.A. Application of agro-waste for sustainable construction materials: A review. Constr. Build. Mater. 2013, 38, 872–878. [Google Scholar] [CrossRef]
- Yu, T.; Ren, J.; Li, S.; Yuan, H.; Li, Y. Effect of fiber surface-treatments on the properties of poly (lactic acid)/ramie composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 499–505. [Google Scholar] [CrossRef]
- Song, Y.S.; Lee, J.T.; Ji, D.S.; Kim, M.W.; Lee, S.H.; Youn, J.R. Viscoelastic and thermal behavior of woven hemp fiber reinforced poly (lactic acid) composites. Compos. Part B Eng. 2012, 43, 856–860. [Google Scholar] [CrossRef]
- Law, K.L.; Morét-Ferguson, S.E.; Goodwin, D.S.; Zettler, E.R.; DeForce, E.; Kukulka, T.; Proskurowski, G. Distribution of surface plastic debris in the eastern Pacific Ocean from an 11-year data set. Environ. Sci. Technol. 2014, 48, 4732–4738. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [Green Version]
- Gall, S.C.; Thompson, R.C. The impact of debris on marine life. Mar. Pollut. Bull. 2015, 92, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Andrady, A.L. Plastics and Environmental Sustainability; Wiley Online Library; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 145–176. [Google Scholar]
- Islam, M.S.; Pickering, K.L.; Foreman, N.J. Influence of hygrothermal ageing on the physico-mechanical properties of alkali treated industrial hemp fibre reinforced polylactic acid composites. J. Polym. Environ. 2010, 18, 696–704. [Google Scholar] [CrossRef]
- Barnes, D.K.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Qin, L.; Qiu, J.; Liu, M.; Ding, S.; Shao, L.; Lü, S.; Zhang, G.; Zhao, Y.; Fu, X. Mechanical and thermal properties of poly (lactic acid) composites with rice straw fiber modified by poly (butyl acrylate). Chem. Eng. J. 2011, 166, 772–778. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, J.; Feng, H.; Zhang, M.; Lei, L.; Wu, X. Improvement of tensile and thermal properties of poly (lactic acid) composites with admicellar-treated rice straw fiber. Chem. Eng. J. 2011, 173, 659–666. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S. Mechanical, dynamic, and thermomechanical properties of coir/pineapple leaf fiber reinforced polylactic acid hybrid biocomposites. Polym. Compos. 2019, 40, 2000–2011. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.; Kenny, J. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Wahit, M.U.; Akos, N.I.; Laftah, W.A. Influence of natural fibers on the mechanical properties and biodegradation of poly (lactic acid) and poly (ε-caprolactone) composites: A review. Polym. Compos. 2012, 33, 1045–1053. [Google Scholar] [CrossRef]
- Yussuf, A.; Massoumi, I.; Hassan, A. Comparison of polylactic acid/kenaf and polylactic acid/rise husk composites: The influence of the natural fibers on the mechanical, thermal and biodegradability properties. J. Polym. Environ. 2010, 18, 422–429. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Kenny, J. Development and thermal behaviour of ternary PLA matrix composites. Polym. Degrad. Stab. 2010, 95, 2200–2206. [Google Scholar] [CrossRef]
- Azwa, Z.; Yousif, B.; Manalo, A.; Karunasena, W. A review on the degradability of polymeric composites based on natural fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Pickering, K.L.; Foreman, N.J. Influence of accelerated ageing on the physico-mechanical properties of alkali-treated industrial hemp fibre reinforced poly (lactic acid) (PLA) composites. Polym. Degrad. Stab. 2010, 95, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Yakubu, M.; Anandjiwala, R. Biodegradation of flax fiber reinforced poly lactic acid. Express Polym. Lett. 2010, 4, 423–430. [Google Scholar] [CrossRef]
