Simple One Pot Preparation of Chemical Hydrogels from Cellulose Dissolved in Cold LiOH/Urea
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Dissolution of Cellulose
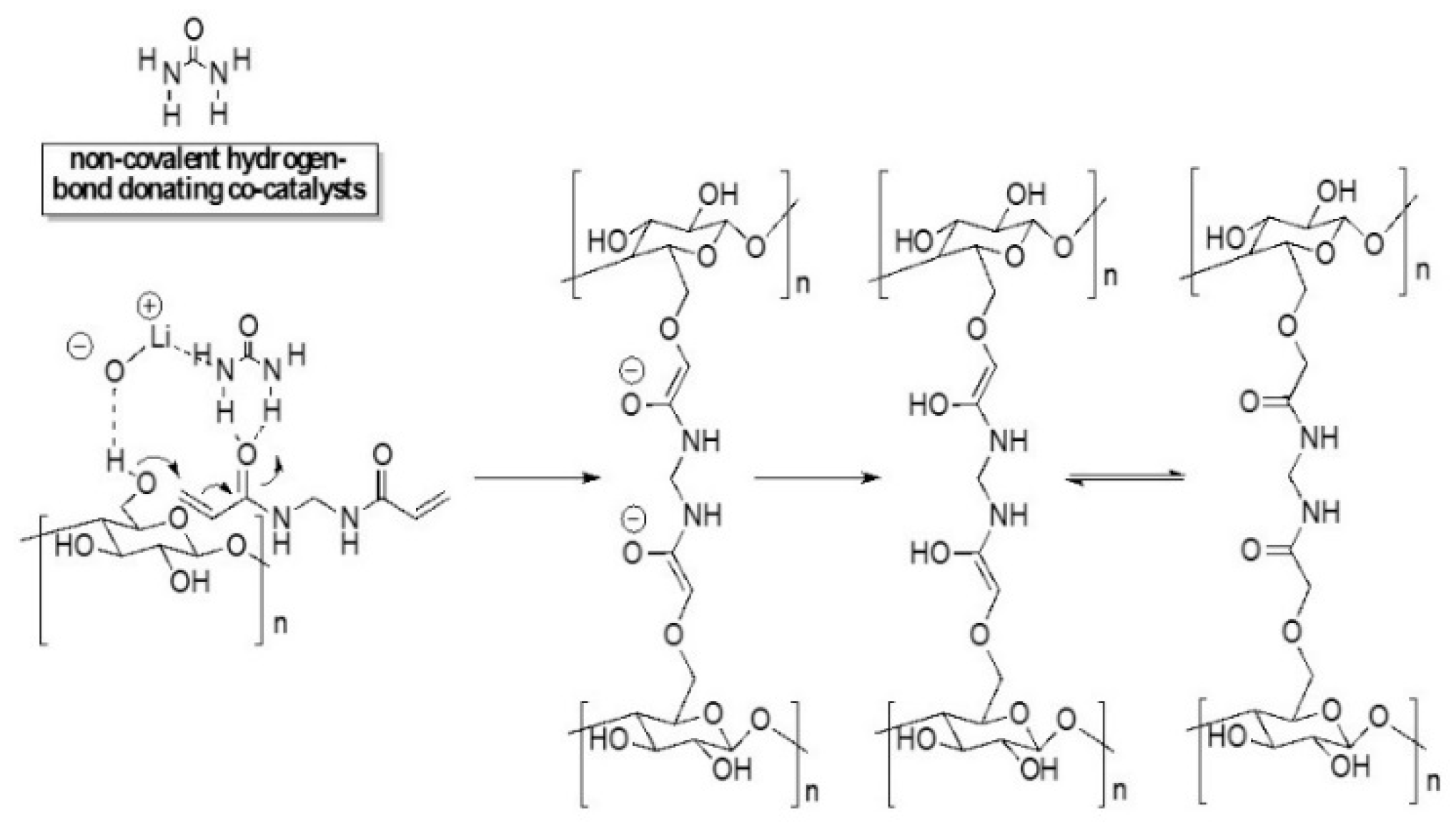
2.3. Preparation of Chemically Crosslinked Cellulose Hydrogels
2.4. Characterization of Cellulose Hydrogels
2.4.1. Swelling Degree
2.4.2. Rheometry
2.4.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.4. Raman Spectroscopy
2.4.5. X-Ray Diffraction (XRD)
2.4.6. Electronic Microscopy
3. Results and Discussion
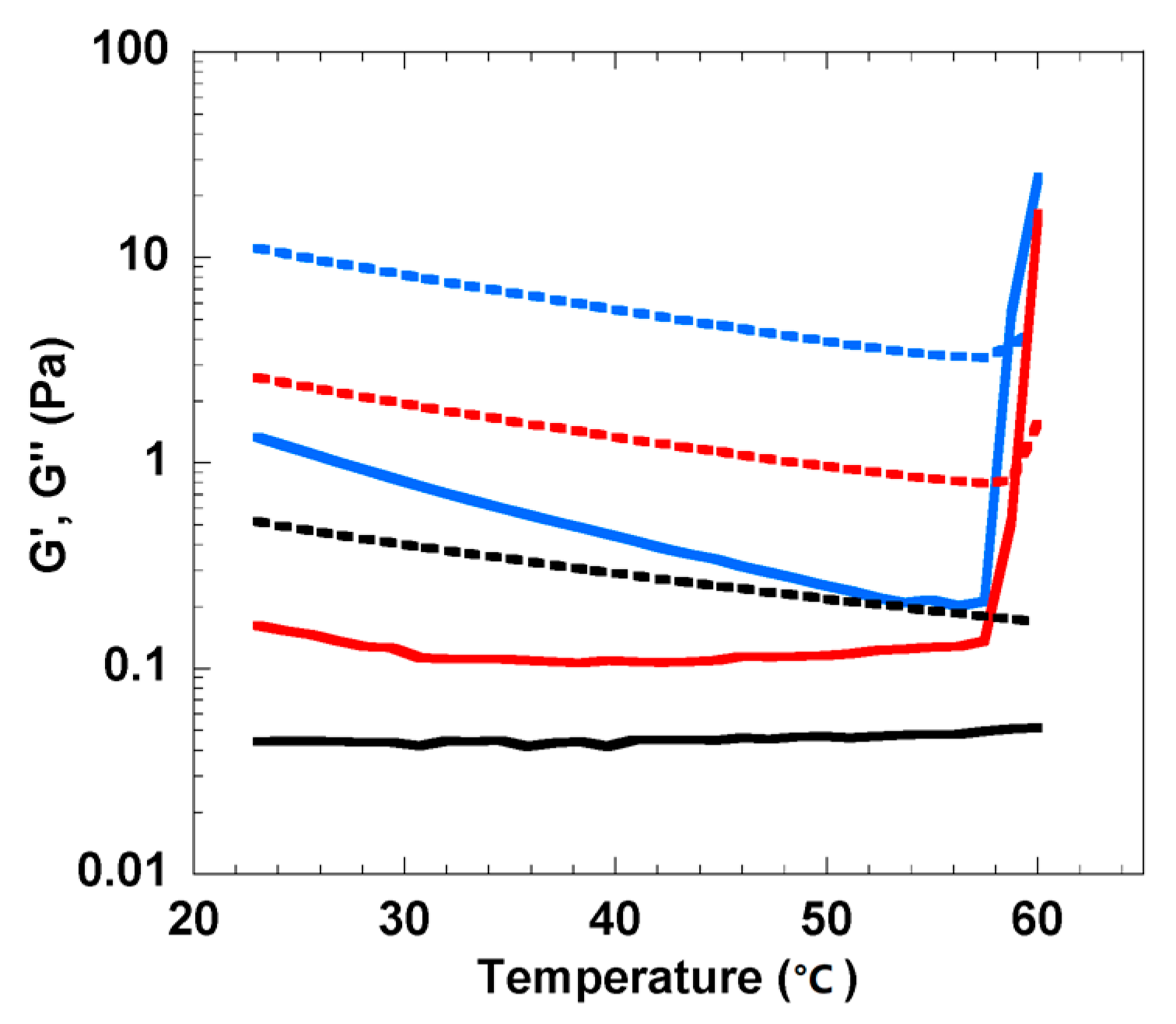
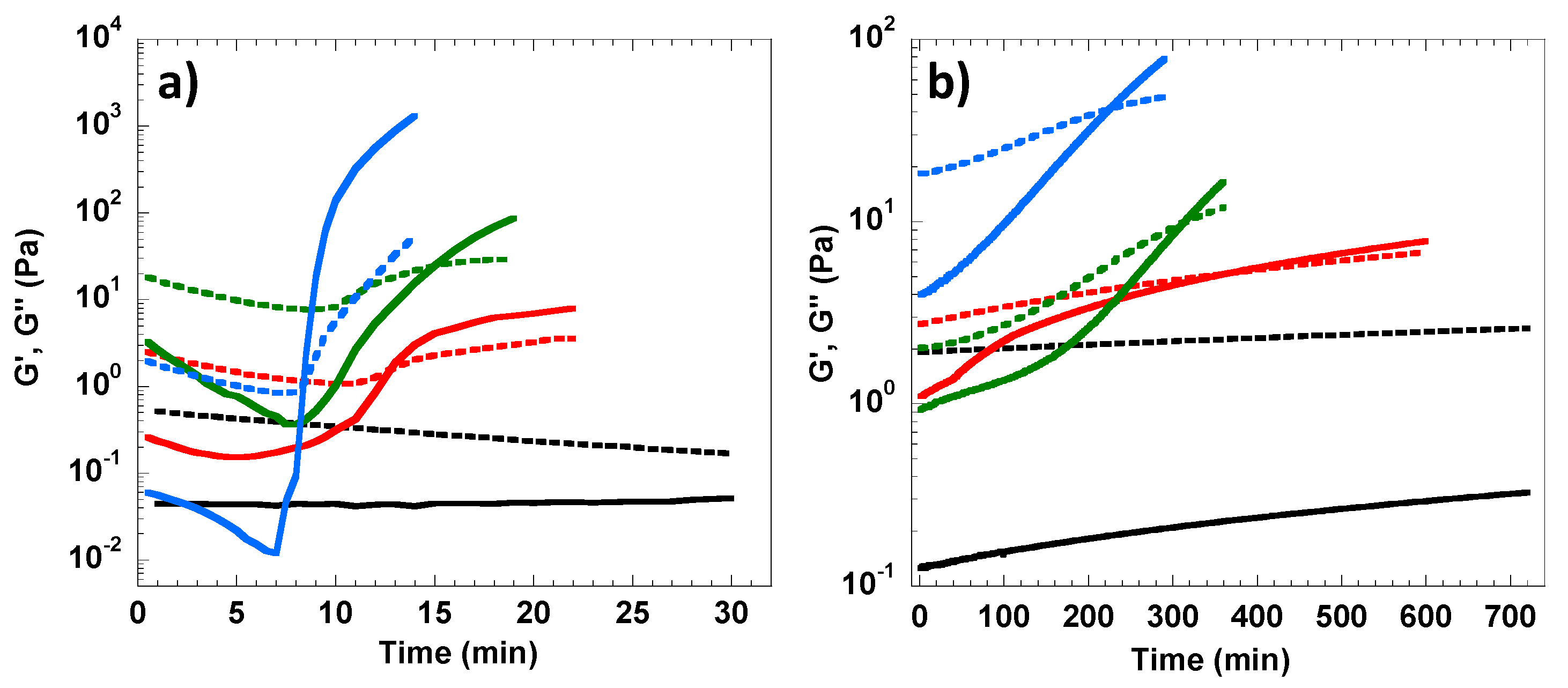
3.1. Rheological Analysis of Cellulose Solution and Cellulose/MBA
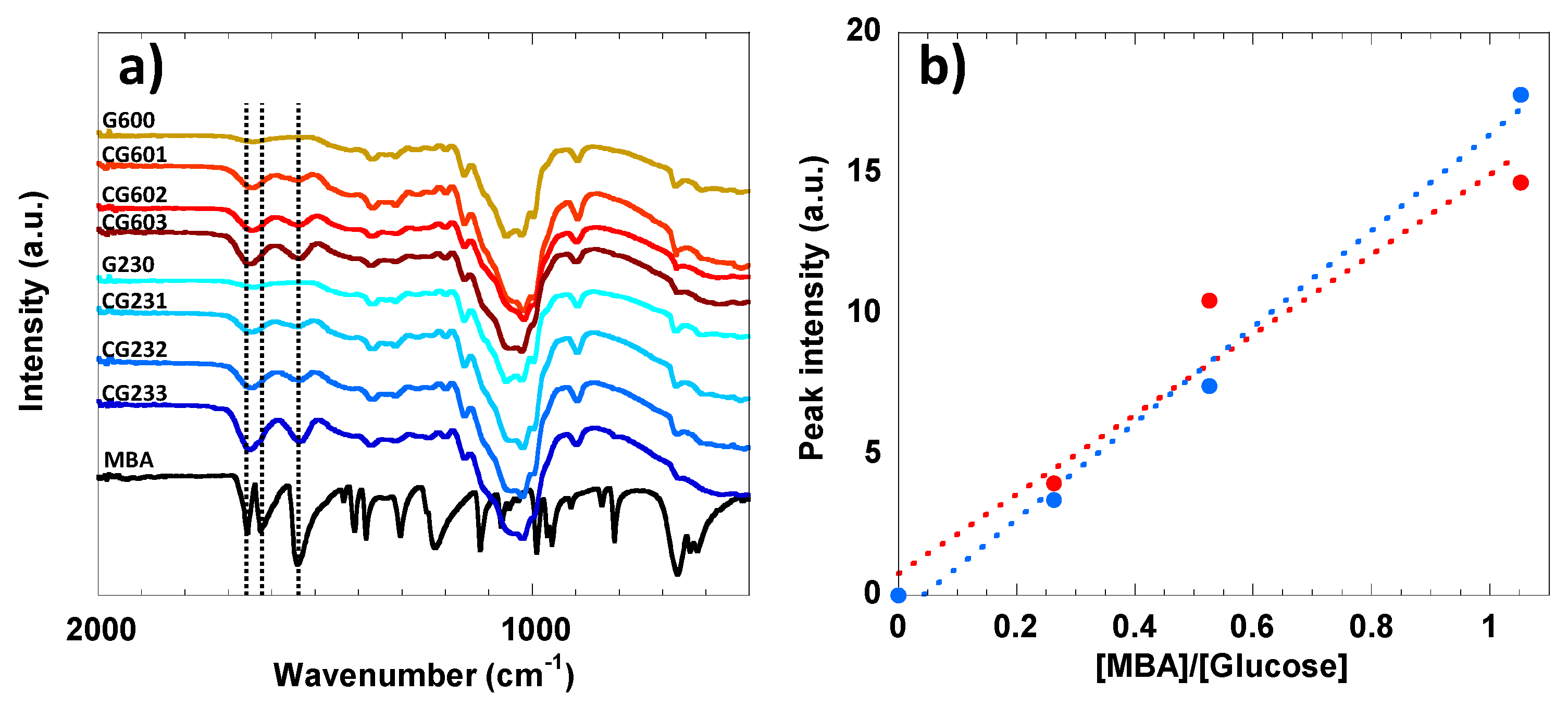
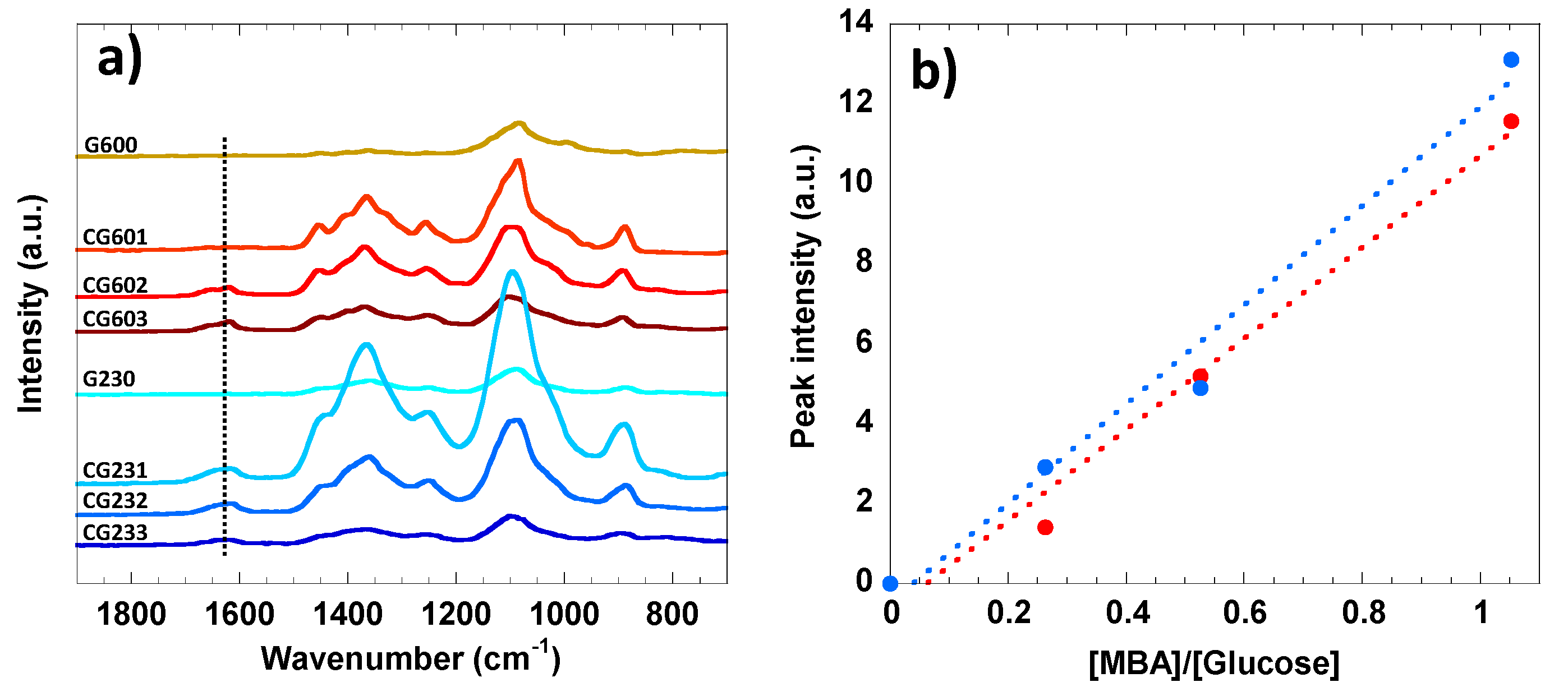
3.2. FTIR and Raman Analysis of Accessibility of Cellulose Hydrogel
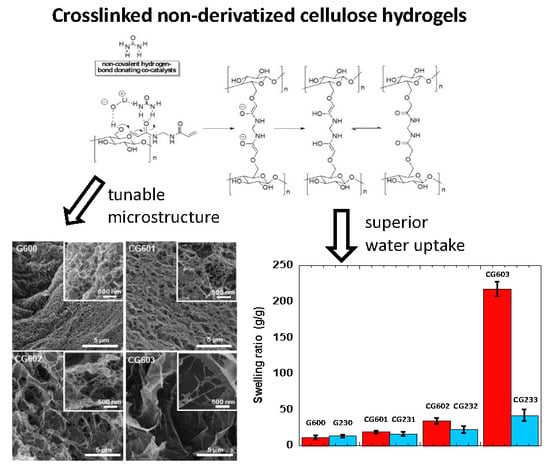
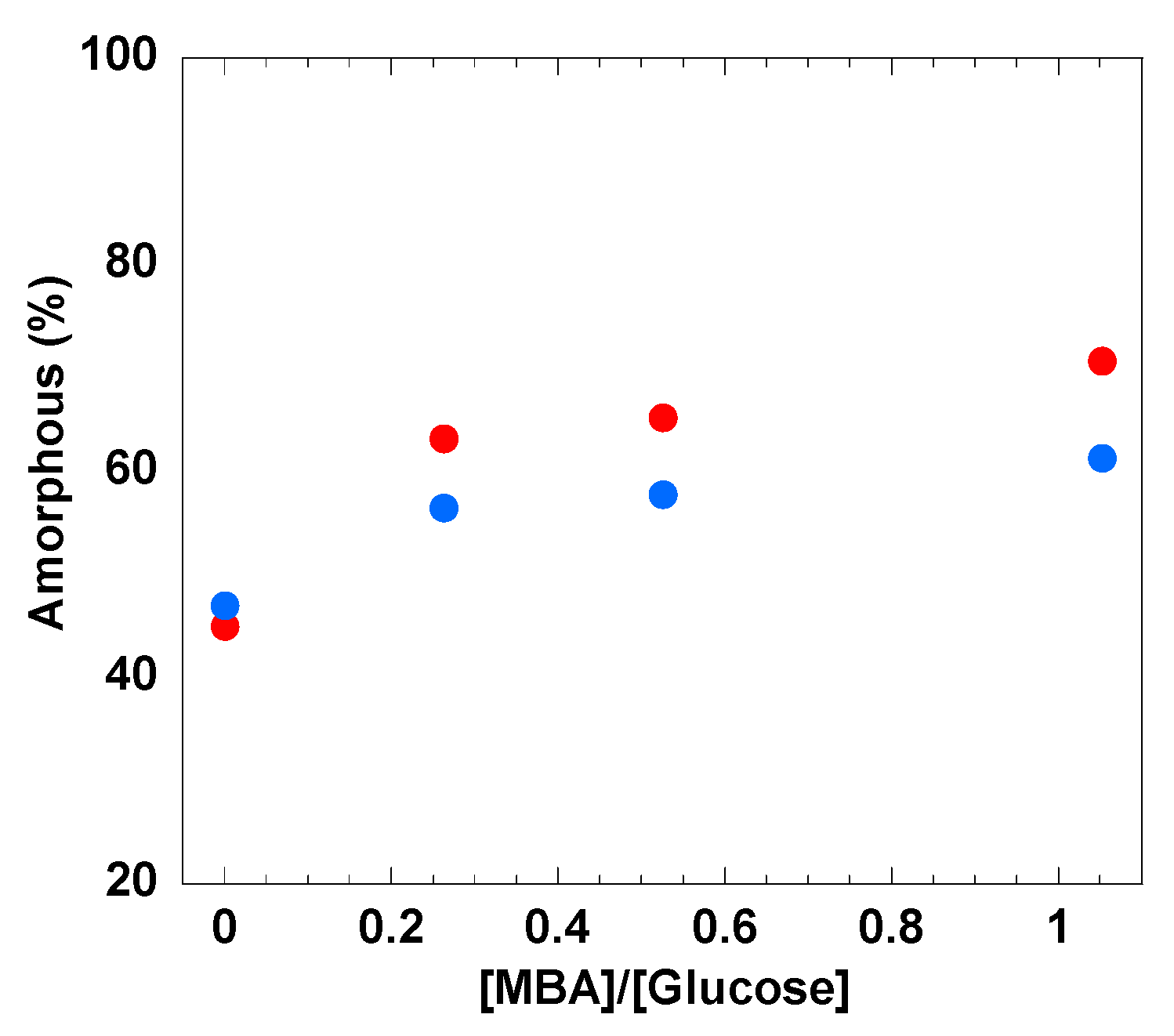
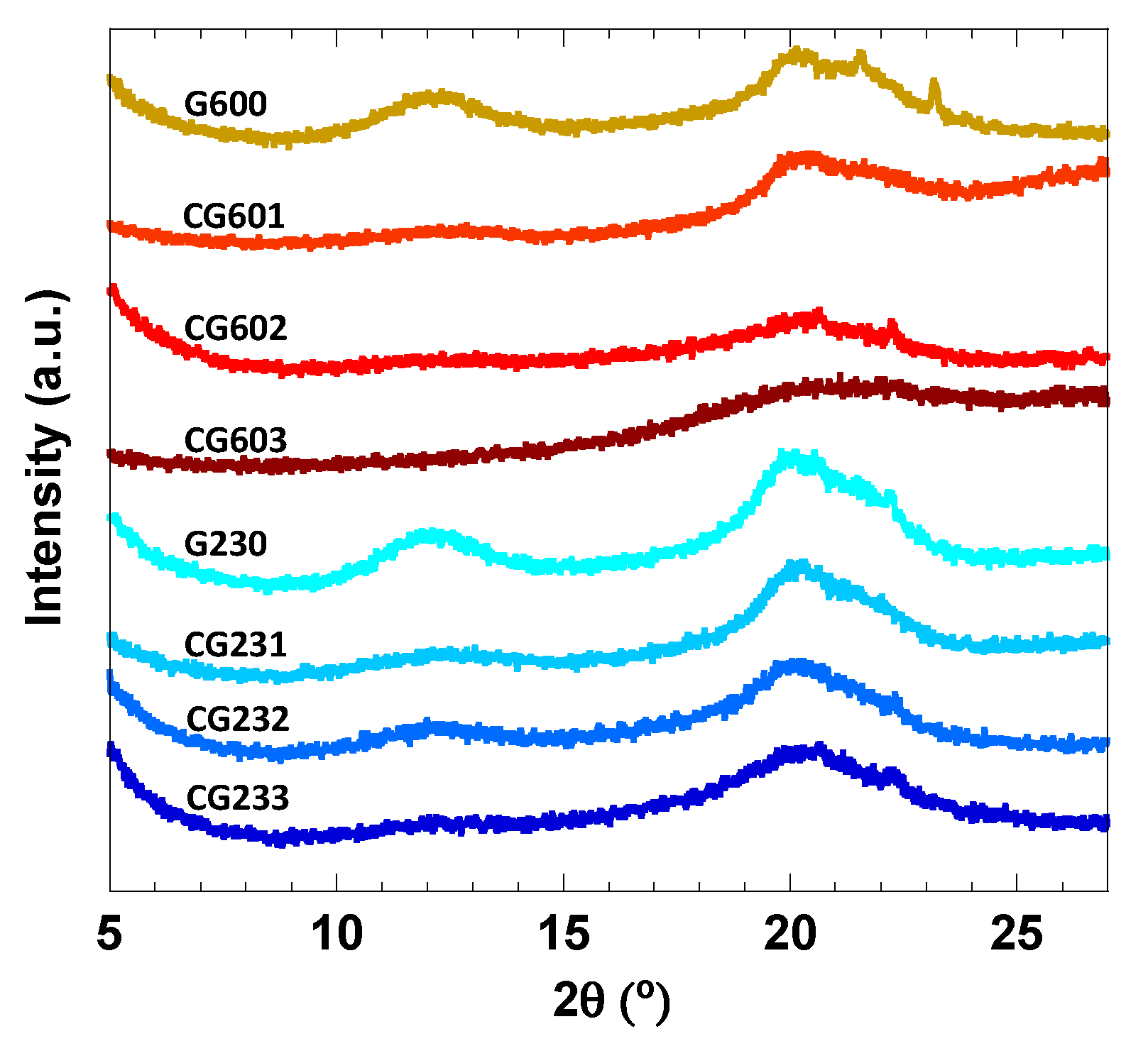
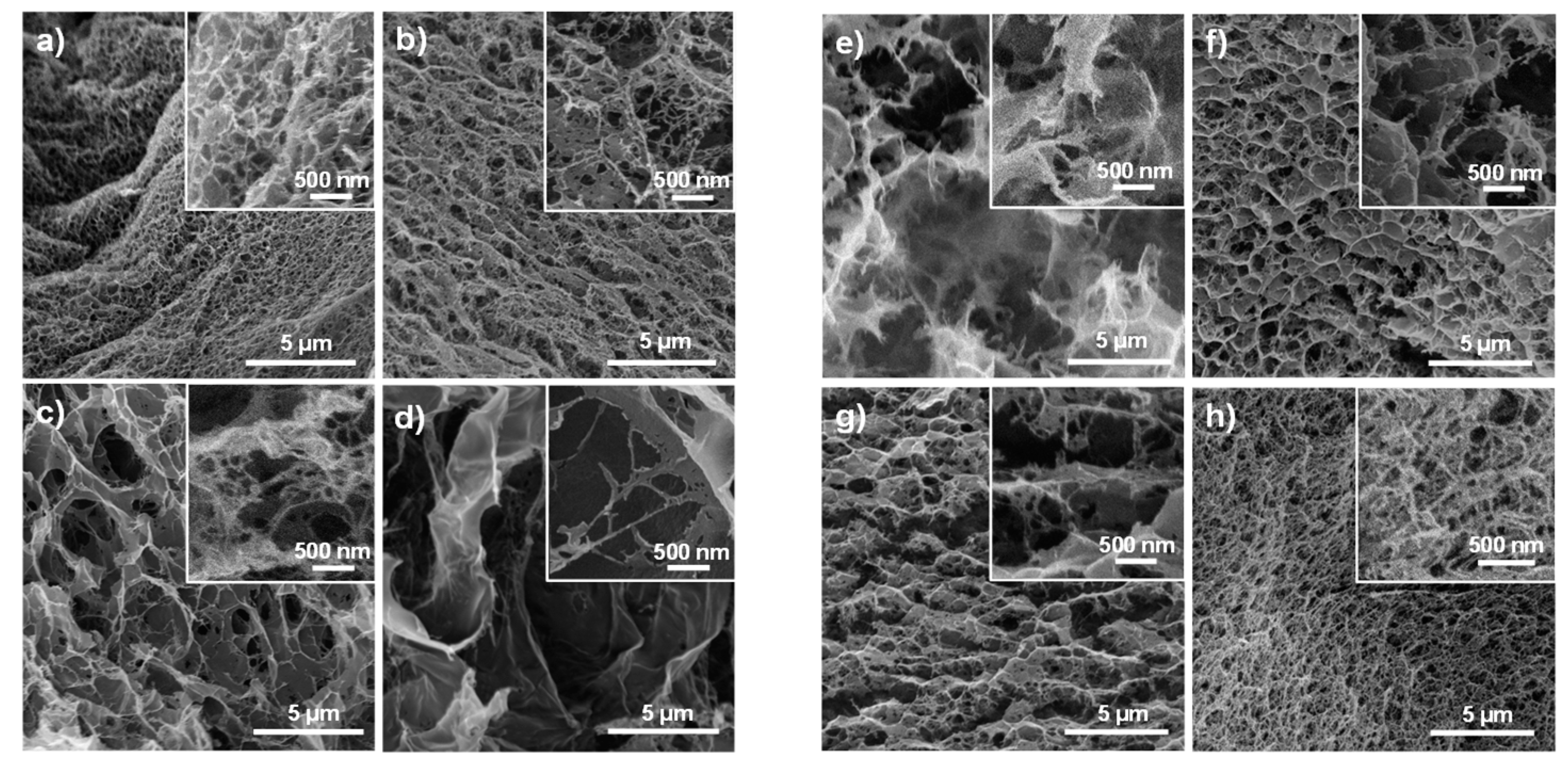
3.3. XRD and Morphology of the Cellulose Hydrogels
3.4. Swelling of the Cellulose Hydrogels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heinze, T. Cellulose: Structure and Properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O.J., Ed.; Springer: Cham, Switzerland, 2015; Volume 271. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Chang, C.Y.; Zhang, L.N. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Geng, H.J. A one-step approach to make cellulose-based hydrogels of various transparency and swelling degrees. Carbohydr. Polym. 2018, 186, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Sadeghi, M.; Hosseinzadeh, H. Synthesis of starch-poly(sodium acrylate-co-acrylamide) superabsorbent hydrogel with salt and pH-Responsiveness properties as a drug delivery system. J. Bioact. Compat. Polym. 2008, 23, 381–404. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. A versatile strategy to fabricate hydrogel-silver nanocomposites and investigation of their antimicrobial activity. J. Colloid Interface Sci. 2007, 315, 389–395. [Google Scholar] [CrossRef]
- Song, X.F.; Wei, J.F.; He, T.S. A method to repair concrete leakage through cracks by synthesizing super-absorbent resin in situ. Constr. Build. Mater. 2009, 23, 386–391. [Google Scholar] [CrossRef]
