Fluoroalkyl Pentacarbonylmanganese(I) Complexes as Initiators for the Radical (co)Polymerization of Fluoromonomers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. VDF Polymerizations with [Mn(RF)(CO)5]
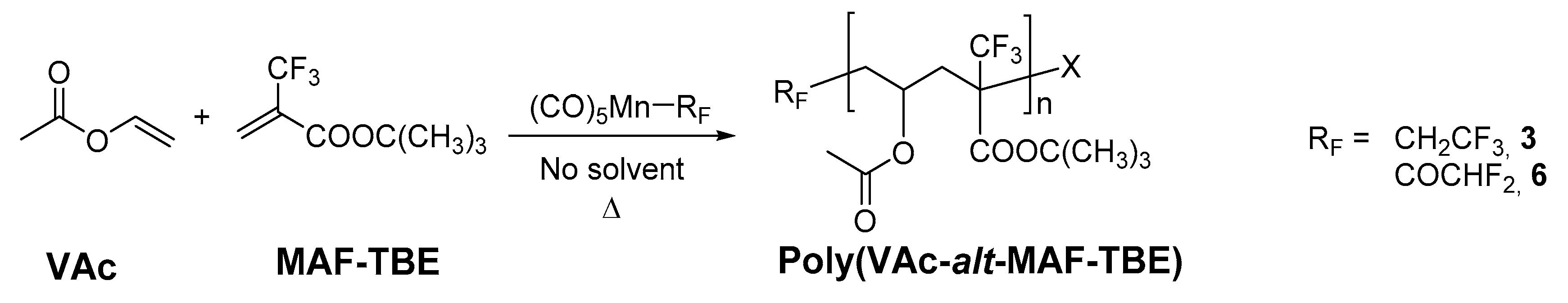
2.4. Copolymerization of VAc and MAF-TBE
3. Results
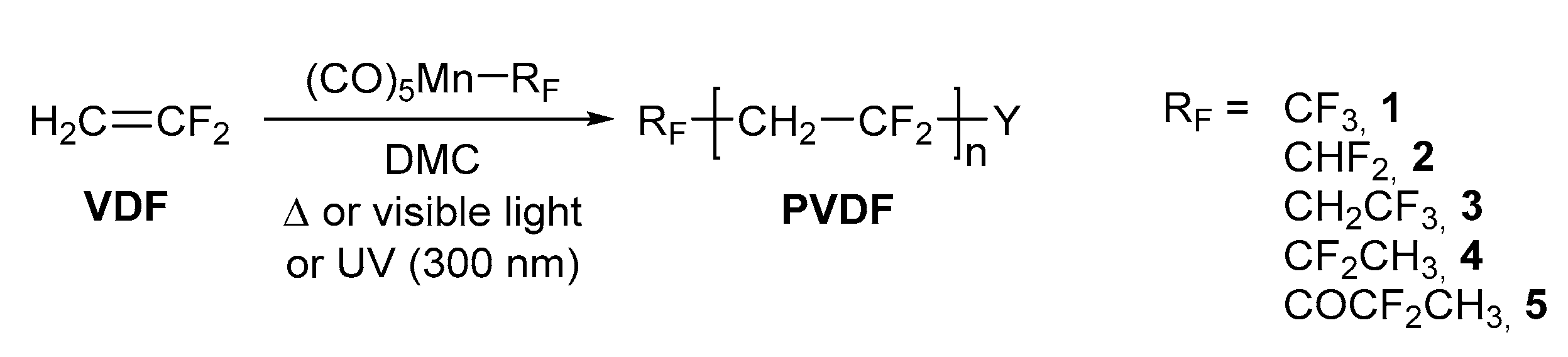
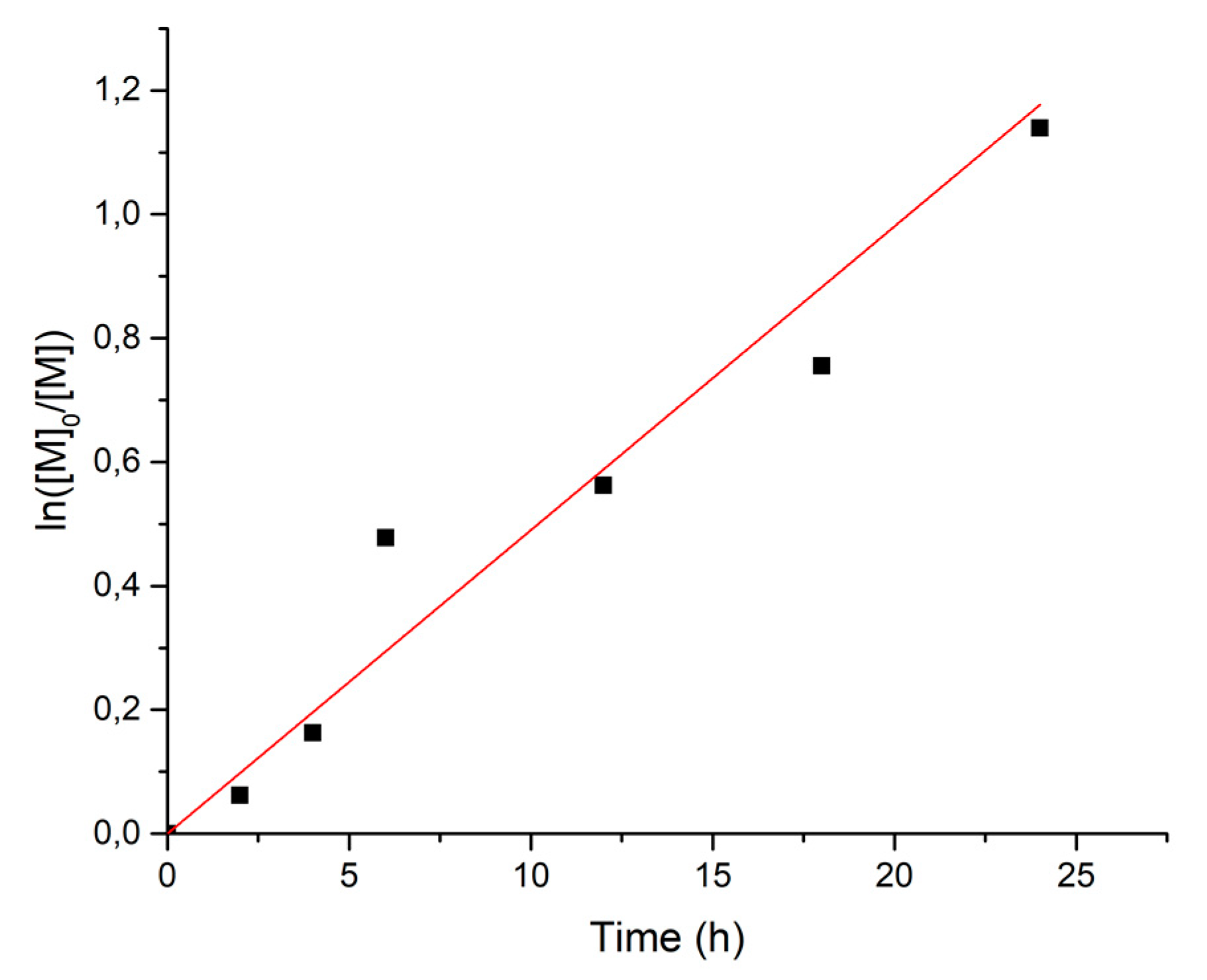
3.1. Radical Polymerization of VDF with 1 as an Initiator
3.2. NMR Characterization of PVDFs
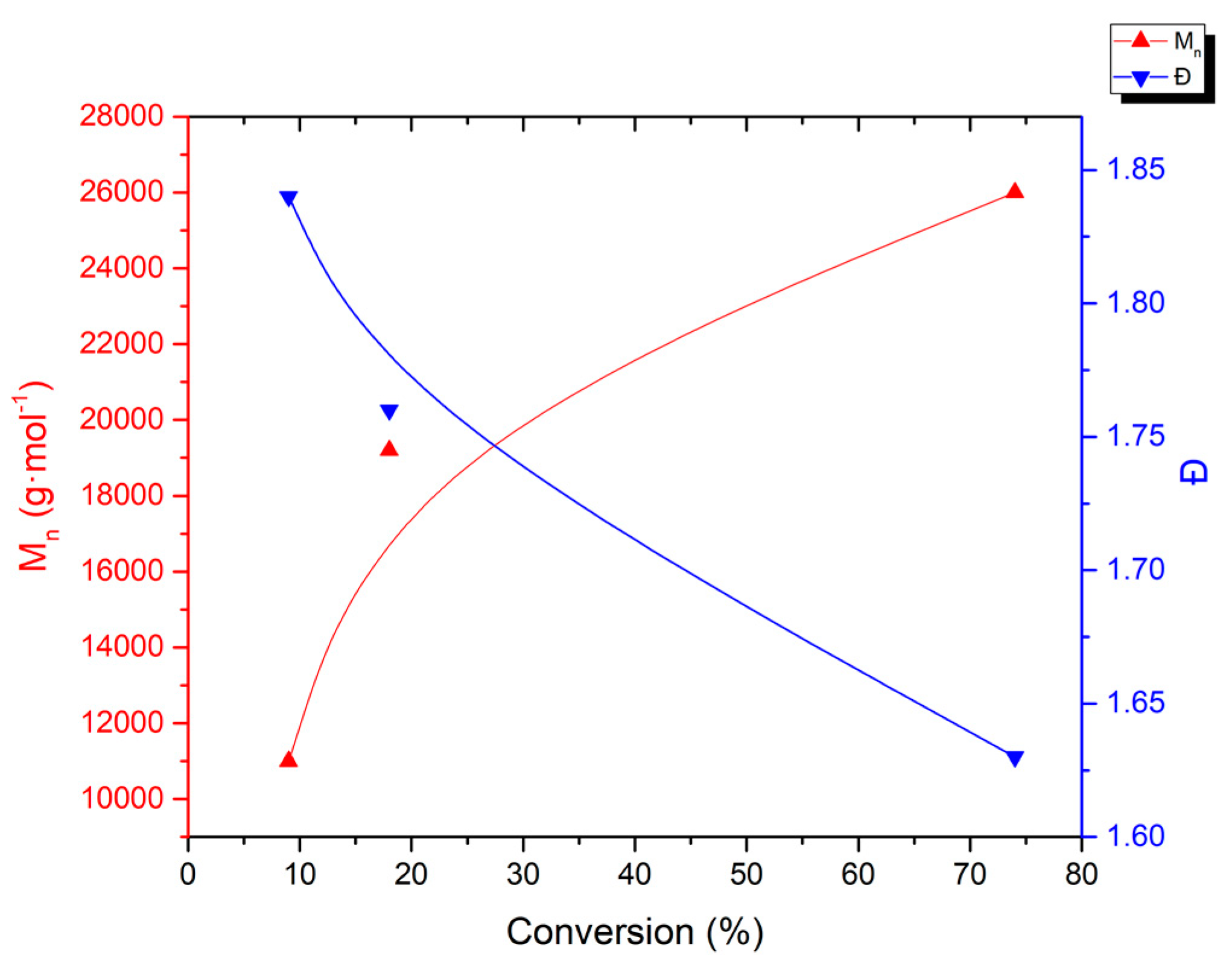
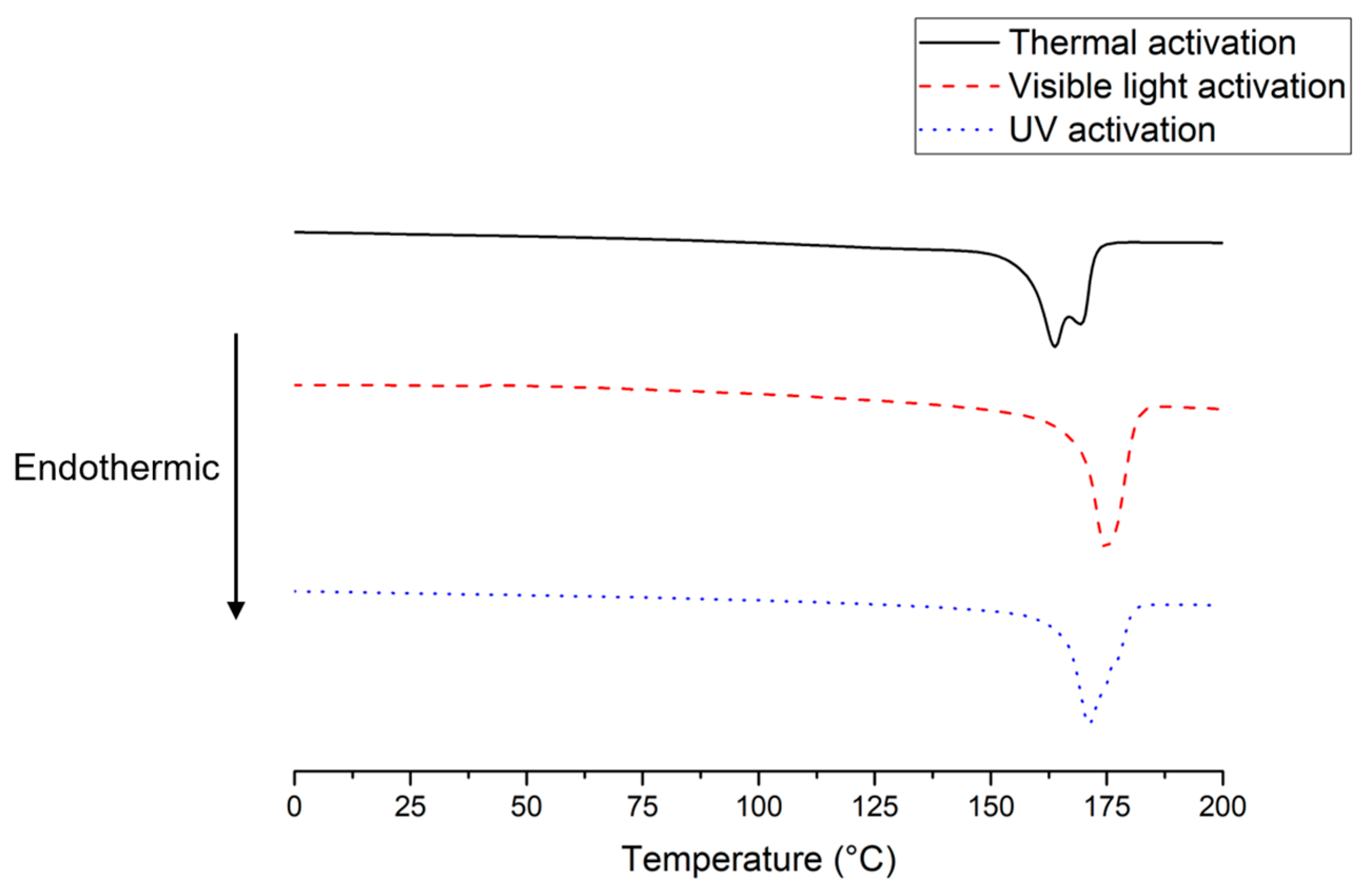
3.3. Thermal Properties of the Resulting PVDFs
3.4. Radical Polymerization of VDF with 2, 3, and 5 as Initiator