- Batista, K.; Silva, D.; Coelho, L.; Pezzin, S.; Pezzin, A. Soil biodegradation of PHBV/peach palm particles biocomposites. J. Polym. Environ. 2010, 18, 346–354. [Google Scholar] [CrossRef]
- Beninia, K.; Voorwald, H.J.C.; Cioffi, M. Mechanical properties of HIPS/sugarcane bagasse fiber composites after accelerated weathering. Procedia Eng. 2011, 10, 3246–3251. [Google Scholar] [CrossRef] [Green Version]
- Dittenber, D.B.; GangaRao, H.V. Critical review of recent publications on use of natural composites in infrastructure. Compos. Part A Appl. Sci. Manuf. 2012, 43, 1419–1429. [Google Scholar] [CrossRef]
- Ochi, S. Mechanical properties of kenaf fibers and kenaf/PLA composites. Mech. Mater. 2008, 40, 446–452. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M.; Sultan, M.; Alothman, O.Y.; Abdullah, L.C. Accelerated weathering and soil burial effects on colour, biodegradability and thermal properties of bamboo/kenaf/epoxy hybrid composites. Polym. Test. 2019, 79, 106054. [Google Scholar] [CrossRef]
- Umar, A.; Zainudin, E.; Sapuan, S. Effect of accelerated weathering on tensile properties of kenaf reinforced high-density polyethylene composites. J. Mech. Eng. Sci. 2012, 2, 198–205. [Google Scholar] [CrossRef]
- Dong, Y.; Ghataura, A.; Takagi, H.; Haroosh, H.J.; Nakagaito, A.N.; Lau, K.-T. Polylactic acid (PLA) biocomposites reinforced with coir fibres: Evaluation of mechanical performance and multifunctional properties. Compos. Part A Appl. Sci. Manuf. 2014, 63, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Van den Oever, M.; Beck, B.; Müssig, J. Agrofibre reinforced poly (lactic acid) composites: Effect of moisture on degradation and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1628–1635. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Rheological and mechanical characterization of poly (lactic acid)/polypropylene polymer blends. J. Polym. Res. 2011, 18, 1799–1806. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Huang, C.-C. Polylactic acid (PLA)/banana fiber (BF) biodegradable green composites. J. Polym. Res. 2011, 18, 2335–2340. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Chen, H.; Zhang, S.; Xiong, C. Preparation and characterization of biodegradable thermoplastic Elastomers (PLCA/PLGA blends). J. Polym. Res. 2010, 17, 77–82. [Google Scholar] [CrossRef]
- Xie, L.; Xu, H.; Wang, Z.-P.; Li, X.-J.; Chen, J.-B.; Zhang, Z.-J.; Yin, H.-M.; Zhong, G.-J.; Lei, J.; Li, Z.-M. Toward faster degradation for natural fiber reinforced poly (lactic acid) biocomposites by enhancing the hydrolysis-induced surface erosion. J. Polym. Res. 2014, 21, 357. [Google Scholar] [CrossRef]
- Yusoff, R.B.; Takagi, H.; Nakagaito, A.N. Tensile and flexural properties of polylactic acid-based hybrid green composites reinforced by kenaf, bamboo and coir fibers. Ind. Crop. Prod. 2016, 94, 562–573. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Salit, M.S. Effects of surface treatments on tensile, thermal and fibre-matrix bond strength of coir and pineapple leaf fibres with poly lactic acid. J. Bionic Eng. 2018, 15, 1035–1046. [Google Scholar] [CrossRef]
- Mehta, G.; Mohanty, A.K.; Drzal, L.T.; Kamdem, D.P.; Misra, M. Effect of accelerated weathering on biocomposites processed by SMC and compression molding. J. Polym. Environ. 2006, 14, 359–368. [Google Scholar] [CrossRef]