- Shen, X.P.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Apostolov, A.A.; Boneva, D.; Vassileva, E.; Mark, J.E.; Fakirov, S. Mechanical properties of native and crosslinked gelatins in a bending deformation. J. Appl. Polym. Sci. 2000, 76, 2041. [Google Scholar] [CrossRef]
- Rajagopal, K.; Schneider, J.P. Self-assembling peptides and proteins for nanotechnological applications. Curr. Opin. Struct. Biol. 2004, 14, 480–486. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Beebe, D.J.; Moore, J.S.; Bauer, J.M.; Yu, Q.; Liu, R.H.; Devadoss, C.; Jo, B.H. Functional hydrogel structures for autonomous flow control inside microfluidic channels. Nature 2000, 404, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Eddington, D.T.; Beebe, D.J. Flow control with hydrogels. Adv. Drug Deliv. Rev. 2004, 56, 199–210. [Google Scholar] [CrossRef]
- Giordano, C.; Albani, D.; Gloria, A.; Tunesi, M.; Rodilossi, S.; Russo, T.; Forloni, G.; Ambrosio, L.; Cigada, A. Nanocomposites for neurodegenerative diseases: Hydrogel-nanoparticle combinations for a challenging drug delivery. Int. J. Artif. Organs 2011, 34, 1115–1127. [Google Scholar] [CrossRef]
- Yan, C.Q.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef]
- Liebert, T. Cellulose Solvents—Remarkable History, Bright Future. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; Lieber, T., Heinze, T., Edgar, K., Eds.; Oxford university press: Northants, UK, 2009; Volume 1033, pp. 3–54. [Google Scholar]
- Singh, P.; Duarte, H.; Alves, L.; Antunes, F.; Le Moigne, N.; Dormanns, J.; Duchemin, B.; Staiger, M.P.; Medronho, M. Cellulose—Fundamental Aspects and Current Trends. In From Cellulose Dissolution and Regeneration to Added Value Applications—Synergism Between Molecular Understanding and Material Development; Poletto, D.M., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Medronho, B.; Lindman, B. Competing forces during cellulose dissolution: From solvents to mechanisms. Curr. Opin. Colloid Interface Sci. 2014, 19, 32–40. [Google Scholar] [CrossRef]