3.5. Copolymerization of Vinyl Acetate with tert-Butyl 2-(Trifluoromethyl)acrylate Initiated by Complexes 3 and 6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends†. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [Green Version]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Asandei, A.D. Photomediated Controlled Radical Polymerization and Block Copolymerization of Vinylidene Fluoride. Chem. Rev. 2016, 116, 2244–2274. [Google Scholar] [CrossRef]
- Goldbach, J.T.; Amin-Sanayei, R.; He, W.; Henry, J.; Kosar, W.; Lefebvre, A.; O’Brien, G.; Vaessen, D.; Wood, K.; Zerafati, S. Chapter 6 Commercial Synthesis and Applications of Poly(Vinylidene Fluoride). In Fluorinated Polymers: Volume 2; Applications, A.B., Sawada, H., Eds.; The Royal Society of Chemistry: London, UK, 2017; Volume 2, pp. 127–157. [Google Scholar]
- Golzari, N.; Adams, J.; Beuermann, S. Inducing β Phase Crystallinity in Block Copolymers of Vinylidene Fluoride with Methyl Methacrylate or Styrene. Polymer 2017, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Guiot, J.; Ameduri, B.; Boutevin, B. Radical homopolymerization of vinylidene fluoride initiated by tert-butyl peroxypivalate. Investigation of the microstructure by 19F and 1H NMR spectroscopies and mechanisms. Macromolecules 2002, 35, 8694–8707. [Google Scholar] [CrossRef]
- Mladenov, G.; Ameduri, B.; Kostov, G.; Mateva, R. Synthesis and characterization of fluorinated telomers containing vinylidene fluoride and hexafluoropropene from 1,6-diiodoperfluorohexane. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 1470–1485. [Google Scholar] [CrossRef]
- Pladis, P.; Alexopoulos, A.H.; Kiparissides, C.; Pladis, P. Mathematical Modeling and Simulation of Vinylidene Fluoride Emulsion Polymerization. Ind. Eng. Chem. Res. 2014, 53, 7352–7364. [Google Scholar] [CrossRef]
- Tatemoto, M. In Proceedings of the First Regular Meeting of Soviet-Japanese Fluorine Chemists, Tokyo, Japan, 14–15 February 1979.
