Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) for Cell Culture Applications
Abstract
1. Introduction
2. Preparation of PNIPAAm Medicated Substrates for Cell Culture
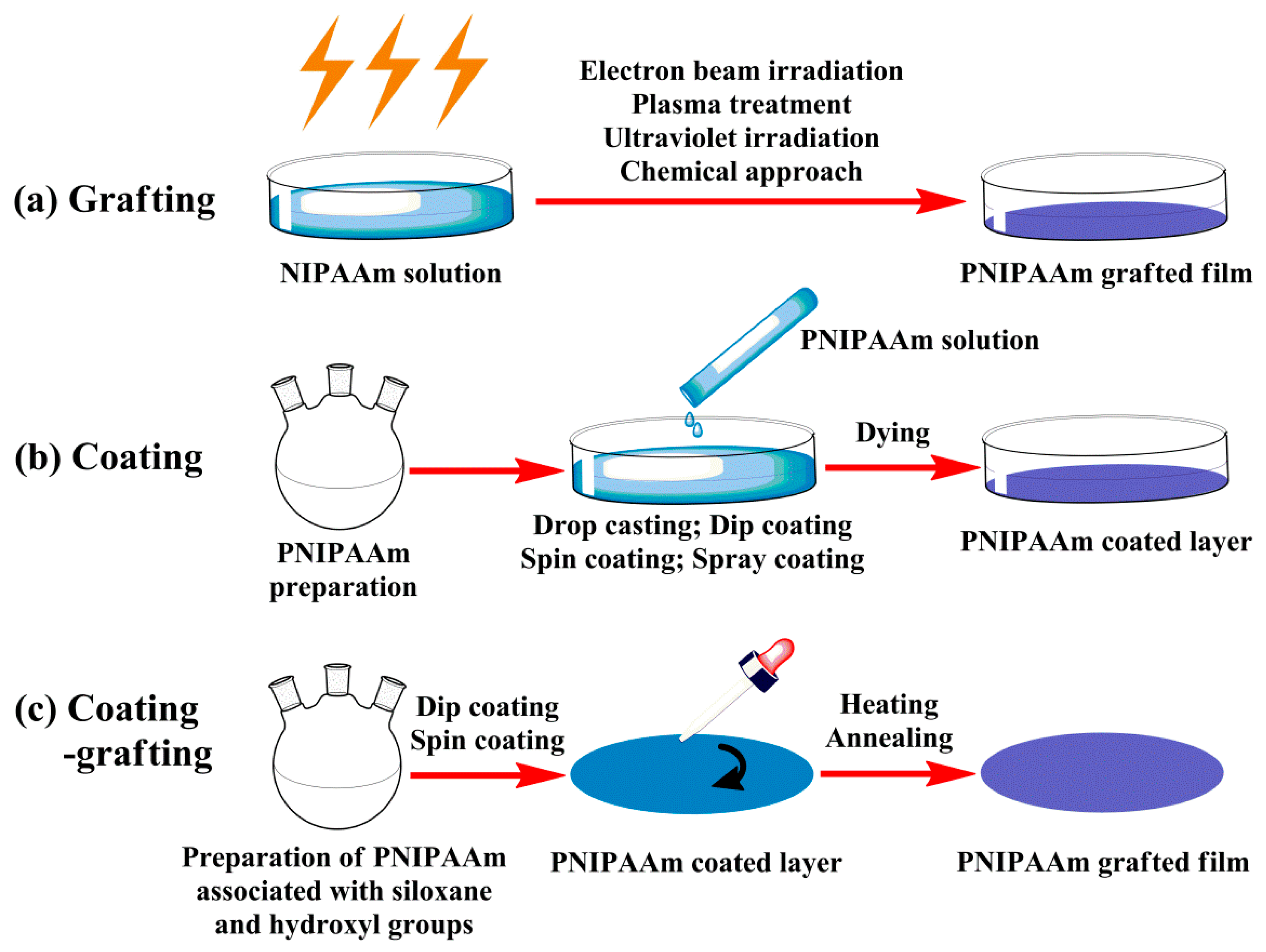
2.1. Planar Films
2.1.1. Covalent Grafting Films
2.1.2. Physically Adsorbed Coating Films
2.1.3. Grafted Coating Films
2.2. Spatial Supports
2.2.1. Thermoresponsive Hydrogels
2.2.2. Thermoresponsive Carriers
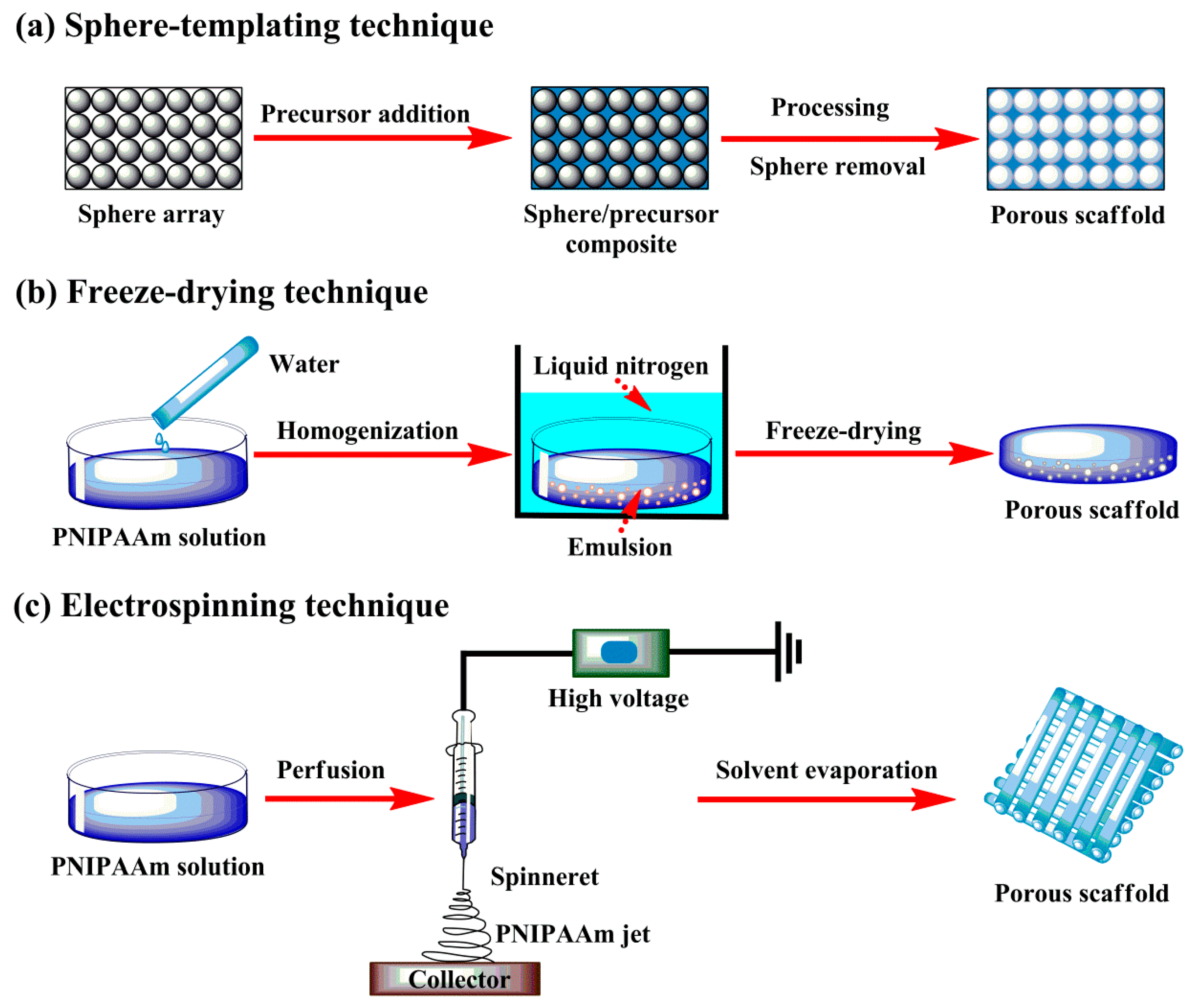
2.2.3. Thermoresponsive Scaffolds
3. Characterization of PNIPAAm Medicated Substrates for Cell Culture
3.1. Identification of PNIPAAm Homopolymers and Copolymers
3.1.1. Chemical Composition
3.1.2. Molecular Weight
3.1.3. Volume Phase Transition Temperature/Lower Critical Solution Temperature
3.2. Characterization of Thermoresponsive Planar Films
3.2.1. Chemical Composition
3.2.2. Grafting Amount
3.2.3. Surface Topography
3.2.4. Surface Wettability
3.2.5. Film Thickness
3.3. Characterization of Thermoresponsive Hydrogels
3.3.1. Thermoresponsive Swelling
3.3.2. Mechanical Properties
3.3.3. Architecture
3.4. Characterization of Thermoresponsive Carriers
3.4.1. Chemical Features
3.4.2. Size and Morphology
3.4.3. Thermoresponsive Swelling
3.5. Characterization of Thermoresponsive Scaffolds
3.5.1. Chemical Features
3.5.2. Morphology and Structure
3.5.3. Surface Wettability
3.5.4. Thermoresponsive Swelling
3.5.5. Mechanical Properties
3.6. Biocompatibility Assessment of Thermoresponsive Substrates
3.6.1. Cell Viability
3.6.2. Cell Attachment and Detachment
4. PNIPAAm Medicated Substrates Used for Cell Culture
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scarpa, J.S.; Mueller, D.D.; Klotz, I.M. Slow hydrogen-deuterium exchange in a non-a-helical polyamide. J. Am. Chem. Soc. 1967, 89, 6024–6030. [Google Scholar] [CrossRef]
- Heskins, M.; Guillet, J.E. Solution properties of poly(N-isopropylacrylamide). J. Macromol. Sci. Chem. A 1968, 2, 1441–1455. [Google Scholar] [CrossRef]
- Bae, Y.H.; Okano, T.; Kim, S.W. Temperature dependence of swelling of crosslinked poly(N,N’-alkyl substituted acrylamides) in water. J. Polym. Sci. B Polym. Phys. 1990, 28, 923–936. [Google Scholar] [CrossRef]
- Ekerdt, B.L.; Fuentes, C.M.; Lei, Y.; Adil, M.M.; Ramasubramanian, A.; Segalman, R.A.; Schaffer, D.V. Thermoreversible hyaluronic acid-PNIPAAm hydrogel systems for 3D stem cell culture. Adv. Healthc. Mater. 2018, 7, 1800225. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Seo, K.D.; Yoon, H.; Han, S.J.; Kim, D.S. Bulk poly(N-isopropylacrylamide) (PNIPAAm) thermoresponsive cell culture platform: Toward a new horizon in cell sheet engineering. Biomater. Sci. 2019, 7, 2277–2287. [Google Scholar] [CrossRef]
- Rosenthal, A.; Rauch, S.; Eichhorn, K.-J.; Stamm, M.; Uhlmann, P. Enzyme immobilization on protein-resistant PNIPAAm brushes: Impact of biotin linker length on enzyme amount and catalytic activity. Colloid Surf. B 2018, 171, 351–357. [Google Scholar] [CrossRef]
- Cheaburu-Yilmaz, C.N.; Lupuşoru, C.E.; Vasile, C. New alginate/PNIPAAm matrices for drug delivery. Polymers 2019, 11, 366. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, J.; Han, H.; Zhang, J.; Wu, F.; Qin, X.; Yu, J. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: In vitro and in vivo study. Acta Biomater. 2018, 65, 305–316. [Google Scholar] [CrossRef]
- Wei, Y.; Zeng, Q.; Hu, Q.; Wang, M.; Tao, J.; Wang, L. Self-cleaned electrochemical protein imprinting biosensor basing on a thermo-responsive memory hydrogel. Biosens. Bioelectron. 2018, 99, 136–141. [Google Scholar] [CrossRef]
- Majd, H.; Wipff, P.-J.; Buscemi, L.; Bueno, M.; Vonwil, D.; Quinn, T.M.; Hinz, B. A novel method of dynamic culture surface expansion improves mesenchymal stem cell proliferation and phenotype. Stem Cells 2009, 27, 200–209. [Google Scholar] [CrossRef]