- Olsson, C.; Westman, G. Direct Dissolution of Cellulose: Background, Means and Applications. Cellul. Fundam. Asp. 2013, 10, 52144. [Google Scholar]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Ciolacu, D.; Oprea, A.M.; Anghel, N.; Cazacu, G.; Cazacu, M. New cellulose-lignin hydrogels and their application in controlled release of polyphenols. Mater. Sci. Eng. C 2012, 32, 452–463. [Google Scholar] [CrossRef]
- Haque, A.; Morris, E.R. Thermogelation of Methylcellulose. Part 1: Molecular-Structures and Processes. Carbohydr. Polym. 1993, 22, 161–173. [Google Scholar] [CrossRef]
- Sarkar, N. Kinetics of Thermal Gelation of Methylcellulose and Hydroxypropylmethylcellulose in Aqueous-Solutions. Carbohydr. Polym. 1995, 26, 195–203. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takacs, E.; Wojnarovits, L. Synthesis of cellulose derivative based superabsorbent hydrogels by radiation induced crosslinking. Cellulose 2014, 21, 4157–4165. [Google Scholar] [CrossRef]
- Ranganathan, N.; Joseph Bensingh, R.; Abdul Kader, M.; Nayak, S.K. Synthesis and Properties of Hydrogels Prepared by Various Polymerization Reaction Systems. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Shokri, J.; Adibkia, K. Application of Cellulose and Cellulose Derivatives in Pharmaceutical Industries; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Zhang, W.M.; Sha, Z.; Huang, Y.; Bai, Y.P.; Xi, N.; Zhang, Y.C. Glow discharge electrolysis plasma induced synthesis of cellulose-based ionic hydrogels and their multiple response behaviors. RSC Adv. 2015, 5, 6505–6511. [Google Scholar] [CrossRef]
- Fei, B.; Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of biodegradable cellulose derivatives. I. Radiation-induced crosslinking of CMC. J. Appl. Polym. Sci. 2000, 78, 278–283. [Google Scholar] [CrossRef]
- Wach, R.A.; Rokita, B.; Bartoszek, N.; Katsumura, Y.; Ulanski, P.; Rosiak, J.M. Hydroxyl radical-induced crosslinking and radiation-initiated hydrogel formation in dilute aqueous solutions of carboxymethylcellulose. Carbohydr. Polym. 2014, 112, 412–415. [Google Scholar] [CrossRef]
- Liu, P.F.; Zhai, M.L.; Li, J.Q.; Peng, J.; Wu, J.L. Radiation preparation and swelling behavior of sodium carboxymethyl cellulose hydrogels. Radiat. Phys. Chem. 2002, 63, 525–528. [Google Scholar] [CrossRef]