- Spiridon, I.; Darie, R.N.; Kangas, H. Influence of fiber modifications on PLA/fiber composites. Behavior to accelerated weathering. Compos. Part B Eng. 2016, 92, 19–27. [Google Scholar] [CrossRef]
- Ray, B.C.; Rathore, D. Environmental damage and degradation of FRP composites: A review report. Polym. Compos. 2015, 36, 410–423. [Google Scholar] [CrossRef]
- Campos, A.D.; Marconcini, J.; Martins-Franchetti, S.; Mattoso, L. The influence of UV-C irradiation on the properties of thermoplastic starch and polycaprolactone biocomposite with sisal bleached fibers. Polym. Degrad. Stab. 2012, 97, 1948–1955. [Google Scholar] [CrossRef]
- Jawaid, M.; Saba, N.; Alothman, O.; Paridah, M. Effect of accelerated environmental aging on tensile properties of oil palm/jute hybrid composites. In Proceedings of AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2016; p. 040007. [Google Scholar]
- Schanda, J. Colorimetry: Understanding the CIE System; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 25–76. [Google Scholar]
- Ergücü, Z.; Türkün, L.; Aladag, A. Color stability of nanocomposites polished with one-step systems. Oper. Dent. 2008, 33, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Spiridon, I.; Leluk, K.; Resmerita, A.M.; Darie, R.N. Evaluation of PLA–lignin bioplastics properties before and after accelerated weathering. Compos. Part B Eng. 2015, 69, 342–349. [Google Scholar] [CrossRef]
- Ohkita, T.; Lee, S.H. Thermal degradation and biodegradability of poly (lactic acid)/corn starch biocomposites. J. Appl. Polym. Sci. 2006, 100, 3009–3017. [Google Scholar] [CrossRef]
- Harmaen, A.S.; Khalina, A.; Azowa, I.; Hassan, M.A.; Tarmian, A.; Jawaid, M. Thermal and biodegradation properties of poly (lactic acid)/fertilizer/oil palm fibers blends biocomposites. Polym. Compos. 2015, 36, 576–583. [Google Scholar] [CrossRef]
- Kim, H.S.; Yang, H.S.; Kim, H.J. Biodegradability and mechanical properties of agro-flour–filled polybutylene succinate biocomposites. J. Appl. Polym. Sci. 2005, 97, 1513–1521. [Google Scholar] [CrossRef]
- Rajesh, G.; Prasad, A.V.R. Tensile properties of successive alkali treated short jute iber reinforced PLA composites. Procedia Mater. Sci. 2014, 5, 2188–2196. [Google Scholar] [CrossRef] [Green Version]
- Gunti, R.; Ratna Prasad, A.; Gupta, A. Mechanical and degradation properties of natural fiber-reinforced PLA composites: Jute, sisal, and elephant grass. Polym. Compos. 2018, 39, 1125–1136. [Google Scholar] [CrossRef]
- Rashdi, A.; Sapuan, S.; Ahmad, M.; Khalina, A. Combined Effects of Water Absorption Due to Water Immersion, Soil Buried and Natural Weather on Mechanical Properties of Kenaf Fibre Unsaturated Polyester Composites (KFUPC). Int. J. Mech. Mater. Eng. 2010, 5, 11–17. [Google Scholar]
- Rudnik, E.; Briassoulis, D. Degradaton behavior of poly (lactic acid) films and fibres in soil under Mediterranean field conditions and laboratory simulation testing. Ind. Crop. Prod. 2011, 3, 648–658. [Google Scholar] [CrossRef]

| Characteristics | Description | Characteristics | Description |
|---|---|---|---|
| Physical state | Pallets | Tensile strength | 14.68 MPa |
| Colour | Translucent white | Young’s Modulus | 3.98 GPa |
| Odour | No odour | Flexural strength | 27.87 MPa |
| Density | 1.25 g/mol | Flexural modulus | 3.75 GPa |
| Melting point | 150–170 °C | Impact strength | 3.43 kJ/m2 |
| Glass transition temperature | 55–60 °C | Water absorption | 2.58% |
| Molecular weight | 74,000 g/mol | Thickness swelling | 1.42% |
| Properties | CF | PALF |