- Boyer, C.; Valade, D.; Sauguet, L.; Ameduri, B.; Boutevin, B. Iodine Transfer Polymerization (ITP) of Vinylidene Fluoride (VDF). Influence of the Defect of VDF Chaining on the Control of ITP. Macromolecules 2005, 38, 10353–10362. [Google Scholar] [CrossRef] [Green Version]
- Beuermann, S.; Imran-Ul-Haq, M. Homogeneous Phase Polymerization of Vinylidene Fluoride in Supercritical CO2: Surfactant Free Synthesis and Kinetics. Macromol. Symp. 2007, 259, 210–217. [Google Scholar] [CrossRef]
- Imran-ul-haq, M.; Förster, N.; Vukicevic, R.; Herrmann, K.; Siegmann, R.; Beuermann, S. Chapter 15: Iodine Transfer Radical Polymerizations of Vinylidene Fluoride in Supercritical Carbon Dioxide and Polymer Functionalization via Click Chemistry. In Controlled/Living Radical Polymerization: Progress in RAFT, DT, NMP & OMRP; Matyjaszewski, K., Ed.; ACS: Washington, DC, USA, 2009; Volume 1024, pp. 233–243. [Google Scholar]
- Asandei, A.D.; Adebolu, O.I.; Simpson, C.P. Mild-Temperature Mn2(CO)10-Photomediated Controlled Radical Polymerization of Vinylidene Fluoride and Synthesis of Well-Defined Poly(vinylidene fluoride) Block Copolymers. J. Am. Chem. Soc. 2012, 134, 6080–6083. [Google Scholar] [CrossRef]
- Guerre, M.; Campagne, B.; Gimello, O.; Parra, K.; Ameduri, B.; Ladmiral, V. Deeper Insight into the MADIX Polymerization of Vinylidene Fluoride. Macromolecules 2015, 48, 7810–7822. [Google Scholar] [CrossRef]
- Guerre, M.; Rahaman, S.M.W.; Améduri, B.; Poli, R.; Ladmiral, V. Limits of Vinylidene Fluoride RAFT Polymerization. Macromolecules 2016, 49, 5386–5396. [Google Scholar] [CrossRef]
- Banerjee, S.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Poli, R.; Améduri, B. Organometallic-Mediated Radical Polymerization of Vinylidene Fluoride. Angew. Chem. Int. Ed. 2018, 57, 2934–2937. [Google Scholar] [CrossRef]
- Falireas, P.G.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Poli, R.; Ameduri, B. Straightforward Synthesis of Well-Defined Poly(vinylidene fluoride) and Its Block Copolymers by Cobalt-Mediated Radical Polymerization. Macromolecules 2019, 52, 1266–1276. [Google Scholar] [CrossRef]
- Debuigne, A.; Poli, R.; Jérôme, C.; Jerôme, R.; Detrembleur, C. Overview of cobalt-mediated radical polymerization: Roots, state of the art and future prospects. Prog. Polym. Sci. 2009, 34, 211–239. [Google Scholar] [CrossRef]
- Poli, R. New Phenomena in Organometallic-Mediated Radical Polymerization (OMRP) and Perspectives for Control of Less Active Monomers. Chem. Eur. J. 2015, 21, 6988–7001. [Google Scholar] [CrossRef]
- Debuigne, A.; Jérôme, C.; Detrembleur, C. Organometallic-mediated radical polymerization of ‘less activated monomers’: Fundamentals, challenges and opportunities. Polymer 2017, 115, 285–307. [Google Scholar] [CrossRef]
- Fliedel, C.; Poli, R. Homolytically weak metal-carbon bonds make robust controlled radical polymerizations systems for “less-activated monomers. ” J. Organomet. Chem. 2019, 880, 241–252. [Google Scholar] [CrossRef]
- Poli, R.; Rahaman, S.; Ladmiral, V.; Améduri, B. Effect of α- and β-H/F substitution on the homolytic bond strength in dormant species of controlled radical polymerization: OMRP vs. ITP and RAFT. J. Organomet. Chem. 2018, 864, 12–18. [Google Scholar] [CrossRef]
