A One-Pot Synthesis and Characterization of Antibacterial Silver Nanoparticle–Cellulose Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ag-NPs Solution in Organic Phase
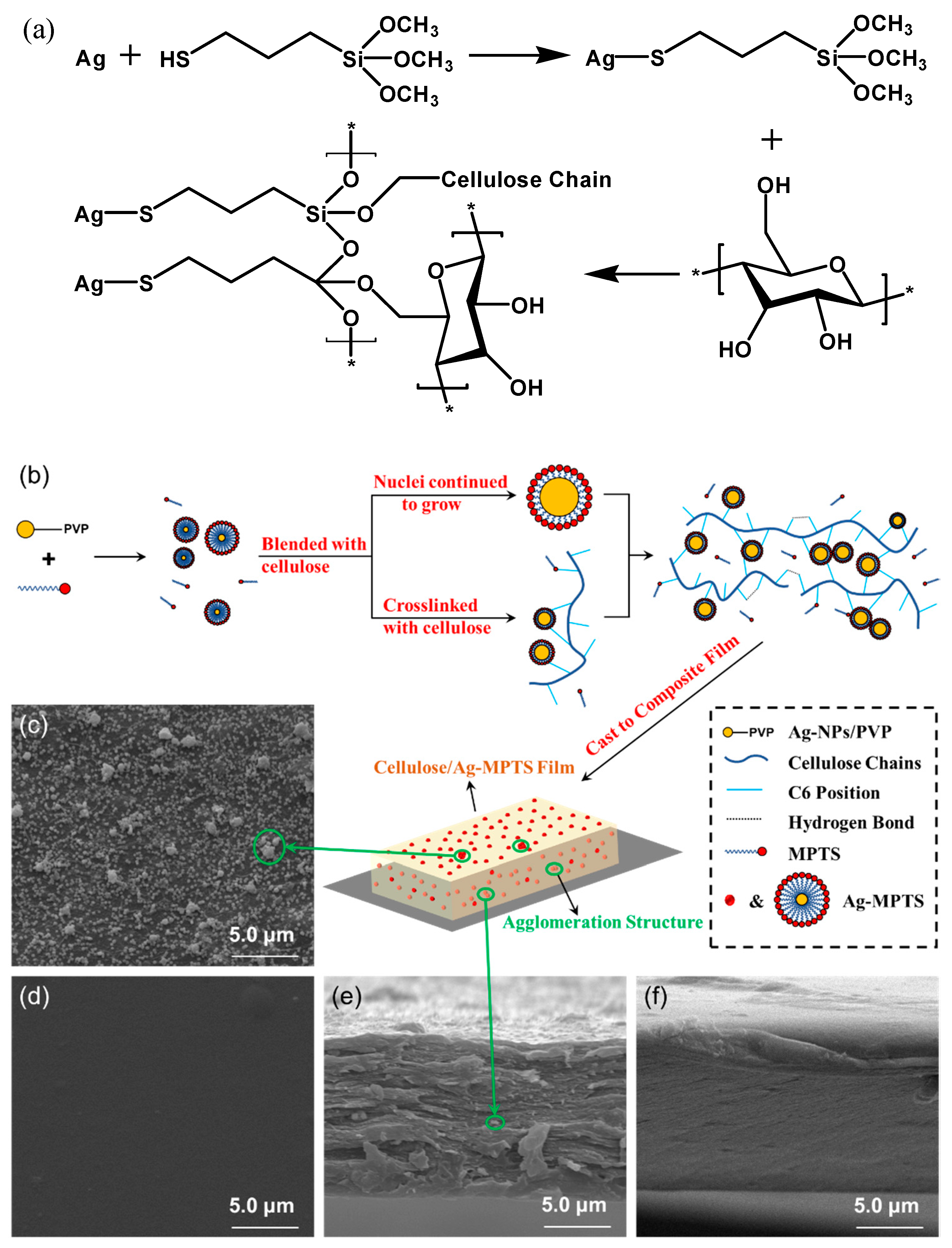
2.3. Preparation of Silver Loaded Cellulose Films
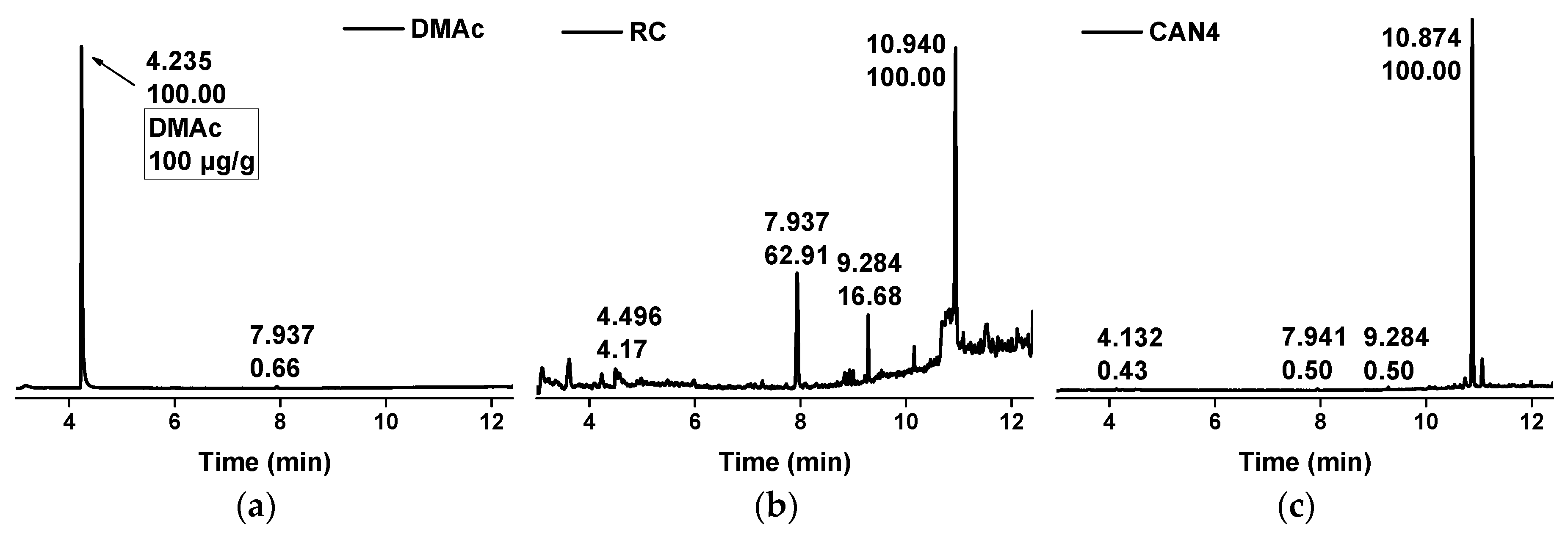
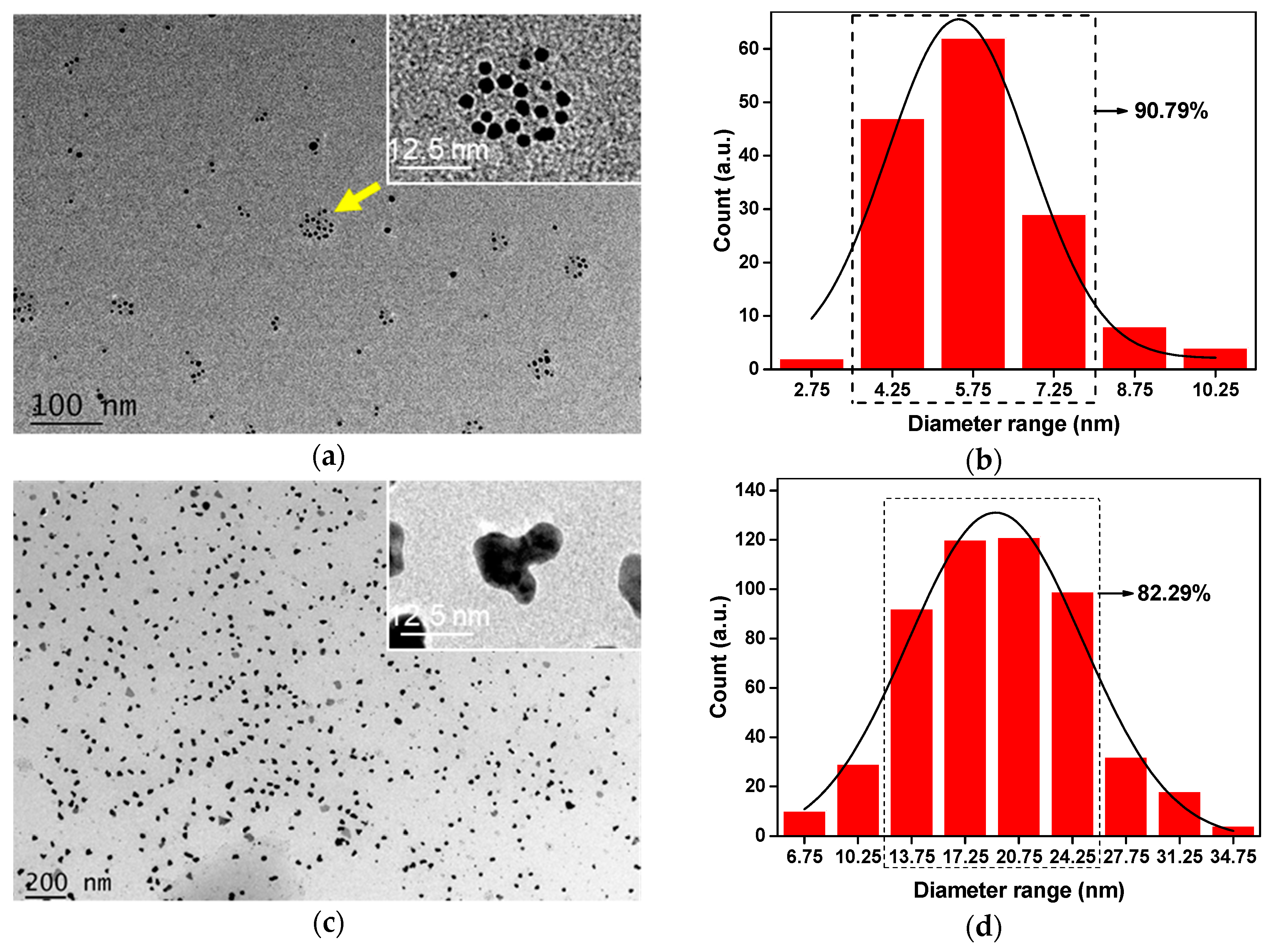
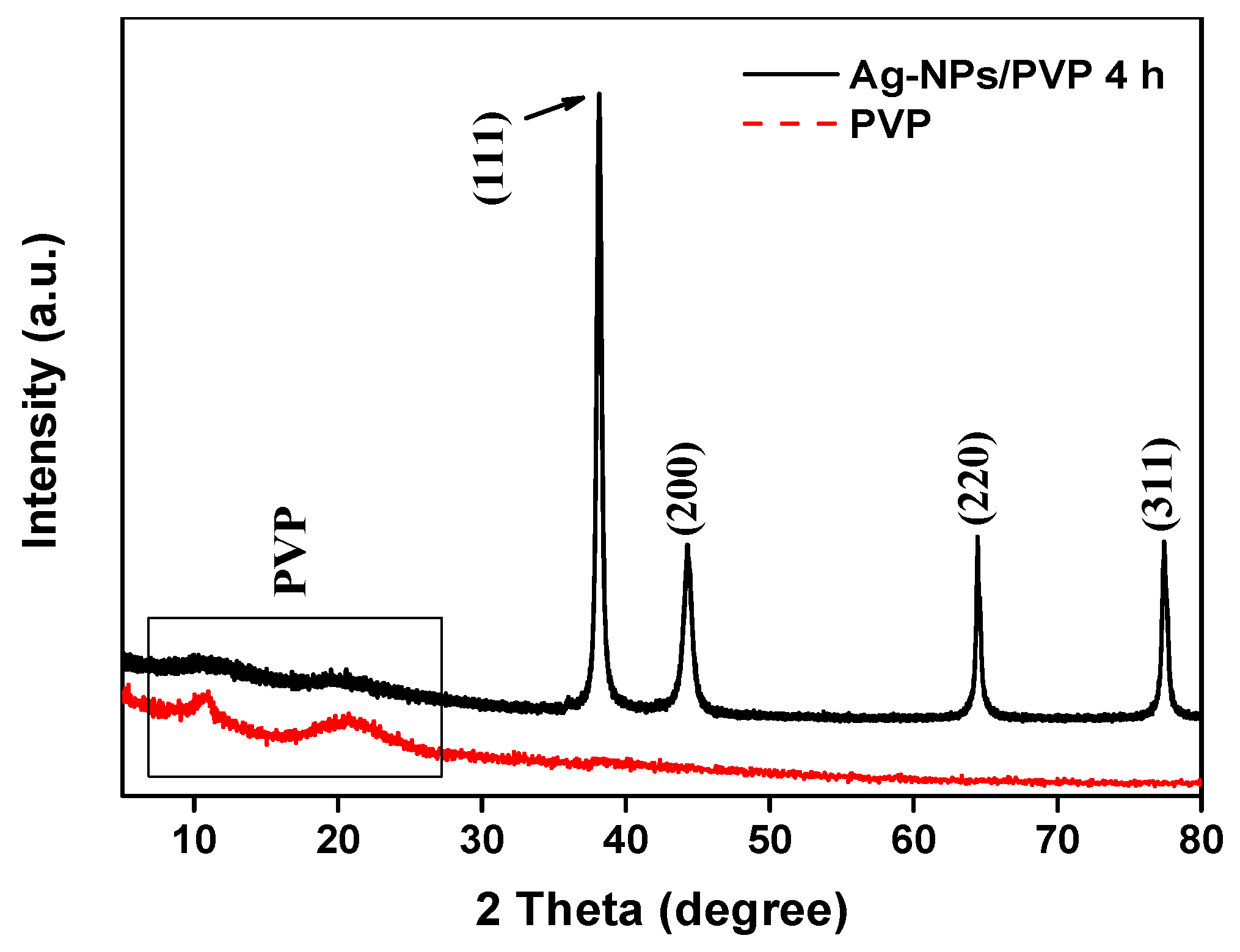
2.4. Morphology and Chemical Structure of Ag-NPs
2.5. Surface Morphology and Properties of Composite Films
2.6. Antibacterial Activities of Ag-NPs and Composite Films
3. Results and Discussion
3.1. Analysis of Ag-NPs Morphology and Chemical Structure
3.2. Analysis of Films’ Surface Morphology and Properties
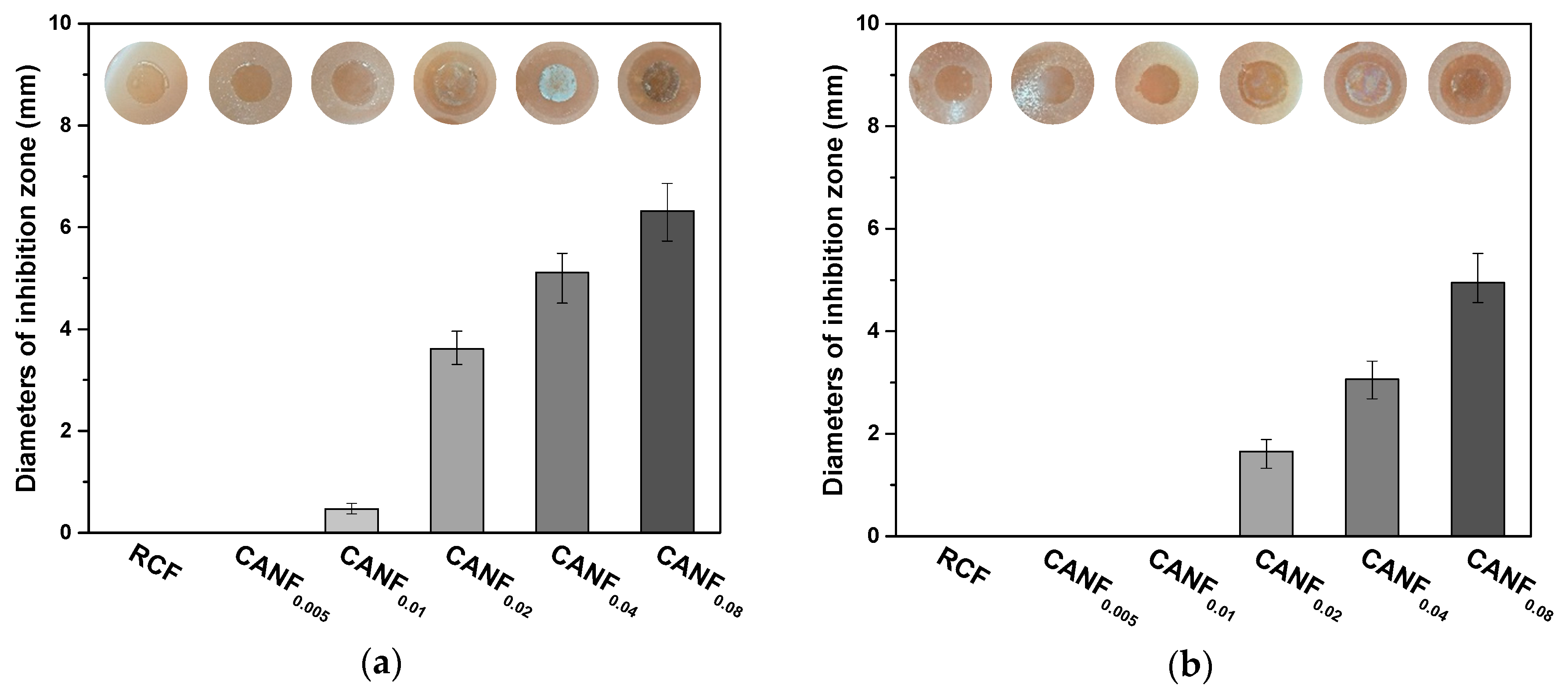
3.3. Analysis of Ag-NPs and Films’ Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Rajesh, S.; Dharanishanthi, V.; Kanna, A.V. Antibacterial mechanism of biogenic silver nanoparticles of Lactobacillus acidophilus. J. Exp. Nanosci. 2015, 10, 1143–1152. [Google Scholar] [CrossRef]
- Hachicho, N.; Hoffmann, P.; Ahlert, K.; Heipieper, H.J. Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS Microbiol. Lett. 2014, 355, 71–77. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Ahmad, M.K.; Mahdi, A.A.; Pal, R.; Cameotra, S.S. Interaction of silver nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Basic Microbiol. 2014, 54, 905–915. [Google Scholar] [CrossRef]