- Macher, B.A.; Yen, T.-Y. Proteins at membrane surfaces-a review of approaches. Mol. Biosyst. 2007, 3, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Canavan, H.E.; Cheng, X.; Graham, D.J.; Ratner, B.D.; Castner, D.G. Surface characterization of the extracellular matrix remaining after cell detachment from a thermoresponsive polymer. Langmuir 2005, 21, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cheng, F.; Liu, T.; Lu, J.R.; Song, K.; Jiang, L.; Wu, S.; Guo, W. Comparison of mesenchymal stem cells released from poly(N-isopropylacrylamide) copolymer film and by trypsinization. Biomed. Mater. 2012, 7, 035003. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.G.; Zhang, G. Responsive systems for cell sheet detachment. Organogenesis 2013, 9, 93–100. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.M.P.; Mano, J.F.; Reis, R.L. Smart thermoresponsive coatings and surfaces for tissue engineering: Switching cell-material boundaries. Trends Biotechnol. 2007, 25, 577–583. [Google Scholar] [CrossRef]
- Nagase, K.; Kobayashi, J.; Okano, T. Temperature-responsive intelligent interfaces for biomolecular separation and cell sheet engineering. J. R. Soc. Interface 2009, 6 (Suppl. 3), S293–S309. [Google Scholar] [CrossRef]
- Ernst, O.; Lieske, A.; Jäger, M.; Lankenau, A.; Duschl, C. Control of cell detachment in a microfluidic device using a thermo-responsive copolymer on a gold substrate. Lab Chip 2007, 7, 1322–1329. [Google Scholar] [CrossRef]
- Mizutani, A.; Kikuchi, A.; Yamato, M.; Kanazawa, H.; Okano, T. Preparation of thermoresponsive polymer brush surfaces and their interaction with cells. Biomaterials 2008, 29, 2073–2081. [Google Scholar] [CrossRef]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Kobayashi, J.; Okano, T. Fabrication of a thermoresponsive cell culture dish: A key technology for cell sheet tissue engineering. Sci. Technol. Adv. Mater. 2010, 11, 014111. [Google Scholar] [CrossRef]
- Hirose, M.; Kwon, O.H.; Yamato, M.; Kikuchi, A.; Okano, T. Creation of designed shape cell sheets that are noninvasively harvested and moved onto another surface. Biomacromolecules 2000, 1, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Tamirisa, P.A.; Koskinen, J.; Hess, D.W. Plasma polymerized hydrogel thin films. Thin Solid Films 2006, 515, 2618–2624. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Liu, L.; Yang, W. Developments and new applications of UV-induced surface graft polymerizations. Prog. Polym. Sci. 2009, 34, 156–193. [Google Scholar] [CrossRef]
- Xu, F.J.; Zhong, S.P.; Yung, L.Y.L.; Tong, Y.W.; Kang, E.T.; Neoh, K.G. Thermoresponsive comb-shaped copolymer-Si (100) hybrids for accelerated temperature-dependent cell detachment. Biomaterials 2006, 27, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.A.; Love, S.A.; Lucero, A.E.; Haynes, C.L.; Canavan, H.E. Effect of polymer deposition method on thermoresponsive polymer films and resulting cellular behavior. Langmuir 2012, 28, 2281–2287. [Google Scholar] [PubMed]
- Vasani, R.B.; Mclnnes, S.J.P.; Cole, M.A.; Jani, A.M.; Ellis, A.V.; Voelcker, N.H. Stimulus-responsiveness and drug release from porous silicon films ATRP-grafted with poly(N-isopropylacrylamide). Langmuir 2011, 27, 7843–7853. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Higuchi, A.; Yamamoto, T.; Sugiyama, K.; Hayashi, S.; Tak, T.M.; Nakagawa, T. Temperature-dependent cell detachment on pluronic gels. Biomacromolecules 2005, 6, 691–696. [Google Scholar] [CrossRef]
- Moran, M.T.; Carroll, W.M.; Gorelov, A.; Rochev, Y. Intact endothelial cell sheet harvesting from thermoresponsive surfaces coated with cell adhesion promoters. J. R. Soc. Interface 2007, 4, 1151–1157. [Google Scholar] [CrossRef]
- Chen, B.; Dang, J.; Tan, T.L.; Fang, N.; Chen, W.N.; Leong, K.W.; Chan, V. Dynamics of smooth muscle cell deadhesion from thermosensitive hydroxybutyl chitosan. Biomaterials 2007, 28, 1503–1514. [Google Scholar] [CrossRef]
- Shi, J.; Biegler, L.T.; Hamdan, I.; Wassick, J. Optimization of grade transitions in polyethylene solution polymerization process under uncertainty. Comput. Chem. Eng. 2016, 95, 260–279. [Google Scholar] [CrossRef]
- Semsarilar, M.; Ladmiral, V.; Perrier, S. Synthesis of a cellulose supported chain transfer agent and its application to RAFT polymerization. J. Polym. Sci. Polym. Chem. 2010, 48, 4361–4365. [Google Scholar] [CrossRef]