- Pekel, N.; Yoshii, F.; Kume, T.; Guven, O. Radiation crosslinking of biodegradable hydroxypropylmethylcellulose. Carbohydr. Polym. 2004, 55, 139–147. [Google Scholar] [CrossRef]
- Wach, R.A.; Mitomo, H.; Nagasawa, N.; Yoshii, F. Radiation crosslinking of methylcellulose and hydroxyethylcellulose in concentrated aqueous solutions. Nucl Instrum Methods B 2003, 211, 533–544. [Google Scholar] [CrossRef]
- Li, R.; Wu, G. Chapter 5—Preparation of polysaccharide-based hydrogels via radiation technique. In Hydrogels Based on Natural Polymers; Chen, Y., Ed.; Elsevier Matthew Deans: Chennai, India, 2020; pp. 119–148. [Google Scholar]
- Baiya, C.; Nannuan, L.; Tassanapukdee, Y.; Chailapakul, O.; Songsrirote, K. The Synthesis of Carboxymethyl Cellulose-Based Hydrogel from Sugarcane Bagasse Using Microwave-Assisted Irradiation for Selective Adsorption of Copper(II) Ions. Environ. Prog. Sustain. Energy 2019, 38, S157–S165. [Google Scholar] [CrossRef]
- Haque, M.O.; Mondal, M.I.H. Synthesis and Characterization of Cellulose-based Eco-Friendly Hydrogels. Rajshahi Univ. J. Sci. Eng. 2016, 44, 45–53. [Google Scholar] [CrossRef]
- Sanson, N.; Rieger, J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 2010, 1, 965–977. [Google Scholar] [CrossRef]
- Chauhan, G.S.; Mahajan, S. Structural aspects and nature of swelling medium as equilibrium swelling determinants of acrylamide and cellulosic-based smart hydrogels. J. Appl. Polym. Sci. 2002, 85, 1161–1169. [Google Scholar] [CrossRef]
- Geng, H.J. A facile approach to light weight, high porosity cellulose aerogels. Int. J. Biol. Macromol. 2018, 118, 921–931. [Google Scholar] [CrossRef]
- Wang, W.B.; Wang, A.Q. Preparation, swelling, and stimuli-responsive characteristics of superabsorbent nanocomposites based on carboxymethyl cellulose and rectorite. Polym. Adv. Technol. 2011, 22, 1602–1611. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Barzegar, S.; Mahdavinia, G.R. MBA-crosslinked Na-Alg/CMC as a smart full-polysaccharide superabsorbent hydrogels. Carbohydr. Polym. 2006, 66, 386–395. [Google Scholar] [CrossRef]