- Devi, L.S.; Joshi, S.R. Evaluation of the antimicrobial potency of silver nanoparticles biosynthesized by using an endophytic fungus, Cryptosporiopsis ericae PS4. J. Microbiol. 2014, 52, 667–674. [Google Scholar] [CrossRef]
- Anas, A.; Jiya, J.; Rameez, M.J.; Anand, P.B.; Anantharaman, M.R.; Nair, S. Sequential interactions of silver–silica nanocomposite (Ag–SiO2NC) with cell wall, metabolism and genetic stability of Pseudomonas aeruginosa, a multiple antibiotic-resistant bacterium. Lett. Appl. Microbiol. 2013, 56, 57–62. [Google Scholar] [CrossRef]
- Gurunathan, S. Biologically synthesized silver nanoparticles enhances antibiotic activity against Gram-negative bacteria. J. Ind. Eng. Chem. 2015, 29, 217–226. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, D.-Y. Silver nanoparticle-induced oxidative stress, genotoxicity and apoptosis in cultured cells and animal tissues. J. Appl. Toxicol. 2013, 33, 78–89. [Google Scholar] [CrossRef]
- Kora, A.J.; Sashidhar, R.B. Biogenic silver nanoparticles synthesized with rhamnogalacturonan gum: Antibacterial activity, cytotoxicity and its mode of action. Arab. J. Chem. 2018, 11, 313–323. [Google Scholar] [CrossRef]