- Yang, L.; Pan, F.; Zhao, X.; Yaseen, M.; Padia, F.; Coffey, P.; Freund, A.; Yang, L.; Liu, T.; Ma, X.; et al. Thermoresponsive copolymer nanofilms for controlling cell adhesion, growth, and detachment. Langmuir 2010, 26, 17304–17314. [Google Scholar] [CrossRef] [PubMed]
- Rusen, L.; Dinca, V.; Mitu, B.; Mustaciosu, C.; Dinescu, M. Temperature responsive functional polymeric thin films obtained by matrix assisted pulsed laser evaporation for cells attachment-detachment study. Appl. Surf. Sci. 2014, 302, 134–140. [Google Scholar] [CrossRef]
- Schmidt, S.; Motschmann, H.; Hellweg, T.; Klitzing, R.V. Thermoresponsive surfaces by spin-coating of PNIPAm-co-PAA microgels: A combined AFM and ellipsometry study. Polymer 2008, 49, 749–756. [Google Scholar] [CrossRef]
- Harmon, M.E.; Kuckling, D.; Pareek, P.; Frank, C.W. Photo-cross-linkable PNIPAAm copolymers. 4. effects of copolymerization and cross-linking on the volume-phase transition in constrained hydrogel layers. Langmuir 2003, 19, 10947–10956. [Google Scholar] [CrossRef]
- Matsuguchi, M.; Tada, A. Fabrication of poly(N-isopropylacrylamide) nanoparticles using a simple spray-coating method and applications for a QCM-based HCl gas sensor coating. Sens. Actuators B Chem. 2017, 251, 821–827. [Google Scholar] [CrossRef]
- Lee, E.L.; von Recum, H.A. Cell culture platform with mechanical conditioning and nondamaging cellular detachment. J. Biomed. Mater. Res. A 2010, 93, 411–418. [Google Scholar] [CrossRef]
- Loh, X.J.; Cheong, W.C.D.; Li, J.; Ito, Y. Novel poly(N-isopropylacrylamide)-poly[(R)-3-hydroxybutyrate]-poly(N-isopropyl acrylamide) triblock copolymer surface as a culture substrate for human mesenchymal stem cells. Soft Matter. 2009, 5, 2937–2946. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization, 4th ed.; John Wiley and Sons, INC.: New York, NY, USA, 2004. [Google Scholar]
- Zhang, J.; Cui, Z.; Field, R.; Moloney, M.G.; Rimmer, S.; Ye, H. Thermo-responsive microcarriers based on poly(N-isopropylacrylamide). Eur. Polym. J. 2015, 67, 346–364. [Google Scholar] [CrossRef]
- Qiu, J.; Charleux, B.; Matyjaszewski, K. Controlled/living radical polymerization in aqueous media: Homogeneous and heterogeneous systems. Prog. Polym. Sci. 2001, 26, 2083–2134. [Google Scholar] [CrossRef]
- Gokmen, M.T.; du Prez, F.E. Porous polymer particles-A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Williams, R.A. Manufacture of large uniform droplets using rotating membrane emulsification. J. Colloid Interface Sci. 2006, 299, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.Y.; Xie, R.; Zhu, J.H.; Chen, W.M.; Yamaguchi, T.; Nakao, S.I. Study of SPG membrane emulsification processes for the preparation of monodisperse core-shell microcapsules. J. Colloid Interface Sci. 2003, 265, 187–196. [Google Scholar] [CrossRef]
- Ma, G.H.; Nagai, M.; Omi, S. Study on preparation and morphology of uniform artificial polystyrene-poly(methyl methacrylate) composite microspheres by employing the SPG membrane emulsification technique. J. Colloid Interface Sci. 1999, 214, 264–282. [Google Scholar] [CrossRef] [PubMed]
- You, J.O.; Park, S.B.; Park, H.Y.; Haam, S.; Chung, C.H.; Kim, W.S. Preparation of regular sized Ca-alginate microspheres using membrane emulsification method. J. Microencapsul. 2001, 18, 521–532. [Google Scholar]
- Li, J.; Fan, X.; Yang, L.; Wang, F.; Zhang, J.; Wang, Z. A review on thermoresponsive cell culture systems based on poly(N-isopropylacrylamide) and derivatives. Int. J. Polym. Biomater. 2018, 67, 371–382. [Google Scholar] [CrossRef]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Sakurai, Y.; Okano, T. Dynamic contact angle measurement of temperature-responsive surface properties for poly(N-isopropylacrylamide) grafted surfaces. Macromolecules 1994, 27, 6163–6166. [Google Scholar] [CrossRef]
- Yang, H.S.; Jeon, O.; Bhang, S.H.; Lee, S.H.; Kim, B.S. Suspension culture of mammalian cells using thermosensitive microcarrier that allows cell detachment without proteolytic enzyme treatment. Cell Transpl. 2010, 19, 1123–1132. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.C.; Lee, H.Y.; Kim, J.D.; Yang, J.H. Release property of temperature-sensitive alginate beads containing poly(N-isopropylacrylamide). Colloid Surf. B 2005, 46, 57–61. [Google Scholar] [CrossRef]