- El Salmawi, K.M.; Ibrahim, S.M. Characterization of superabsorbent carboxymethylcellulose/clay hydrogel prepared by electron beam irradiation. Macromol. Res. 2011, 19, 1029–1034. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Thermal responsive hydrogels based on semi interpenetrating network of poly(NIPAm) and cellulose nanowhiskers. Carbohydr. Polym. 2014, 102, 159–166. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Fu, S.Y.; Zhang, L.L.; Zhan, H.Y. Superabsorbent nanocomposite hydrogels made of carboxylated cellulose nanofibrils and CMC-g-p(AA-co-AM). Carbohydr. Polym. 2013, 97, 429–435. [Google Scholar] [CrossRef]
- Pandey, M.; Amin, M.C.I.M.; Ahmad, N.; Abeer, M.M. Rapid Synthesis of Superabsorbent Smart-Swelling Bacterial Cellulose/Acrylamide-Based Hydrogels for Drug Delivery. Int. J. Polym. Sci. 2013. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Hu, S.W. “Smart” Materials Based on Cellulose: A Review of the Preparations, Properties, and Applications. Materials 2013, 6, 738–781. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, P.; Dhara, S.; Panda, A.B.; Pal, S. Dextrin and poly(acrylic acid)-based biodegradable, non-cytotoxic, chemically cross-linked hydrogel for sustained release of ornidazole and ciprofloxacin. ACS Appl. Mater. Interfaces 2015, 7, 4791–4803. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, D.; Maitland, D.; Oszustowicz, T.; Hoare, T. Tuning drug release from smart microgel-hydrogel composites via cross-linking. J. Colloid Interface Sci. 2013, 392, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Budtova, T.; Navard, P. Cellulose in NaOH-water based solvents: A review. Cellulose 2016, 23, 5–55. [Google Scholar] [CrossRef]
- Ruan, D.; Lue, A.; Zhang, L. Gelation behaviors of cellulose solution dissolved in aqueous NaOH/thiourea at low temperature. Polymer 2008, 49, 1027–1036. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Unique gelation behavior of cellulose in NaOH/Urea aqueous solution. Biomacromolecules 2006, 7, 183–189. [Google Scholar] [CrossRef]