- Vishnupriya, S.; Chaudhari, K.; Jagannathan, R.; Pradeep, T. Single-Cell Investigations of Silver Nanoparticle–Bacteria Interactions. Part. Part. Syst. Charact. 2013, 30, 1056–1062. [Google Scholar] [CrossRef]
- Ouda, S.M. Some nanoparticles effects on Proteus sp. and Klebsiella sp. isolated from water. Am. J. Infect. Dis. Microbiol. 2014, 2, 4–10. [Google Scholar]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; OU-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, J.H.; Chae, Y.J.; Kim, Y.S.; Kim, B.C.; Sang, B.-I.; Gu, M.B. Analysis of the Toxic Mode of Action of Silver Nanoparticles Using Stress-Specific Bioluminescent Bacteria. Small 2008, 4, 746–750. [Google Scholar] [CrossRef]
- Naranjo, L.P.; de Araújo, C.B. Enhanced blue photoluminescence of B2O3-CaF2 glass-ceramics containing silver nanoparticles. J. Alloy Compd. 2018, 749, 40–43. [Google Scholar] [CrossRef]
- Marassi, V.; Di Cristo, L.; Smith, S.G.J.; Ortelli, S.; Blosi, M.; Costa, A.L.; Reschiglian, P.; Volkov, Y.; Prina-Mello, A. Silver nanoparticles as a medical device in healthcare settings: A five-step approach for candidate screening of coating agents. R. Soc. Open Sci. 2018, 5, 171113. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Zaikova, T.; Dankovich, T.; Yun, J.; Ryan, M.; Hutchison, J.E.; Lowry, G.V. Effect of silver concentration and chemical transformations on release and antibacterial efficacy in silver-containing textiles. NanoImpact 2018, 11, 51–57. [Google Scholar] [CrossRef]
- Volova, T.G.; Shumilova, A.A.; Shidlovskiy, I.P.; Nikolaeva, E.D.; Sukovatiy, A.G.; Vasiliev, A.D.; Shishatskaya, E.I. Antibacterial properties of films of cellulose composites with silver nanoparticles and antibiotics. Polym. Test. 2018, 65, 54–68. [Google Scholar] [CrossRef]
- Zhai, L.; Park, J.; Lee, J.Y.; Kim, D.; Kim, J. Synthesis, characterization, and antibacterial property of eco-friendly Ag/cellulose nanocomposite film. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 420–426. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Antimicrobial poly(lactic acid)/cellulose bionanocomposite for food packaging application: A review. Food Packag. Shelf Life 2018, 17, 150–161. [Google Scholar] [CrossRef]
- Berndt, S.; Wesarg, F.; Wiegand, C.; Kralisch, D.; Müller, F.A. Antimicrobial porous hybrids consisting of bacterial nanocellulose and silver nanoparticles. Cellulose 2013, 20, 771–783. [Google Scholar] [CrossRef]