- Morales-Cerrada, R.; Fliedel, C.; Daran, J.-C.; Gayet, F.; Ladmiral, V.; Améduri, B.; Poli, R. Fluoroalkyl Radical Generation by Homolytic Bond Dissociation in Pentacarbonylmanganese Derivatives. Chem. Eur. J. 2019, 25, 296–308. [Google Scholar] [CrossRef]
- Nakagawa, K.; Ishida, Y. Annealing effects in poly(vinylidene fluoride) as revealed by specific volume measurements, differential scanning calorimetry, and electron microscopy. J. Polym. Sci. Part A-2 Polym. Phys. 1973, 11, 2153–2171. [Google Scholar] [CrossRef]
- Morales-Cerrada, R.; Fliedel, C.; Gayet, F.; Ladmiral, V.; Améduri, B.; Poli, R. Thermal Decomposition of Fluoroalkyl Pentacarbonylmanganese(I) Derivatives by α-Fluorine Elimination. Organometallics 2019, 38, 1021–1030. [Google Scholar] [CrossRef]
- Fawcett, J.P.; Poe, A.; Sharma, K.R. Reaction mechanisms of metal-metal bonded carbonyls. X. Thermal decomposition of decacarbonyldimanganese and decacarbonylmanganeserhenium in decalin. J. Am. Chem. Soc. 1976, 98, 1401–1407. [Google Scholar] [CrossRef]
- Aliwi, S.M.; Bamford, C.H.; Mullik, S.U. Recent studies in photoinitiated polymerization. J. Polym. Sci. Part C 1975, 50, 33–50. [Google Scholar] [CrossRef]
- Allan, L.E.; Perry, M.R.; Shaver, M.P. Organometallic mediated radical polymerization. Prog. Polym. Sci. 2012, 37, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Destarac, M.; Matyjaszewski, K.; Silverman, E.; Ameduri, B.; Boutevin, B. Atom Transfer Radical Polymerization Initiated with Vinylidene Fluoride Telomers. Macromolecules 2000, 33, 4613–4615. [Google Scholar] [CrossRef]
- Girard, E.; Marty, J.-D.; Ameduri, B.; Destarac, M. Direct Synthesis of Vinylidene Fluoride-Based Amphiphilic Diblock Copolymers by RAFT/MADIX Polymerization. ACS Macro Lett. 2012, 1, 270–274. [Google Scholar] [CrossRef]
- Duc, M.; Ameduri, B.; Boutevin, B.; Kharroubi, M.; Sage, J.M. Telomerization of vinylidene fluoride with methanol. Elucidation of the reaction process and mechanism by a structural analysis of the telomers. Macromol. Chem. Physic. 1998, 199, 1271–1289. [Google Scholar] [CrossRef]
- Boschet, F.; Ono, T.; Ameduri, B. Novel Source of Trifluoromethyl Radical As Efficient Initiator for the Polymerization of Vinylidene Fluoride. Macromol. Rapid Commun. 2012, 33, 302–308. [Google Scholar] [CrossRef]
- Pianca, M.; Barchiesi, E.; Esposto, G.; Radice, S. End groups in fluoropolymers. J. Fluor. Chem. 1999, 95, 71–84. [Google Scholar] [CrossRef]
- Ameduri, B.; Ladaviere, C.; Delolme, F.; Boutevin, B. First MALDI-TOF Mass Spectrometry of Vinylidene Fluoride Telomers Endowed with Low Defect Chaining. Macromolecules 2004, 37, 7602–7609. [Google Scholar] [CrossRef]
- Kim, J.-S.; Dutta, A.; Vasu, V.; Adebolu, O.I.; Asandei, A.D. Universal Group 14 Free Radical Photoinitiators for Vinylidene Fluoride, Styrene, Methyl Methacrylate, Vinyl Acetate, and Butadiene. Macromolecules 2019, 52, 8895–8909. [Google Scholar] [CrossRef]
- Mattern, D.E.; Lin, F.T.; Hercules, D.M. Laser mass spectrometry of poly(fluoroethylenes). Anal. Chem. 1984, 56, 2762–2769. [Google Scholar] [CrossRef]