- Yuan, Z.W.; Zhang, J.J.; Jiang, A.N.; Lv, W.T.; Wang, Y.W.; Geng, H.J.; Wang, J.; Qin, M.H. Fabrication of cellulose self-assemblies and high-strength ordered cellulose films. Carbohydr. Polym. 2015, 117, 414–421. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Rapid dissolution of cellulose in LiOH/Urea and NaOH/Urea aqueous solutions. Macromol. Biosci. 2005, 5, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dahlström, C.; Edlund, H.; Lindman, B.; Norgren, M. pH-responsive cellulose–chitosan nanocomposite films with slow release of chitosan. Cellulose 2019. [Google Scholar] [CrossRef]
- Yang, J.; Duan, J.; Zhang, L.; Lindman, B.; Edlund, H.; Norgren, M. Spherical nanocomposite particles prepared from mixed cellulose–chitosan solutions. Cellulose 2016, 23, 3105–3115. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S.; Zhang, L. Cellulose aerogels from aqueous alkali hydroxide-urea solution. Chemsuschem 2008, 1, 149–154. [Google Scholar] [CrossRef]
- Lavoine, N.; Bergstrom, L. Nanocellulose-based foams and aerogels: Processing, properties, and applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II aerogels: A review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Roy, C.; Budtova, T.; Navard, P. Rheological properties and gelation of aqueous cellulose-NaOH solutions. Biomacromolecules 2003, 4, 259–264. [Google Scholar] [CrossRef]
- Pereira, A.; Duarte, H.; Nosrati, P.; Gubitosi, M.; Gentile, L.; Romano, A.; Medronho, B.; Olsson, U. Cellulose gelation in NaOH solutions is due to cellulose crystallization. Cellulose 2018, 25, 3205–3210. [Google Scholar] [CrossRef]
- Weng, L.H.; Zhang, L.N.; Ruan, D.; Shi, L.H.; Xu, J. Thermal gelation of cellulose in a NaOH/thiourea aqueous solution. Langmuir 2004, 20, 2086–2093. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Filipe, A.; Antunes, F.E.; Lindman, B.; Topgaard, D.; Davidovich, I.; Talmon, Y. New Insights on the Role of Urea on the Dissolution and Thermally-Induced Gelation of Cellulose in Aqueous Alkali. Gels 2018, 4, 87. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Kimura, S.; Wada, M.; Kuga, S. Mechanism of cellulose gelation from aqueous alkali-urea solution. Carbohydr. Polym. 2012, 89, 1298–1300. [Google Scholar] [CrossRef] [PubMed]