- Yang, G.; Xie, J.; Hong, F.; Cao, Z.; Yang, X. Antimicrobial activity of silver nanoparticle impregnated bacterial cellulose membrane: Effect of fermentation carbon sources of bacterial cellulose. Carbohydr. Polym. 2012, 87, 839–845. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Arul, H.; Bosak, V.Z.; Burban, A.F.; Voice, T.C.; Bruening, M.L.; Tarabara, V.V. Effect of filler incorporation route on the properties of polysulfone–silver nanocomposite membranes of different porosities. J. Membr. Sci. 2008, 325, 58–68. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.; Yue, X.; Lu, W. Review of Silver Nanoparticles (AgNPs)-Cellulose Antibacterial Composites. BioResources 2018, 13, 2150–2170. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, S.; Shi, S.Q.; Cai, L. Isolation of Cellulose from Poplar Wood by Nitric Acid-Ethanol Treatment and Its Effect on the Quality of Films Cast from Ionic Liquid. BioResources 2018, 13, 8943–8955. [Google Scholar] [CrossRef]
- McCormick, C.L.; Callais, P.A.; Hutchinson, B.H. Solution studies of cellulose in lithium chloride and N,N-dimethylacetamide. Macromolecules 1985, 18, 2394–2401. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. Formation and Stabilization of Silver Nanoparticles through Reduction by N,N-Dimethylformamide. Langmuir 1999, 15, 948–951. [Google Scholar] [CrossRef]
