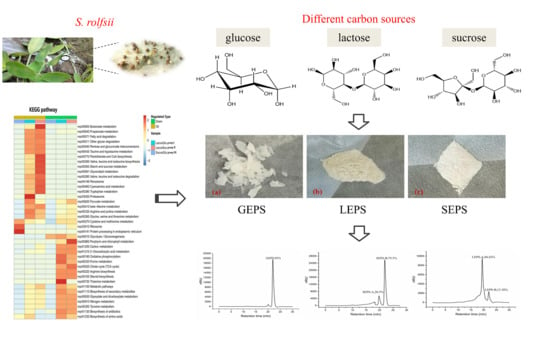
Initial Analysis on the Characteristics and Synthesis of Exopolysaccharides from Sclerotium rolfsii with Different Sugars as Carbon Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fungal Strains and Growth Media
2.3. Rheological Property Measurement
2.4. Extraction and Purification of Exopolysaccharides
2.5. Physicochemical Characterization of EPS
2.5.1. Morphology Observation
2.5.2. Chemical Composition Analysis
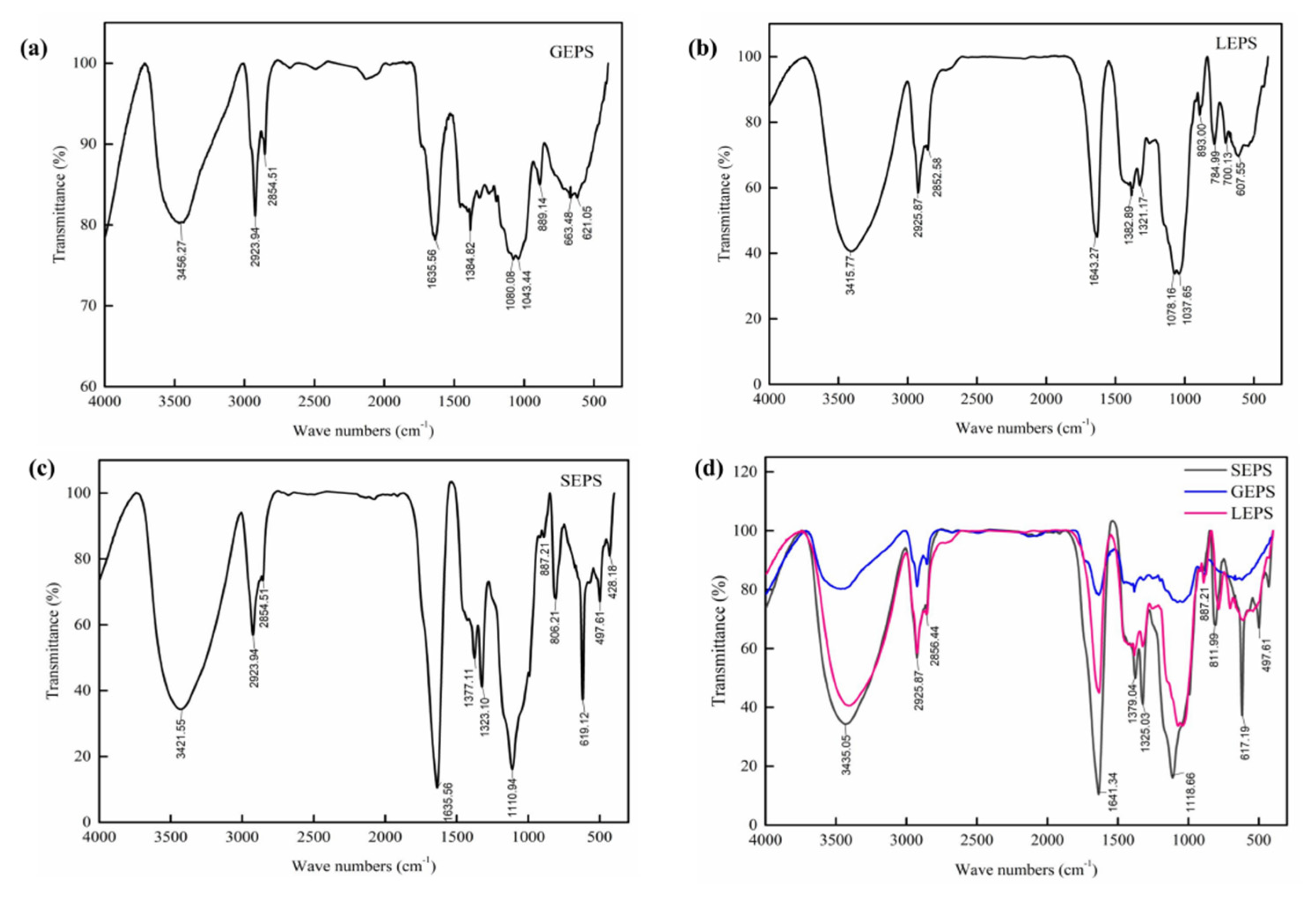
2.5.3. Fourier Transform Infrared Spectra Analysis
2.6. Molecular Weight Distribution and Monosaccharide Composition