- Tedder, J.M.; Walton, J.C. The kinetics and orientation of free-radical addition to olefins. Acc. Chem. Res. 1976, 9, 183–191. [Google Scholar] [CrossRef]
- Dolbier, W.R. Structure, Reactivity, and Chemistry of Fluoroalkyl Radicals. Chem. Rev. 1996, 96, 1557–1584. [Google Scholar] [CrossRef]
- Dolbier, W.R.; Duan, J.-X.; Abboud, K.; Ameduri, B. Synthesis and Reactivity of a Novel, Dimeric Derivative of Octafluoro[2.2]paracyclophane. A New Source of Trifluoromethyl Radicals. J. Am. Chem. Soc. 2000, 122, 12083–12086. [Google Scholar] [CrossRef]
- Otazaghine, B.; Sauguet, L.; Ameduri, B. Synthesis and copolymerisation of fluorinated monomers bearing a reactive lateral group Part 21. Radical copolymerisation of vinylidene fluoride with 2-hydroperfluorooct-1-ene. J. Fluor. Chem. 2005, 126, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Ladmiral, V.; Totée, C.; Améduri, B. Alternating radical copolymerization of vinyl acetate and tert-butyl-2-trifluoromethacrylate. Eur. Polym. J. 2018, 104, 164–169. [Google Scholar] [CrossRef]
- Banerjee, S.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Rahaman, S.M.W.; Poli, R.; Ameduri, B. Organometallic-Mediated Alternating Radical Copolymerization of tert -Butyl-2-Trifluoromethacrylate with Vinyl Acetate and Synthesis of Block Copolymers Thereof. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef]
- Banerjee, S.; Guerre, M.; Ameduri, B.; Ladmiral, V. Syntheses of 2-(trifluoromethyl)acrylate-containing block copolymers via RAFT polymerization using a universal chain transfer agent. Polym. Chem. 2018, 9, 3511–3521. [Google Scholar] [CrossRef]
- Banerjee, S.; Bellan, E.V.; Gayet, F.; Debuigne, A.; Detrembleur, C.; Poli, R.; Améduri, B.; Ladmiral, V. Bis(formylphenolato)cobalt(II)-Mediated Alternating Radical Copolymerization of tert-Butyl 2-Trifluoromethylacrylate with Vinyl Acetate. Polymers 2017, 9, 702. [Google Scholar] [CrossRef] [Green Version]
- Tedder, J.M.; Walton, J.C. The Importance of Polarity and Steric Effects in Determining the Rate and Orientation of Free-Radical Addition to Olefins-Rules for Determining the Rate and Preferred Orientation. Tetrahedron 1980, 36, 701–707. [Google Scholar] [CrossRef]
- Tedder, J.M. Which Factors Determine the Reactivity and Regioselectivity of Free Radical Substitution and Addition Reactions? Angew. Chem. Int. Ed. 1982, 21, 401–410. [Google Scholar] [CrossRef]
- Serov, S.I.; Zhuravlev, M.V.; Sass, V.P.; Sokolov, S.V. Reactions of fluoro-containing free radicals in solution. II. Kinetics of the addition of perfluoroalkyl radicals to olefins in heptane. J. Org. Chem. USSR 1981, 17, 53–58. [Google Scholar]


| Entry | Activation Method | Reaction Time (h) | Yield a (%) | Mn b (g·mol−1) | Ɖ |
|---|---|---|---|---|---|
| 1 | Thermal (50 °C) | 24 | 0 | - | - |
| 2 | Thermal (100 °C) | 2 | 6 | 16,000 | 1.42 |
| 3 | Thermal (100 °C) | 4 | 15 | 23,000 | 1.48 |
| 4 | Thermal (100 °C) | 6 | 38 | 20,100 | 1.50 |
| 5 | Thermal (100 °C) | 12 | 40 | ND | ND |
| 6 | Thermal (100 °C) | 18 | 49 | ND | ND |
| 7 | Thermal (100 °C) | 24 | 68 | 16,900 | 1.53 |
| 8 | Visible light | 2 | 6 | ND | ND |
| 9 | Visible light | 4 | 14 | 53,000 | 1.65 |
| 10 | Visible light | 6 | 19 | 48,300 | 1.61 |
| 11 | Visible light | 24 | 60 | 40,300 | 1.47 |