- Tapdiqov, S.; Zeynalov, N.; Babayeva, D.; Nasiyyati, E.; Humbatova, S. Copolymerization of N-vinylpyrrolidone with N,N’-methylen-bis-acrylamide: Properties and Structure. Am. J. Polym. Sci. 2015, 5, 18–23. [Google Scholar] [CrossRef]
- Jallapuram, R.; Naydenova, I.; Byrne, H.J.; Martin, S.; Howard, R.G.; Toal, V. Investigation of polymerization rate in an acrylamide-based photopolymer using Raman spectroscopy. Appl. Opt. 2008, 47, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Sundaraganesan, N.; Puviarasan, N.; Mohan, S. Vibrational spectra, assignments and normal coordinate calculation of acrylamide. Talanta 2001, 54, 233–241. [Google Scholar] [CrossRef]
- Wibowo, S.; Velazquez, G.; Savant, V.; Torres, J.A. Surimi wash water treatment for protein recovery: Effect of chitosan–alginate complex concentration and treatment time on protein adsorption. Bioresour. Technol. 2005, 96, 665–671. [Google Scholar] [CrossRef]
- Janko, M.; Jocher, M.; Boehm, A.; Babel, L.; Bump, S.; Biesalski, M.; Meckel, T.; Stark, R.W. Cross-Linking Cellulosic Fibers with Photoreactive Polymers: Visualization with Confocal Raman and Fluorescence Microscopy. Biomacromolecules 2015, 16, 2179. [Google Scholar] [CrossRef]
- Sanchez-Marquez, J.A.; Fuentes-Ramirez, R.; Cano-Rodriguez, I.; Gamino-Arroyo, Z.; Rubio-Rosas, E.; Kenny, J.M.; Rescignano, N. Membrane Made of Cellulose Acetate with Polyacrylic Acid Reinforced with Carbon Nanotubes and Its Applicability for Chromium Removal. Int. J. Polym. Sci. 2015, 2015, 12. [Google Scholar] [CrossRef]
- Agarwal, U.P. Nanocelluloses: Their Preparation, Properties, and Applications; American Chemical Society: Washington, DC, USA, 2017; Volume 1251, pp. 75–90. [Google Scholar]
- Agarwal, U.P.; Ralph, S.A.; Reiner, R.S.; Baez, C. Probing crystallinity of never-dried wood cellulose with Raman spectroscopy. Cellulose 2016, 23, 125–144. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gidley, M.J.; Gilbert, E.P. Application of X-ray and neutron small angle scattering techniques to study the hierarchical structure of plant cell walls: A review. Carbohydr. Polym. 2015, 125, 120–134. [Google Scholar] [CrossRef]