- Da Costa, L.P.; Formiga, A.L.B.; Mazali, I.O.; Sigoli, F.A. Spontaneous formation of highly dispersed spheroidal metallic silver nanoparticles in surfactant-free N,N-dimethylacetamide. Synth. Met. 2011, 161, 1517–1521. [Google Scholar] [CrossRef]
- Chen, D.; Qiao, X.; Qiu, X.; Chen, J. Synthesis and electrical properties of uniform silver nanoparticles for electronic applications. J. Mater. Sci. 2009, 44, 1076–1081. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Liz-Marzán, L.M. N,N-Dimethylformamide as a Reaction Medium for Metal Nanoparticle Synthesis. Adv. Funct. Mater. 2009, 19, 679–688. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kim, H.-M.; Shim, S.; Kim, T.H.; Jeong, D.H.; Lee, Y.-S.; Jun, B.-H. Silver Nanoparticle-Embedded Thin Silica-Coated Graphene Oxide as an SERS Substrate. Nanomaterials 2016, 6, 176. [Google Scholar] [CrossRef]
- Cellulose modified fibres in cement based composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 2046–2053. [CrossRef]
- Johansson, L.-S.; Campbell, J.M.; Koljonen, K.; Stenius, P. Evaluation of surface lignin on cellulose fibers with XPS. Appl. Surf. Sci. 1999, 144–145, 92–95. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Chen, W.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Facile extraction of cellulose nanocrystals from wood using ethanol and peroxide solvothermal pretreatment followed by ultrasonic nanofibrillation. Green Chem. 2016, 18, 1010–1018. [Google Scholar] [CrossRef]