- Minko, S. Grafting on solid surfaces: “grafting to” and “grafting from” methods. In Polymer Surfaces and Interfaces; Stamm, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 215–234. [Google Scholar]
- Nagase, K.; Kobayashi, J.; Kikuchi, A.; Akiyama, Y.; Kanazawa, H.; Okano, T. Effects of graft densities and chain lengths on separation of bioactive compounds by nanolayered thermoresponsive polymer brush surfaces. Langmuir 2007, 24, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Zajac, R.; Chakrabarti, A. Irreversible polymer adsorption from semidilute and moderately dense solutions. Phys. Rev. E 1995, 52, 6536–6549. [Google Scholar] [CrossRef] [PubMed]
- Kopf, A.; Baschnagel, J.; Wittmer, J.; Binder, K. On the adsorption process in polymer brushes: a monte carlo study. Macromolecules 1996, 29, 1433–1441. [Google Scholar] [CrossRef]
- Stein, A. Sphere templating methods for periodic porous solids. Microporous Mesoporous Mater. 2001, 44, 227–239. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cell Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Singh, O.; Arora, S.; Murthy, R.S.R. Scaffold: A novel carrier for cell and drug delivery. Crit. Rev. Drug 2012, 29, 1–63. [Google Scholar] [CrossRef]
- Sponchioni, M.; Palmiero, U.C.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, S.; Xiao, L. Synthesis and characterization of PNIPAm core cross-linked star polymers and their functionalization with cyclodextrin. Macromol. Chem. Phys. 2015, 216, 749–760. [Google Scholar] [CrossRef]
- Tang, Z.; Guan, Y.; Zhang, Y. Contraction-type glucose-sensitive microgel functionalized with a 2-substituted phenylboronic acid ligand. Polym. Chem. 2014, 5, 1782–1790. [Google Scholar] [CrossRef]
- Ding, A.; Lu, G.; Guo, H.; Zheng, X.; Huang, X. SET-LRP synthesis of PMHDO-g-PNIPAM well-defined amphiphilic graft copolymer. J. Polym. Sci. Polym. Chem. 2013, 51, 1091–1098. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Ye, G.; Zhang, B.; Zhu, D.; Yao, K.; Liu, Z.; Sheng, X. Thermosensitive N-isopropylacrylamide-N-propylacrylamide-vinyl pyrrolidone terpolymers: Synthesis, characterization and preliminary application as embolic agents. Biomaterials 2005, 26, 7002–7011. [Google Scholar] [CrossRef] [PubMed]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angewandte Chemie Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Saghebasl, S.; Davaran, S.; Rahbarghazi, R.; Montaseri, A.; Salehi, R.; Ramazani, A. Synthesis and in vitro evaluation of thermosensitive hydrogel scaffolds based on (PNIPAAm-PCL-PEG-PCL-PNIPAAm)/Gelatin and (PCL-PEG-PCL)/Gelatin for use in cartilage tissue engineering. J. Biomater. Sci. Polym. E 2018, 29, 1185–1206. [Google Scholar] [CrossRef]
- Nojima, R.; Sato, T.; Qiu, X.; Winnik, F.M. Light scattering evidence for the random association of flower micelles of a telechelic hydrophobically modified poly(N-isopropylacrylamide) in dilute aqueous solution. Macromolecules 2008, 41, 292–294. [Google Scholar] [CrossRef]
- David, S.O. Mass spectrometry desk reference 2. J. Am. Soc. Mass Spectrom. 2000, 11, 1144. [Google Scholar]
- Cui, X.; Hartanto, Y.; Wu, C.; Bi, J.; Dai, S.; Zhang, H. Tuning microenvironment for multicellular spheroid formation in thermoresponsive anionic microgel scaffolds. J. Biomed. Mater. Res. A 2018, 106, 2899–2909. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, J.; Ding, Y.; Ye, X. Effect of urea on phase transition of poly(N-isopropylacrylamide) investigated by differential scanning calorimetry. J. Phys. Chem. B 2014, 118, 9460–9466. [Google Scholar] [CrossRef]
- Kokufuta, E.; Ogawa, K.; Doi, R.; Kikuchi, R.; Farinato, R.S. Geometrical characteristics of polyelectrolyte nanogel particles and their polyelectrolyte complexes studied by dynamic and static light scattering. J. Phys. Chem. B 2007, 111, 8634–8640. [Google Scholar] [CrossRef]
- Xie, D.; Ye, X.; Ding, Y.; Zhang, G.; Zhao, N.; Wu, K.; Cao, Y.; Zhu, X.X. Multistep thermosensitivity of poly(N-n-propylacrylamide)-block-poly(N-isopropylacrylamide)-block-poly(N,N-ethylmethylacrylamide) triblock terpolymers in aqueous solutions as studied by static and dynamic light scattering. Macromolecules 2009, 42, 2715–2720. [Google Scholar] [CrossRef]