2.6.1. Molecular Weight Distribution
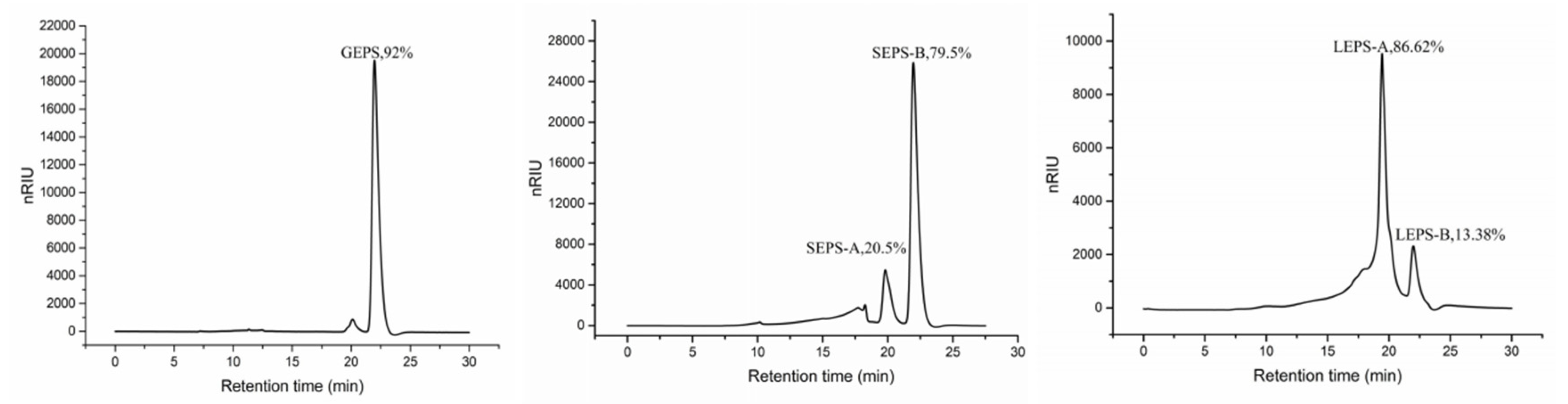
2.6.2. Monosaccharide Composition
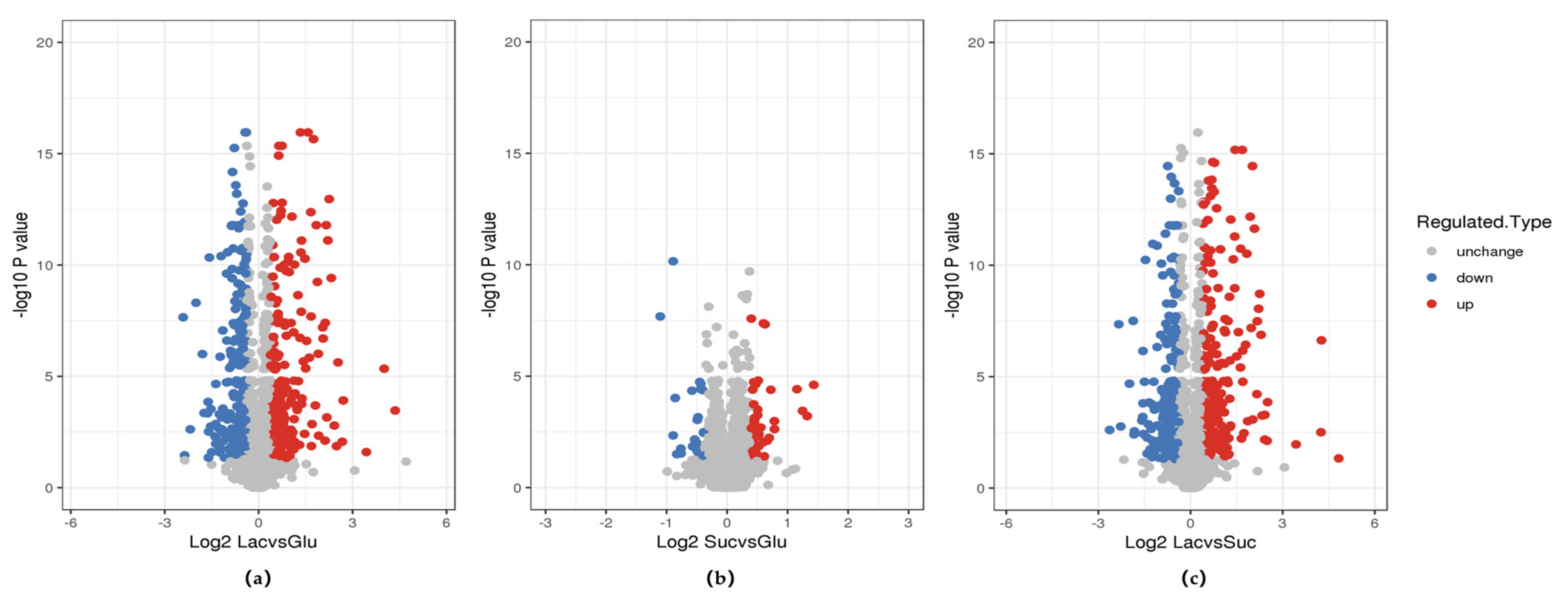
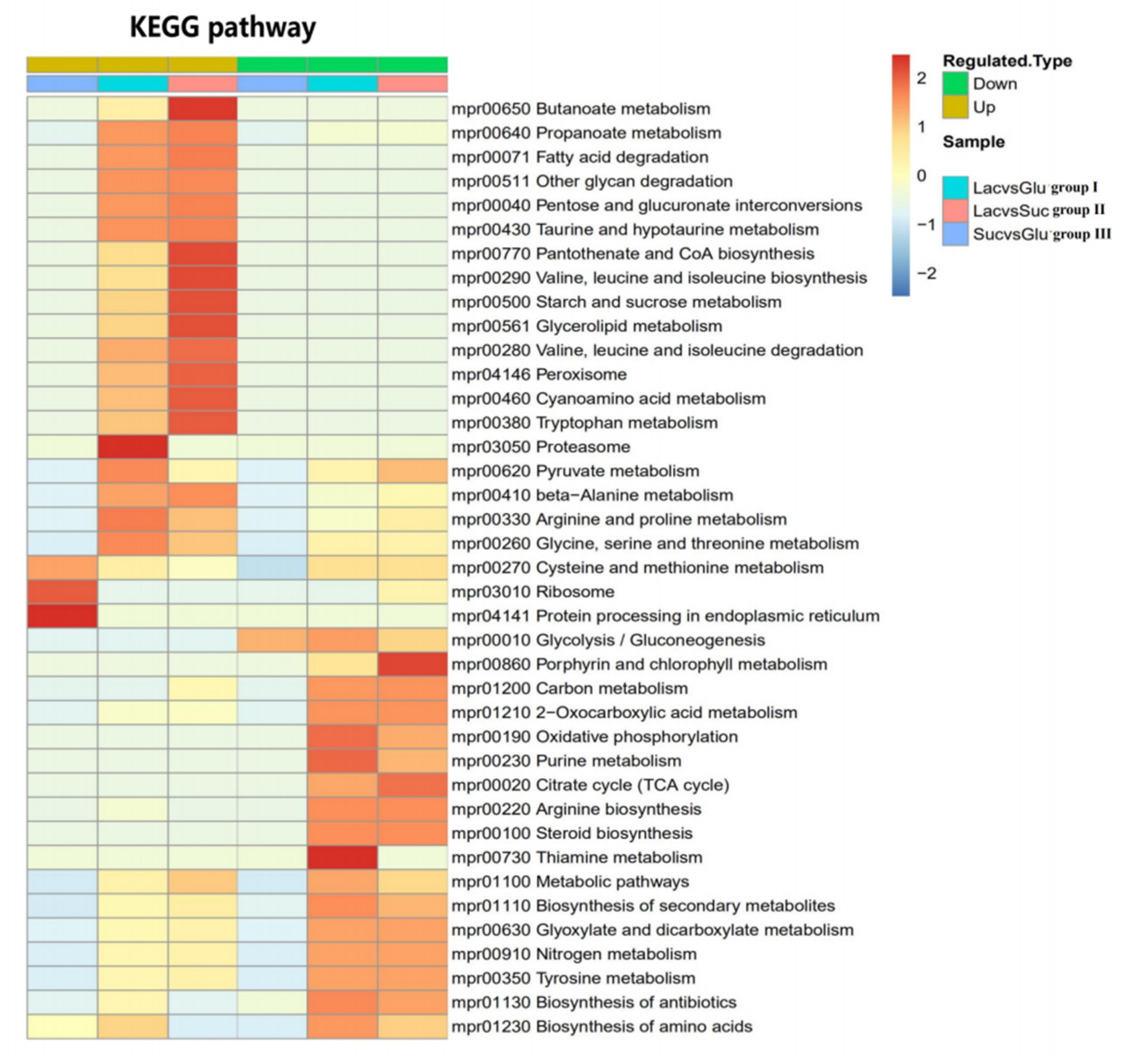
2.7. Comparative Proteome Analysis