| 12 | UV (300 nm) | 2 | 9 | 11,000 | 1.84 |
| 13 | UV (300 nm) | 6 | 18 | 19,200 | 1.76 |
| 14 | UV (300 nm) | 24 | 74 | 26,000 | 1.63 |
| Activation Method | Mn (g·mol−1) | T2% | T5% | T10% |
|---|---|---|---|---|
| Thermal (100 °C) | 16,900 | 343 | 391 | 424 |
| Visible light | 40,300 | 412 | 444 | 459 |
| UV (300 nm) | 26,000 | 364 | 414 | 441 |
| Activation Method | Mn (g·mol−1) | Melting Point (°C) | Enthalpy of Fusion (J·g−1) | Degree of Crystallinity * (%) |
|---|---|---|---|---|
| Thermal (100 °C) | 16,900 | 164 | 46.1 | 44 |
| Visible light | 40,300 | 175 | 55.3 | 53 |
| UV (300 nm) | 26,000 | 171 | 45.9 | 44 |
| Entry | Complex | Activation Method | Reaction Time (h) | Yield a (%) | [VDF]/[Mn(CO)5RF] | Mn b (g·mol−1) | Ɖ |
|---|---|---|---|---|---|---|---|
| 1 | 2 | Thermal (80 °C) | 24 | 0 | 50 | - | - |
| 2 | 2 | hν (visible light) | 24 | 2 | 50 | ND | ND |
| 3 | 2 | hν (UV 300 nm) | 24 | 5 | 50 | ND | ND |
| 4 | 3 | Thermal (90 °C) | 24 | 2 | 50 | 32,200 | 1.11 |
| 5 | 3 | Thermal (90 °C) | 24 | 3 | 100 | 32,200 | 1.16 |
| 6 | 3 | Thermal (100 °C) | 24 | 3 | 100 | 31,600 | 1.15 |
| 7 | 5 | Thermal (80 °C) | 24 | 7 | 50 | 16,200 | 1.38 |
| 8 | 5 | hν (visible light) | 4 | 3 | 50 | 12,600 | 1.48 |
| 9 | 5 | hν (visible light) | 8 | 8 | 50 | 22,500 | 1.44 |
| 10 | 5 | hν (visible light) | 12 | 13 | 50 | 24,000 | 1.40 |
| 11 | 5 | hν (visible light) | 24 | 23 | 50 | 23,000 | 1.45 |
| 12 | 5 | hν (UV 300 nm) | 4 | 16 | 50 | 11,100 | 1.57 |
| 13 | 5 | hν (UV 300 nm) | 8 | 18 | 50 | 10,000 | 1.69 |
| 14 | 5 | hν (UV 300 nm) | 12 | 20 | 50 | 9500 | 1.81 |
| 15 | 5 | hν (UV 300 nm) | 24 | 21 | 50 | 7000 | 1.94 |
| Entry | Initiator | Temperature (°C) | Reaction Time (h) | [Mn(RF)(CO)5] (mg) | Mn:VAc: MAF-TBE Molar Ratio | Yield (%) | Mn (g·mol−1) | Ɖ |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 70 | 72 | 53.0 | 1:50:50 | 17 | 58,300 | 1.53 |
| 2 | 6 | 85 | 3 | 50.5 | 1:50:50 | 38 | 41,300 | 1.43 |
| 3 | 3 | 70 | 72 | 22.5 | 1:50:50 | 21 | 7000 | 1.69 |
| 4 | 3 | 80 | 18 | 93.3 | 1:50:50 | 83 | 76,000 | 2.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Cerrada, R.; Ladmiral, V.; Gayet, F.; Fliedel, C.; Poli, R.; Améduri, B. Fluoroalkyl Pentacarbonylmanganese(I) Complexes as Initiators for the Radical (co)Polymerization of Fluoromonomers. Polymers 2020, 12, 384. https://doi.org/10.3390/polym12020384
Morales-Cerrada R, Ladmiral V, Gayet F, Fliedel C, Poli R, Améduri B. Fluoroalkyl Pentacarbonylmanganese(I) Complexes as Initiators for the Radical (co)Polymerization of Fluoromonomers. Polymers. 2020; 12(2):384. https://doi.org/10.3390/polym12020384
Chicago/Turabian StyleMorales-Cerrada, Roberto, Vincent Ladmiral, Florence Gayet, Christophe Fliedel, Rinaldo Poli, and Bruno Améduri. 2020. "Fluoroalkyl Pentacarbonylmanganese(I) Complexes as Initiators for the Radical (co)Polymerization of Fluoromonomers" Polymers 12, no. 2: 384. https://doi.org/10.3390/polym12020384
APA StyleMorales-Cerrada, R., Ladmiral, V., Gayet, F., Fliedel, C., Poli, R., & Améduri, B. (2020). Fluoroalkyl Pentacarbonylmanganese(I) Complexes as Initiators for the Radical (co)Polymerization of Fluoromonomers. Polymers, 12(2), 384. https://doi.org/10.3390/polym12020384