- Zheng, S.; Shi, S.; Xia, Y.; Wu, Q.; Su, Z.; Chen, X. Study on micellization of poly(N-isopropylacrylamide- butyl acrylate) macromonomers in aqueous solution. J. Appl. Polym. Sci. 2010, 118, 671–677. [Google Scholar] [CrossRef]
- Cole, M.A.; Voelcker, N.H.; Thissen, H.; Griesser, H.J. Stimuli-responsive interfaces and systems for the control of protein-surface and cell-surface interactions. Biomaterials 2009, 30, 1827–1850. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Canavan, H.E.; Stein, M.J.; Hull, J.R.; Kweskin, S.J.; Wagner, M.S.; Somorjai, G.A.; Castner, D.G.; Ratner, B.D. Surface chemical and mechanical properties of plasma-polymerized N-isopropylacrylamide. Langmuir 2005, 21, 7833–7841. [Google Scholar] [CrossRef] [PubMed]
- Canavan, H.E.; Graham, D.J.; Cheng, X.; Ratner, B.D.; Castner, D.G. Comparison of native extracellular matrix with adsorbed protein films using secondary ion mass spectrometry. Langmuir 2007, 23, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.A.; Jasieniak, M.; Thissen, H.; Voelcker, N.H.; Griesser, H.J. Time-of-flight-secondary ion mass spectrometry study of the temperature dependence of protein adsorption onto poly(N-isopropylacrylamide) graft coatings. Anal. Chem. 2009, 81, 6905–6912. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Ultrathin poly(N-isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir 2004, 20, 5506–5511. [Google Scholar] [CrossRef]
- Na, K.; Jung, J.; Kim, O.; Lee, J.; Lee, T.G.; Park, Y.H.; Hyun, J. “Smart” biopolymer for a reversible stimuli-responsive platform in cell-based biochips. Langmuir 2008, 24, 4917–4923. [Google Scholar] [CrossRef]
- Xu, F.J.; Zhong, S.P.; Yung, L.Y.L.; Kang, E.T.; Neoh, K.G. Surface-active and stimuli-responsive polymer-Si (100) hybrids from surface-initiated atom transfer radical polymerization for control of cell adhesion. Biomacromolecules 2004, 5, 2392–2403. [Google Scholar] [CrossRef]
- Chen, B.; Xu, F.J.; Fang, N.; Neoh, K.G.; Kang, E.T.; Chen, W.N.; Chan, V. Engineering cell de-adhesion dynamics on thermoresponsive poly(N-isopropylacrylamide). Acta Biomater. 2008, 4, 218–229. [Google Scholar] [CrossRef]
- Elashnikov, R.; Radocha, M.; Rimpelova, S.; Švorčíka, V.; Lyutakov, O. Thickness and substrate dependences of phase transition, drug release and antibacterial properties of PNIPAm-co-AAC films. RSC Adv. 2015, 5, 86825–86831. [Google Scholar] [CrossRef]
- Curti, P.S.; de Moura, M.R.; Veiga, W.; Radovanovic, E.; Rubira, A.F.; Muniz, E.C. Characterization of PNIPAAm photografted on PET and PS surfaces. Appl. Surf. Sci. 2005, 245, 223–233. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Thermoresponsive surfaces for cell culture and enzyme-free cell detachment. Prog. Polym. Sci. 2010, 35, 1311–1324. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, J.R.; Lewis, A.L.; Vick, T.A.; Stratford, P.W. Swelling of zwitterionic polymer films characterized by spectroscopic ellipsometry. Macromolecules 2001, 34, 8768–8776. [Google Scholar] [CrossRef]
- Li, P.; Hou, X.; Qu, L.; Dai, X.; Zhang, C. PNIPAM-MAPOSS hybrid hydrogels with excellent swelling behavior and enhanced mechanical performance: Preparation and drug release of 5-fluorouracil. Polymers 2018, 10, 137. [Google Scholar]
- Thomas, J.D.; Fussell, G.; Sarkar, S.; Lowman, A.M.; Marcolongo, M. Synthesis and recovery characteristics of branched and grafted PNIPAAm-PEG hydrogels for the development of an injectable load-bearing nucleus pulposus replacement. Acta Biomater. 2010, 6, 1319–1328. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, C.; Keller, T.; Bhat, R.; Gottschaldt, M.; Schubert, U.S.; Jandt, K.D. Monodisperse, temperature-sensitive microgels crosslinked by Si-O-Si bonds. Macromol. Mater. Eng. 2009, 294, 396–404. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Dong, L.; Hoffman, A.S. A novel approach for preparation of pH-sensitive hydrogels for enteric drug delivery. J. Control. Release 1991, 15, 141–152. [Google Scholar] [CrossRef]