- Michael, A. Ueber die Addition von Natriumacetessig- und Natriummalonsäureäthern zu den Aethern ungesättigter Säuren. J. Prakt. Chem. 1887, 35, 349–356. [Google Scholar] [CrossRef]
- Isogai, A. NMR analysis of cellulose dissolved in aqueous NaOH solutions. Cellulose 1997, 4, 99–107. [Google Scholar] [CrossRef]
- Sinnott, M. Carbohydrate Chemistry and Biochemistry: Structure and Mechanism; Royal Society of Chemistry: London, UK, 2007. [Google Scholar]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Cellulose 2016, 23, 247–258. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.T.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Ramiah, K.V.; Puranik, P.G. Hydrogen bonding in alcohols, acids and secondary amides. In Proceedings of the Indian Academy of Sciences—Section A; Springer India: New Delhi, India, 1962; pp. 155–163. [Google Scholar]
- Chavda, H.V.; Patel, C.N. Effect of crosslinker concentration on characteristics of superporous hydrogel. Int. J. Pharm. Investig. 2011, 1, 17–21. [Google Scholar] [CrossRef] [PubMed]
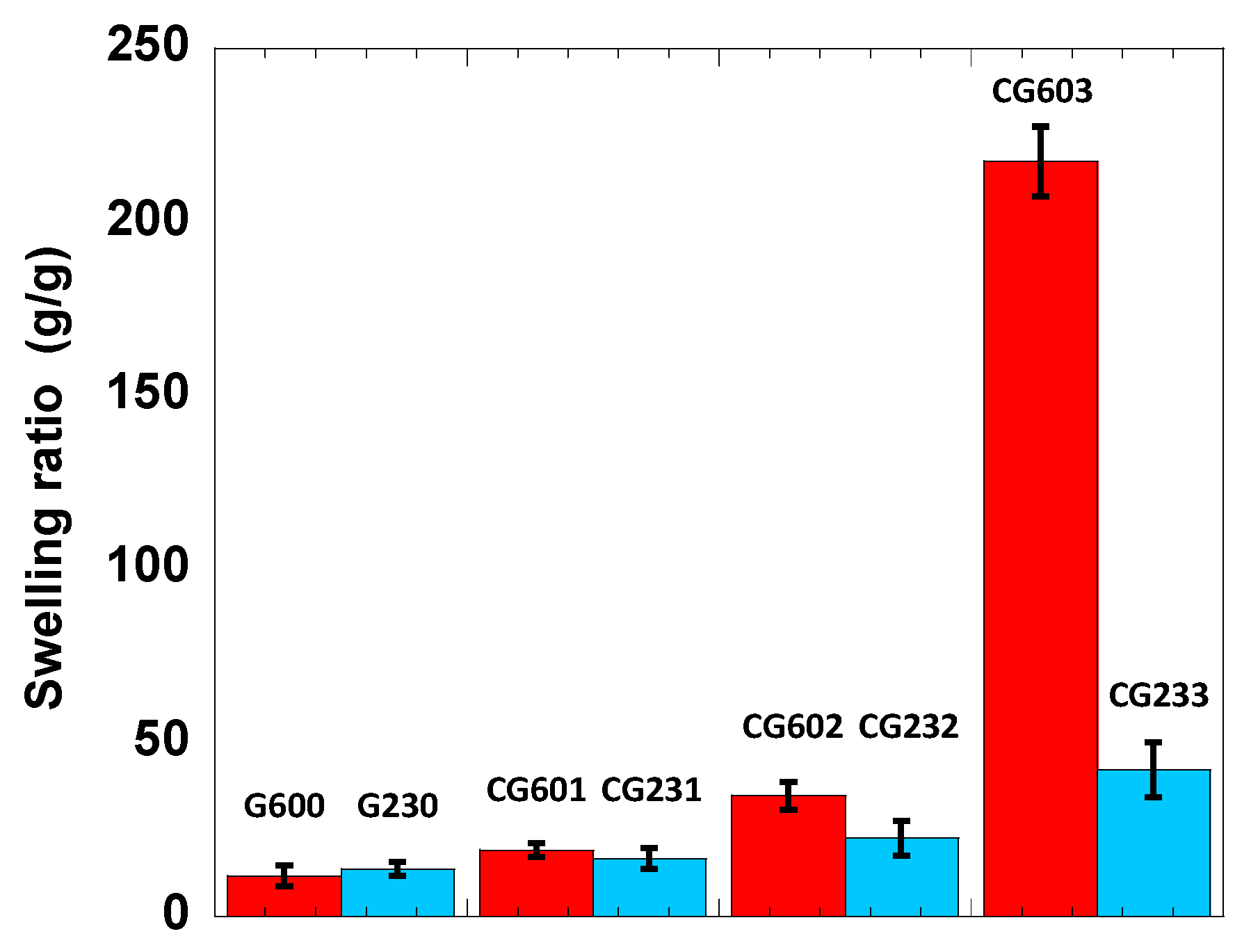
| Reaction Conditions | n[MBA]/n[Glucose] | ||||
|---|---|---|---|---|---|
| 0 | 0.26 | 0.53 | 1.05 | ||
| Temperature/°C | Time/h | Sample Code | |||
| 60 | 0.5 | G600 | CG601 | CG602 | CG603 |
| 23 | 12 | G230 | CG231 | CG232 | CG233 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Medronho, B.; Lindman, B.; Norgren, M. Simple One Pot Preparation of Chemical Hydrogels from Cellulose Dissolved in Cold LiOH/Urea. Polymers 2020, 12, 373. https://doi.org/10.3390/polym12020373
Yang J, Medronho B, Lindman B, Norgren M. Simple One Pot Preparation of Chemical Hydrogels from Cellulose Dissolved in Cold LiOH/Urea. Polymers. 2020; 12(2):373. https://doi.org/10.3390/polym12020373
Chicago/Turabian StyleYang, Jiayi, Bruno Medronho, Björn Lindman, and Magnus Norgren. 2020. "Simple One Pot Preparation of Chemical Hydrogels from Cellulose Dissolved in Cold LiOH/Urea" Polymers 12, no. 2: 373. https://doi.org/10.3390/polym12020373
APA StyleYang, J., Medronho, B., Lindman, B., & Norgren, M. (2020). Simple One Pot Preparation of Chemical Hydrogels from Cellulose Dissolved in Cold LiOH/Urea. Polymers, 12(2), 373. https://doi.org/10.3390/polym12020373