|---|---|---|
| Density (g/m3) | 1.20 | 1.07 |
| Diameter (µm) | 100–450 | 20–80 |
| Cellulose (%) | 42.14 | 81.27 |
| Hemicelluloses (%) | 15.17 | 12.31 |
| Lignin (%) | 35.25 | 3.46 |
| Moisture absorption (%) | 10.00 | 11.80 |
| Microfibrillar angle (°) | 30–45 | 8–15 |
| Elongation at break (%) | 17–47 | 1.6–4 |
| Tensile Strength (MPa) | 105–175 | 413–1627 |
| Youngs Modulus (GPa) | 4–6 | 34.5–82.5 |
| Designation | PLA (Weight %) | CF (Weight %) | PALF (Weight %) |
|---|---|---|---|
| Pure PLA | 100 | _ | _ |
| C-30 | 70 | 30 | _ |
| C7P3 | 70 | 21 | 9 |
| C1P1 | 70 | 15 | 15 |
| C3P7 | 70 | 9 | 21 |
| P-30 | 70 | _ | 30 |
| Sample | Before | After | ||||
|---|---|---|---|---|---|---|
| Ra (µm) | Rmax (µm) | Rz (µm) | Ra (µm) | Rmax (µm) | Rz (µm) | |
| PLA | 0.4041 | 4.1468 | 2.9805 | 0.5611 | 5.8276 | 3.9426 |
| C30 | 0.4878 | 18.0561 | 4.2805 | 2.7034 | 57.5719 | 23.4164 |
| P30 | 1.6913 | 39.2247 | 19.9636 | 1.3076 | 20.4452 | 9.4624 |
| C3P7 | 1.1726 | 30.6461 | 22.0001 | 2.0524 | 47.5884 | 18.2854 |
| C1P1 | 1.0534 | 22.6111 | 14.3713 | 2.3452 | 34.8313 | 21.6659 |
| C7P3 | 2.2285 | 23.7508 | 16.5913 | 0.1773 | 10.6666 | 3.0401 |
| C30 A | 0.6067 | 16.4739 | 6.0132 | 1.9237 | 66.6887 | 22.2321 |
| P30 A | 1.7618 | 13.5416 | 11.4903 | 3.2163 | 64.6825 | 35.9024 |
| C3P7 A | 0.8875 | 7.2143 | 3.3644 | 1.3577 | 62.3672 | 20.7893 |
| C1P1 A | 0.4864 | 16.4976 | 3.7916 | 3.9073 | 62.2191 | 36.5664 |
| C7P3 A | 0.4231 | 11.0194 | 4.7770 | 0.6759 | 28.9814 | 9.6606 |
| Sample | Before Weathering | After 250 h Weathering | ||||||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | dE | L* | a* | b* | dE | |
| PLA | 82.34 | 0.52 | 3.07 | 85.93 | 58.65 | 1.29 | 0.55 | 60.49 |
| C30 | 24.92 | 7.52 | 8.36 | 40.80 | 64.13 | 3.84 | 7.85 | 75.82 |
| P30 | 23.27 | 7.75 | 8.33 | 39.35 | 75.48 | 1.27 | 6.76 | 83.51 |
| C3P7 | 25.50 | 6.65 | 7.41 | 39.56 | 75.46 | 1.89 | 6.37 | 83.51 |
| C1P1 | 23.56 | 6.44 | 6.89 | 36.89 | 73.64 | 2.03 | 5.51 | 81.18 |
| C7P3 | 37.66 | 9.23 | 15.81 | 62.70 | 65.24 | 5.78 | 13.39 | 84.41 |
| C30 A | 25.02 | 7.65 | 9.40 | 42.07 | 63.23 | 4.40 | 9.79 | 77.42 |
| P30 A | 51.75 | 9.93 | 29.94 | 91.62 | 71.33 | 3.84 | 11.20 | 86.37 |
| C3P7 A | 28.29 | 6.51 | 8.06 | 42.86 | 60.15 | 3.69 | 10.18 | 74.02 |
| C1P1 A | 31.42 | 8.71 | 17.13 | 57.26 | 63.04 | 3.18 | 8.26 | 74.48 |
| C7P3 A | 28.78 | 7.54 | 10.76 | 47.08 | 66.54 | 3.58 | 8.98 | 79.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siakeng, R.; Jawaid, M.; Asim, M.; Siengchin, S. Accelerated Weathering and Soil Burial Effect on Biodegradability, Colour and Textureof Coir/Pineapple Leaf Fibres/PLA Biocomposites. Polymers 2020, 12, 458. https://doi.org/10.3390/polym12020458
Siakeng R, Jawaid M, Asim M, Siengchin S. Accelerated Weathering and Soil Burial Effect on Biodegradability, Colour and Textureof Coir/Pineapple Leaf Fibres/PLA Biocomposites. Polymers. 2020; 12(2):458. https://doi.org/10.3390/polym12020458
Chicago/Turabian StyleSiakeng, Ramengmawii, Mohammad Jawaid, Mohammad Asim, and Suchart Siengchin. 2020. "Accelerated Weathering and Soil Burial Effect on Biodegradability, Colour and Textureof Coir/Pineapple Leaf Fibres/PLA Biocomposites" Polymers 12, no. 2: 458. https://doi.org/10.3390/polym12020458
APA StyleSiakeng, R., Jawaid, M., Asim, M., & Siengchin, S. (2020). Accelerated Weathering and Soil Burial Effect on Biodegradability, Colour and Textureof Coir/Pineapple Leaf Fibres/PLA Biocomposites. Polymers, 12(2), 458. https://doi.org/10.3390/polym12020458