- Öle Kiminta, D.M.; Luckham, P.F.; Lenon, S. The rheology of deformable and thermoresponsive microgel particles. Polymer 1995, 36, 4827–4831. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Cell microcarriers and microcapsules of stimuli-responsive polymers. J. Control. Release 2011, 149, 209–224. [Google Scholar] [CrossRef]
- Almeida, E.A.M.S.; Bellettini, I.C.; Garcia, F.P.; Farinácio, M.T.; Nakamura, C.V.; Rubira, A.F.; Martins, A.F.; Muniz, E.C. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr. Polym. 2017, 171, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Gicquel, E.; Martin, C.; Heux, L.; Jean, B.; Bras, J. Adsorption versus grafting of poly(N-isopropylacrylamide) in aqueous conditions on the surface of cellulose nanocrystals. Carbohydr. Polym. 2019, 210, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, S.; Çakmak, A.S.; Gümüşderelioğlu, M. PNIPAAm-grafted thermoresponsive microcarriers: Surface-initiated ATRP synthesis and characterization. Mater. Sci. Eng. C 2013, 33, 3033–3040. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Tsai, A.-C.; Farrance, I.; Rowley, J.A.; Ma, T. Aggregation of culture expanded human mesenchymal stem cells in microcarrier-based bioreactor. Biochem. Eng. J. 2018, 131, 39–46. [Google Scholar] [CrossRef]
- Gümüşderelioğlu, M.; Çakmak, S.; Timuçin, H.Ö.; Çakmak, A.S. Thermosensitive PHEMA microcarriers: ATRP synthesis, characterization, and usabilities in cell cultures. J. Biomater. Sci. Polym. E 2013, 24, 2110–2125. [Google Scholar] [CrossRef]
- Song, K.; Yang, Y.; Wu, S.; Zhang, Y.; Feng, S.; Wang, H.; Wang, Y.; Wang, L.; Liu, T. In vitro culture and harvest of BMMSCs on the surface of a novel thermosensitive glass microcarrier. Mater. Sci. Eng. C 2016, 58, 324–330. [Google Scholar] [CrossRef]
- Nguyen, L.T.B.; Odeleye, A.O.O.; Chui, C.-Y.; Baudequin, T.; Cui, Z.; Ye, H. Development of thermo-responsive polycaprolactone macrocarriers conjugated with Poly(N-isopropyl acrylamide) for cell culture. Sci. Rep. 2019, 9, 3477. [Google Scholar] [CrossRef]
- Nasrazadani, S.; Hassani, S. Modern analytical techniques in failure analysis of aerospace, chemical, and oil and gas industries. In Handbook of Materials Failure Analysis with Case Studies from the Oil and Gas Industry; Makhlouf, A.S.H., Aliofkhazraei, M., Eds.; Elsevier: Oxford, UK, 2016; pp. 39–54. [Google Scholar]
- Kim, Y.-J.; Kim, S.H.; Fujii, T.; Matsunaga, Y.T. Dual stimuli-responsive smart beads that allow “on–off” manipulation of cancer cells. Biomater. Sci. 2016, 4, 953–957. [Google Scholar] [CrossRef]
- Peniche, H.; Reyes-Ortega, F.; Aguilar, M.R.; Rodríguez, G.; Abradelo, C.; García-Fernández, L.; Peniche, C.; Román, J.S. Thermosensitive macroporous cryogels functionalized with bioactive chitosan/bemiparin nanoparticles. Macromol. Biosci. 2013, 13, 1556–1567. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, Y.; Hattori, S.; Terada, D.; Sharma, A.K.; Turner, A.P.F.; Kobayashi, H. Influence of poly(N-isopropylacrylamide)-CNT-polyaniline three-dimensional electrospun microfabric scaffolds on cell growth and viability. Biopolymers 2013, 99, 334–341. [Google Scholar] [CrossRef]
- Chetty, A.S.; Vargha, V.; Maity, A.; Moolman, F.S.; Rossouw, C.; Anandjiwala, R.; Boguslavsky, L.; Mancama, D.; Focke, W.W. Development of thermoresponsive poly(propylene-g-N-isopropylacrylamide) non-woven 3D scaffold for smart cell culture using oxyfluorination-assisted graft polymerization. Colloids Surf. A Phys. Eng. Asp. 2013, 419, 37–45. [Google Scholar] [CrossRef]
- Hashmi, B.; Mammoto, T.; Weaver, J.; Ferrante, T.; Jiang, A.; Jiang, E.; Feliz, J.; Ingber, D.E. Mechanical induction of dentin-like differentiation by adult mouse bone marrow stromal cells using compressive scaffolds. Stem Cell Res. 2017, 24, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Mellati, A.; Kiamahalleh, M.V.; Madani, S.H.; Dai, S.; Bi, J.; Jin, B.; Zhang, H. Poly(N-isopropylacrylamide) hydrogel/chitosan scaffold hybrid for three-dimensional stem cell culture and cartilage tissue engineering. J. Biomed. Mater. Res. A 2016, 104, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Magaz, A.; Maughan, E.; Oliver, N.; Darbyshire, A.; Loizidou, M.; Emberton, M.; Birchall, M.; Song, W. Cellular responses to thermoresponsive stiffness memory elastomer nanohybrid scaffolds by 3D-TIPS. Acta Biomater. 2019, 85, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018, 66, 166–176. [Google Scholar] [CrossRef]