- de Moura, M.R.; Mattoso, L.H.C.; Zucolotto, V. Development of cellulose-based bactericidal nanocomposites containing silver nanoparticles and their use as active food packaging. J. Food Eng. 2012, 109, 520–524. [Google Scholar] [CrossRef]
- Rac-Rumijowska, O.; Maliszewska, I.; Fiedot-Toboła, M.; Karbownik, I.; Teterycz, H. Multifunctional Nanocomposite Cellulose Fibers Doped in Situ with Silver Nanoparticles. Polymers 2019, 11, 562. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Muhler, M.; Schedel-Niedrig, T.; Schlögl, R. Interaction of oxygen with silver at high temperature and atmospheric pressure: A spectroscopic and structural analysis of a strongly bound surface species. Phys. Rev. B 1996, 54, 2249–2262. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Chen, Q.; Xiao, S.; Shi, S.Q.; Cai, L. Synthesis, Characterization, and Antibacterial Activity of N-substituted Quaternized Chitosan and Its Cellulose-based Composite Film. BioResources 2020, 15, 415–428. [Google Scholar]
- Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ. Toxicol. Pharmacol. 2010, 30, 162–168. [CrossRef]

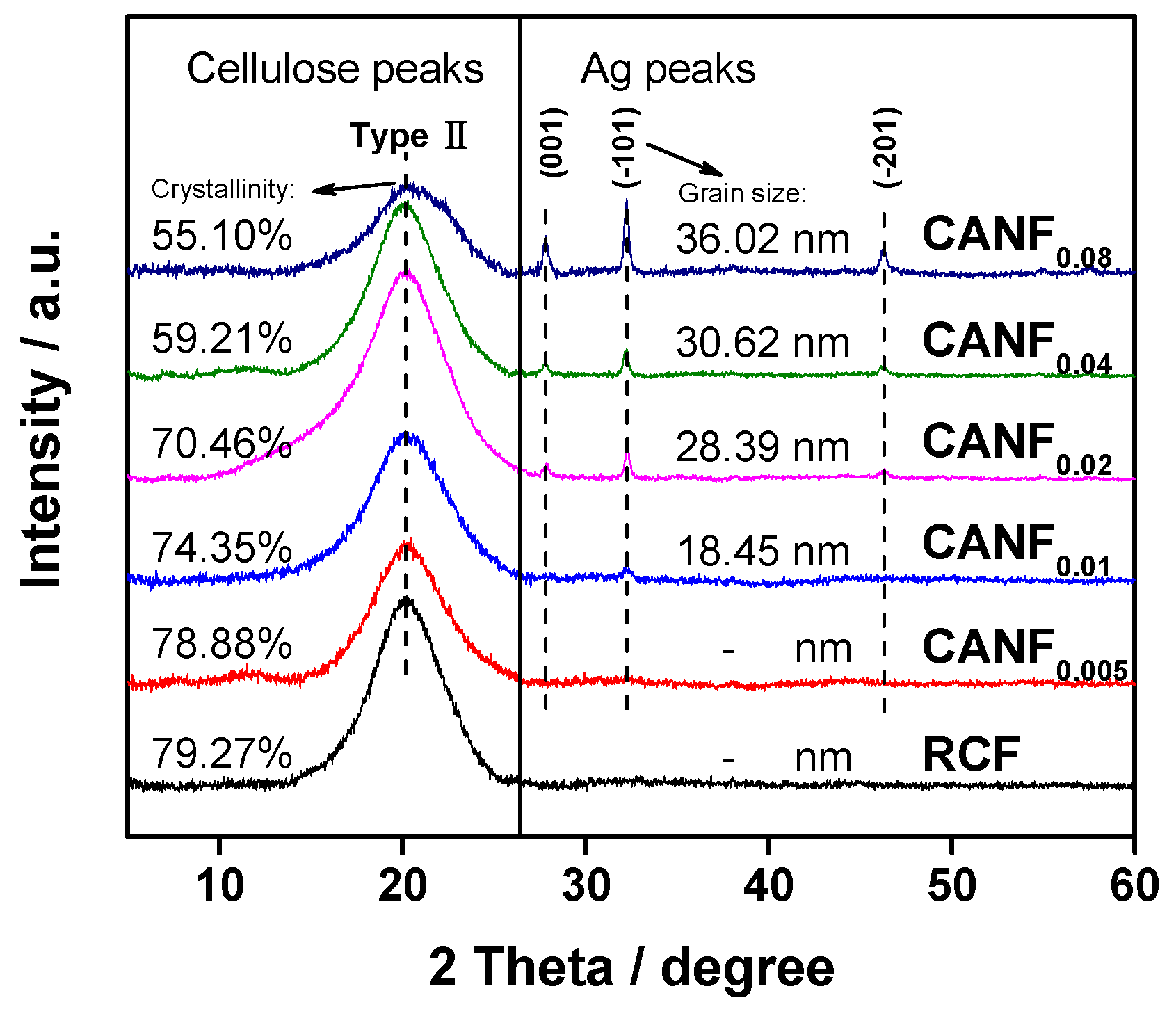