- Jiang, E.; Tang, D.; Yu, Z. Electronspun thermal responsive and photocatalytic Zn(NO3)2/PNIPAAm nanofibers. Adv. Mater. Res. 2013, 710, 50–54. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef]
- Kou, R.; Xu, Z.; Deng, H.; Liu, Z.; Seta, P.; Xu, Y. Surface modification of microporous polypropylene membranes by plasma-induced graft polymerization of α-allyl glucoside. Langmuir 2003, 19, 6869–6875. [Google Scholar] [CrossRef]
- Hendrick, V.; Muniz, E.; Geuskens, G.; Wérenne, J. Adhesion, growth and detachment of cells on modified polystyrene surface. Cytotechnology 2001, 36, 49–53. [Google Scholar] [CrossRef]
- Zhao, T.; Chen, H.; Zheng, J.; Yu, Q.; Wu, Z.; Yuan, L. Inhibition of protein adsorption and cell adhesion on PNIPAAm-grafted polyurethane surface: Effect of graft molecular weight. Colloids Surf. B Biointerfaces 2011, 85, 26–31. [Google Scholar] [CrossRef]
- Sanfilippo, S.; Canis, M.; Ouchchane, L.; Botchorishvili, R.; Artonne, C.; Janny, L.; Brugnon, F. Viability assessment of fresh and frozen/thawed isolated human follicles: Reliability of two methods (Trypan blue and Calcein AM/ethidium homodimer-1). J. Assist. Reprod. Genet. 2011, 28, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Tang, D.; Wu, H.; Wang, X.; Cao, M.; He, H.; Wang, S. Cell attachment/detachment behavior on poly(Nisopropylacrylamide)-based microgel films: The effect of microgel structure and swelling ratio. J. Mater. Sci. 2018, 53, 8795–8806. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, F.; Zhao, Y.; Chen, L.; Zhang, R.; Guo, G. Cell proliferation and thermally induced cell detachment of galactosylated thermo-responsive hydrogels. Carbohydr. Polym. 2010, 82, 578–584. [Google Scholar] [CrossRef]
- He, X.; Nie, P.; Chen, B.; Li, X.; Chen, L.; Guo, G.; Zhang, R. A novel method to fabricate thermoresponsive microstructures with improved cell attachment/detachment properties. J. Biomed. Mater. Res. A 2012, 100, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Iqbal, S.M.; Wan, Y. Cell detachment: Post-isolation challenges. Biotechnol. Adv. 2013, 31, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, D.J.; Bencherif, S.A.; Srinivasan, A.; Hollinger, J.O.; Matyjaszewski, K. Synthesis, characterization, and in vitro cell culture viability of degradable poly(N-isopropylacrylamide-co-5,6- benzo-2-methylene-1,3-dioxepane)-based polymers and crosslinked gels. J. Biomed. Mater. Res. A 2008, 87, 345–358. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Y.; Li, B.; Gao, C. Fabrication of thermoresponsive polymer gradients for study of cell adhesion and detachment. Langmuir 2008, 24, 13632–13639. [Google Scholar] [CrossRef]
- Cooperstein, M.A.; Canavan, H.E. Biological cell detachment from poly(N-isopropyl acrylamide) and its applications. Langmuir 2010, 26, 7695–7707. [Google Scholar] [CrossRef]
- Xia, Y.; He, X.; Cao, M.; Wang, X.; Sun, Y.; He, H.; Xu, H.; Lu, J.R. Self-assembled two-dimensional thermoresponsive microgel arrays for cell growth/detachment control. Biomacromolecules 2014, 15, 4021–4031. [Google Scholar] [CrossRef]
- Gillet, R.; Sakai, H.; Nabae, Y.; Hayakawa, T.; Kakimoto, M. Synthesis of hyperbranched-linear poly(N-isopropylacrylamide) polymers with a poly(siloxysilane) hyperbranched macroinitiator, and their application to cell culture on glass substrates. Polym. J. 2016, 48, 1007–1012. [Google Scholar] [CrossRef]
- Alghunaim, A.; Brink, E.T.; Newby, B.Z. Surface immobilization of thermo-responsive poly(N-isopropylacrylamide) by simple entrapment in a 3-aminopropyltriethoxysilane network. Polymer 2016, 101, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Stanton, M.M.; Lambert, C.R. A thermoresponsive, micro-roughened cell culture surface. Acta Biomater. 2015, 15, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lu, X.; Zhu, D.; Lu, Q. Targeted grafting of thermoresponsive polymers from a penetrative honeycomb structure for cell sheet engineering. Soft Matter. 2015, 11, 7420–7427. [Google Scholar] [CrossRef]
- Healy, D.; Nash, M.E.; Gorelov, A.; Thompson, K.; Dockery, P.; Belochapkine, S.; Madden, J.; Rochev, Y.A. Nanometer-scale physically adsorbed thermoresponsive films for cell culture. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 221–234. [Google Scholar] [CrossRef]
- Bluestein, B.M.; Reed, J.A.; Canavan, H.E. Effect of substrate storage conditions on the stability of “smart” films used for mammalian cell applications. Appl. Surf. Sci. 2017, 392, 950–959. [Google Scholar] [CrossRef]
- Akiyama, Y.; Kikuchi, A.; Yamato, M.; Okano, T. Accelerated cell-sheet recovery from a surface successively grafted with polyacrylamide and poly(N-isopropylacrylamide). Acta Biomater. 2014, 10, 3398–3408. [Google Scholar] [CrossRef]
- Tang, Z.; Akiyama, Y.; Itoga, K.; Kobayashi, J.; Yamato, M.; Okano, T. Shear stress-dependent cell detachment from temperature-responsive cell culture surfaces in a microfluidic device. Biomaterials 2012, 33, 7405–7411. [Google Scholar] [CrossRef]
- Park, B.R.; Nabae, Y.; Surapati, M.; Hayakawa, T.; Kakimoto, M. Poly(N-isopropylacrylamide)-modified silica beads with hyperbranched polysiloxysilane for three-dimensional cell cultivation. Polym. J. 2013, 45, 210–215. [Google Scholar] [CrossRef]
- Chen, J.P.; Tsai, M.J.; Liao, H.T. Incorporation of biphasic calcium phosphate microparticles in injectable thermoresponsive hydrogel modulates bone cell proliferation and differentiation. Colloid Surf. B 2013, 110, 120–129. [Google Scholar] [CrossRef]
- Zhuang, M.; Liu, T.; Ge, D.; Song, K.; Guan, S. Preservation of osteoblasts and BM-MSCs biological properties after consecutive passages with the thermal-liftoff method. RSC Adv. 2016, 6, 91567–91578. [Google Scholar] [CrossRef]



| Substrate Type | Measurement Data | Detection Techniques |
|---|---|---|
| PNIPAAm homopolymers and copolymers | Chemical composition | Element analysis, FTIR, NMR |
| Molecular weight | SEC, SLS, MS | |
| Phase transition temperature Lower critical solution temperature | DSC, DLS, turbidity measurement | |
| Thermoresponsive planar films | Chemical composition | XPS, SFG, TOF-SIMS |
| Grafting density | Gravimetric method, ATR-FTIR | |
| Surface topology | SEM, AFM, profilometer | |
| Surface wettability | Contact angle measurement | |
| Film thickness | SE, SPR | |
| Thermoresponsive hydrogels | Chemical composition | FTIR, NMR, XPS |
| Thermoresponsive swelling | DLS, DSC, ATR-FTIR, dynamic rheology, gravimetric method | |
| Mechanical properties | Shear-flow treatment, compression assay, mechanical stress | |
| Architecture | Raman microscopy, SEM | |
| Thermoresponsive carriers | Chemical composition | Elemental analysis, ATR-FTIR, NMR, XPS, SEM/EDS system |
| Size and morphology | Optical microscopy, SEM, AFM | |
| Thermoresponsive swelling | Dynamic rheology, DLS, optical microscopy | |
| Thermoresponsive scaffolds | Chemical composition | ATR-FTIR, NMR, XPS, SEM/EDS system |
| Morphology and structure | SEM, AFM, optical microscopy, digital calipers, liquid displacement method, BET, BJH | |
| Surface wettability | Contact angle measurement | |
| Thermoresponsive swelling | Gravimetric method | |
| Mechanical properties | Compression assay, mechanical stress |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Fan, X.; Zhang, J.; Ju, J. Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) for Cell Culture Applications. Polymers 2020, 12, 389. https://doi.org/10.3390/polym12020389
Yang L, Fan X, Zhang J, Ju J. Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) for Cell Culture Applications. Polymers. 2020; 12(2):389. https://doi.org/10.3390/polym12020389
Chicago/Turabian StyleYang, Lei, Xiaoguang Fan, Jing Zhang, and Jia Ju. 2020. "Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) for Cell Culture Applications" Polymers 12, no. 2: 389. https://doi.org/10.3390/polym12020389
APA StyleYang, L., Fan, X., Zhang, J., & Ju, J. (2020). Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) for Cell Culture Applications. Polymers, 12(2), 389. https://doi.org/10.3390/polym12020389