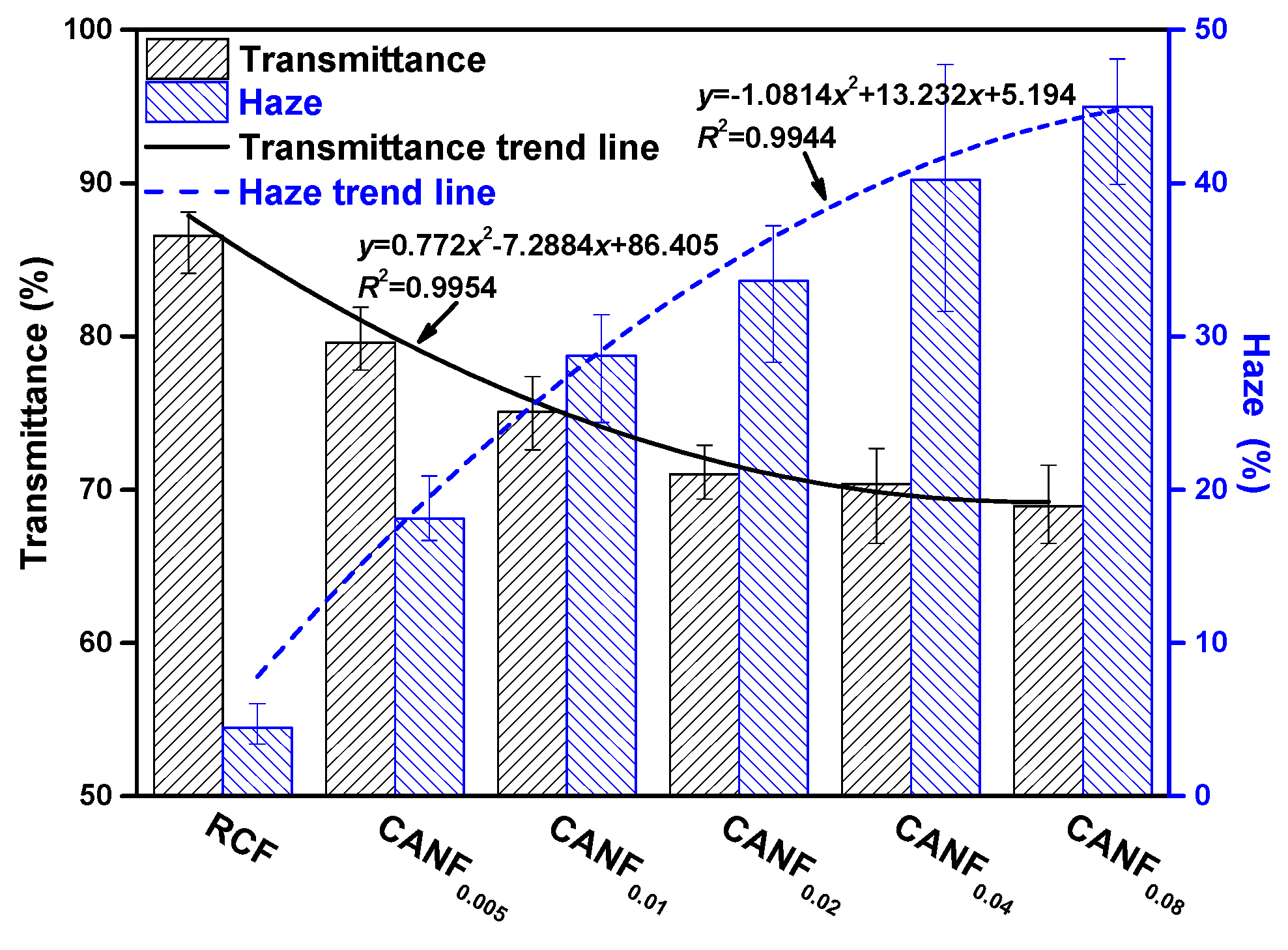
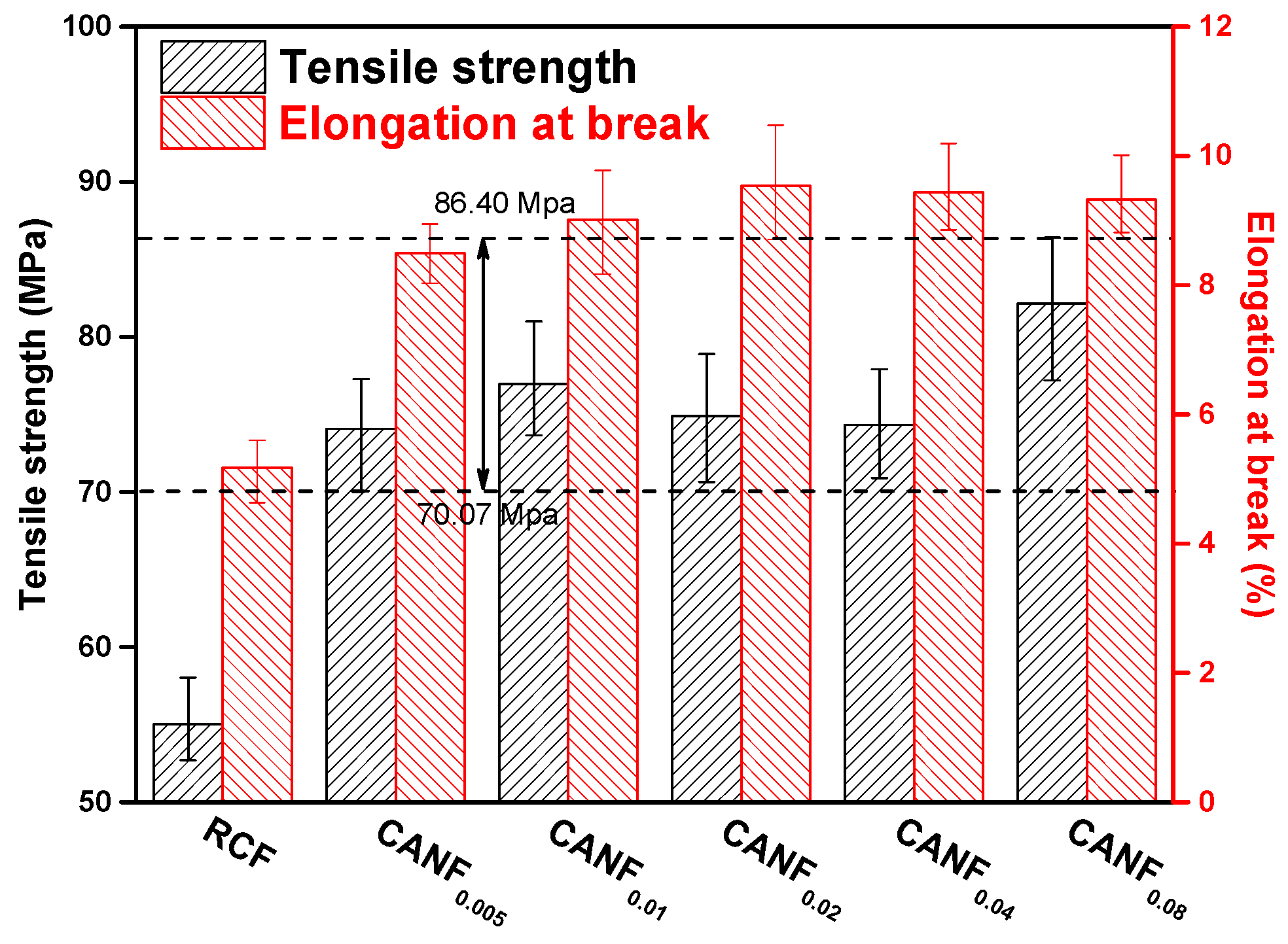
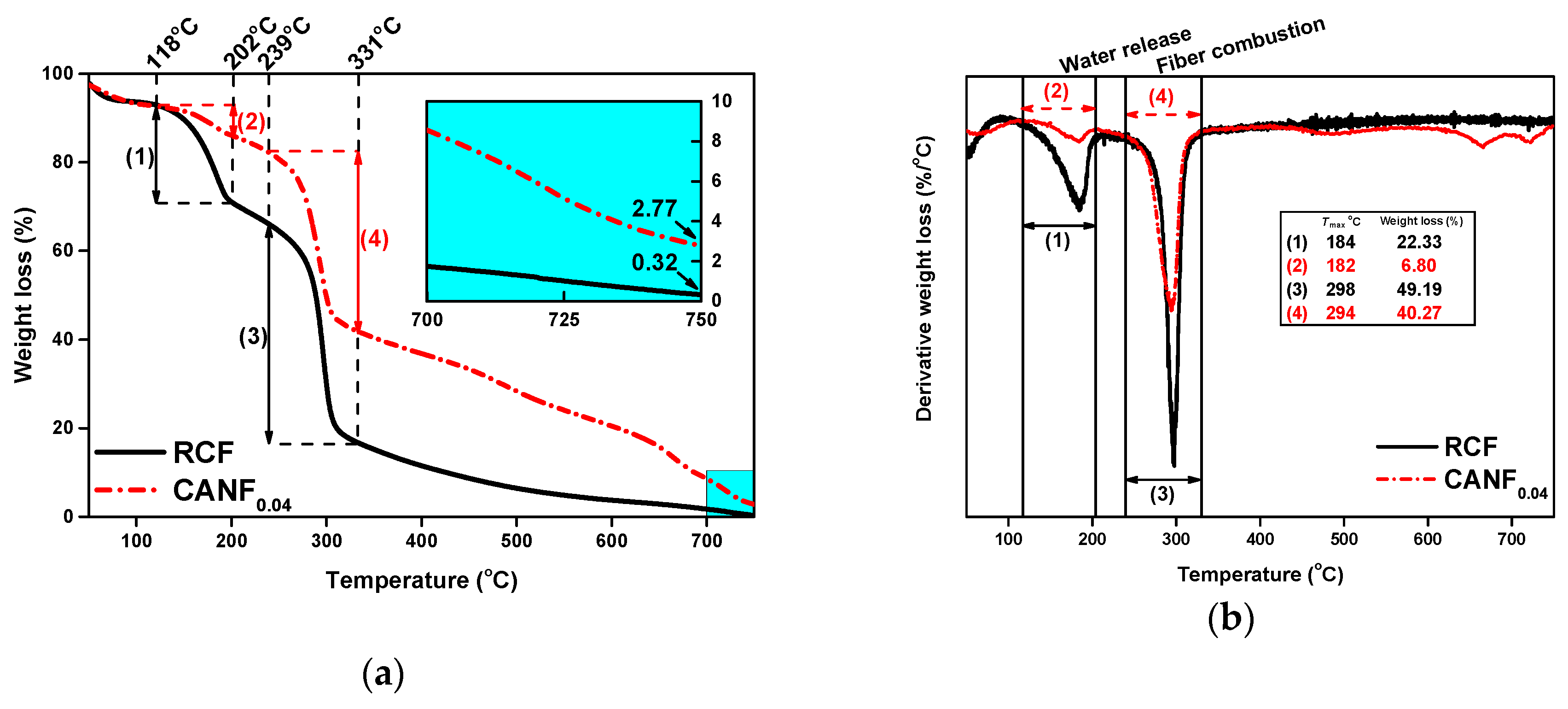
| Sample | E. coli | S. aureus | ||
|---|---|---|---|---|
| Bacterial Growth 1 | Sterilization Rate (%) | Bacterial Growth 1 | Sterilization Rate (%) | |
| Blank control | + | 0 | + | 0 |
| RCF | + | 0 | + | 0 |
| CANF0.04 | - | 100 | - | 99.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.-Y.; Xiao, S.-L.; Shi, S.Q.; Cai, L.-P. A One-Pot Synthesis and Characterization of Antibacterial Silver Nanoparticle–Cellulose Film. Polymers 2020, 12, 440. https://doi.org/10.3390/polym12020440
Chen Q-Y, Xiao S-L, Shi SQ, Cai L-P. A One-Pot Synthesis and Characterization of Antibacterial Silver Nanoparticle–Cellulose Film. Polymers. 2020; 12(2):440. https://doi.org/10.3390/polym12020440
Chicago/Turabian StyleChen, Qi-Yuan, Sheng-Ling Xiao, Sheldon Q. Shi, and Li-Ping Cai. 2020. "A One-Pot Synthesis and Characterization of Antibacterial Silver Nanoparticle–Cellulose Film" Polymers 12, no. 2: 440. https://doi.org/10.3390/polym12020440
APA StyleChen, Q.-Y., Xiao, S.-L., Shi, S. Q., & Cai, L.-P. (2020). A One-Pot Synthesis and Characterization of Antibacterial Silver Nanoparticle–Cellulose Film. Polymers, 12(2), 440. https://doi.org/10.3390/polym12020440