2.8. Statistical Analysis
3. Results and Discussion
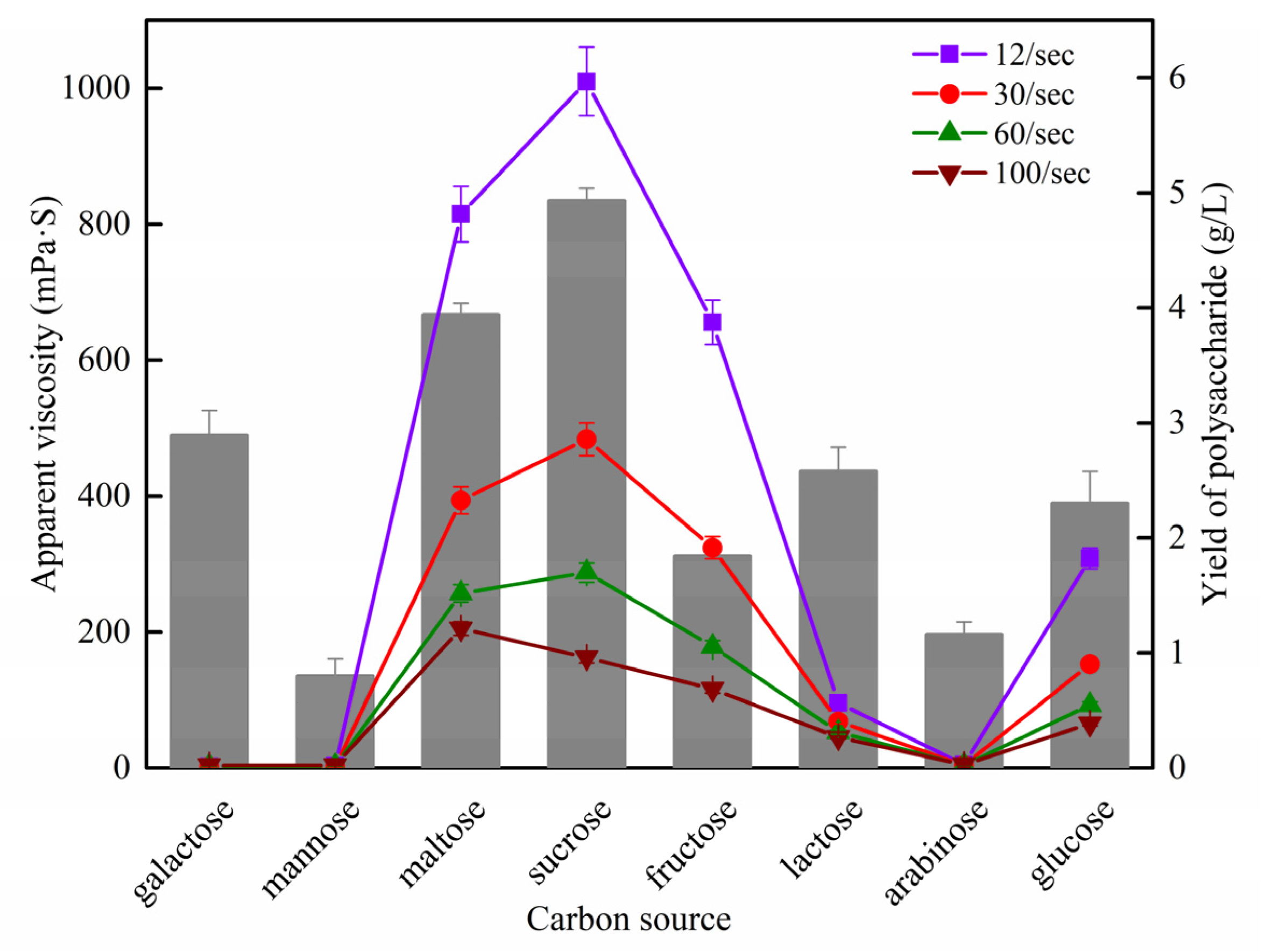
3.1. Rheological Comparison
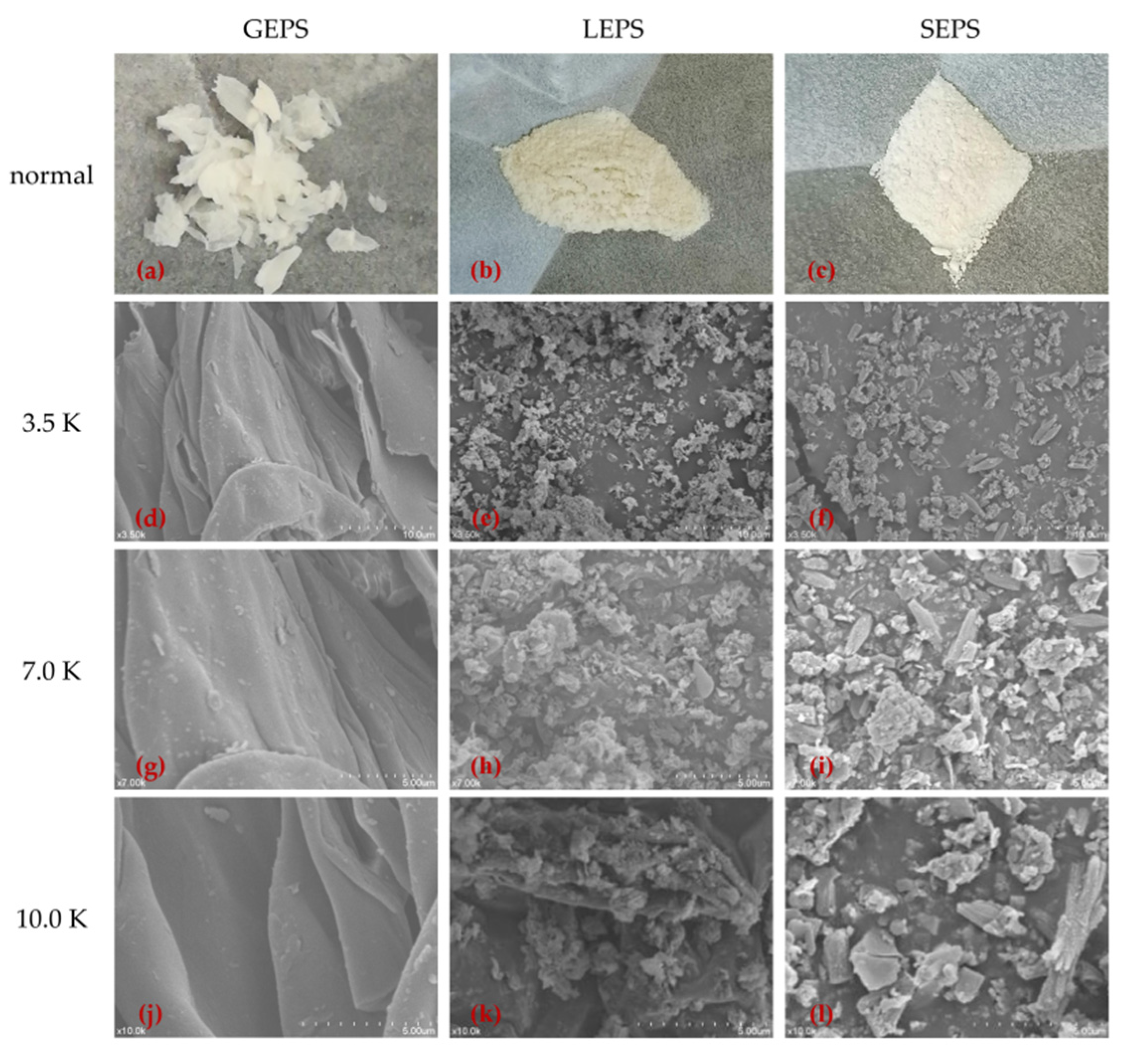
3.2. Scanning Electron Microscopy
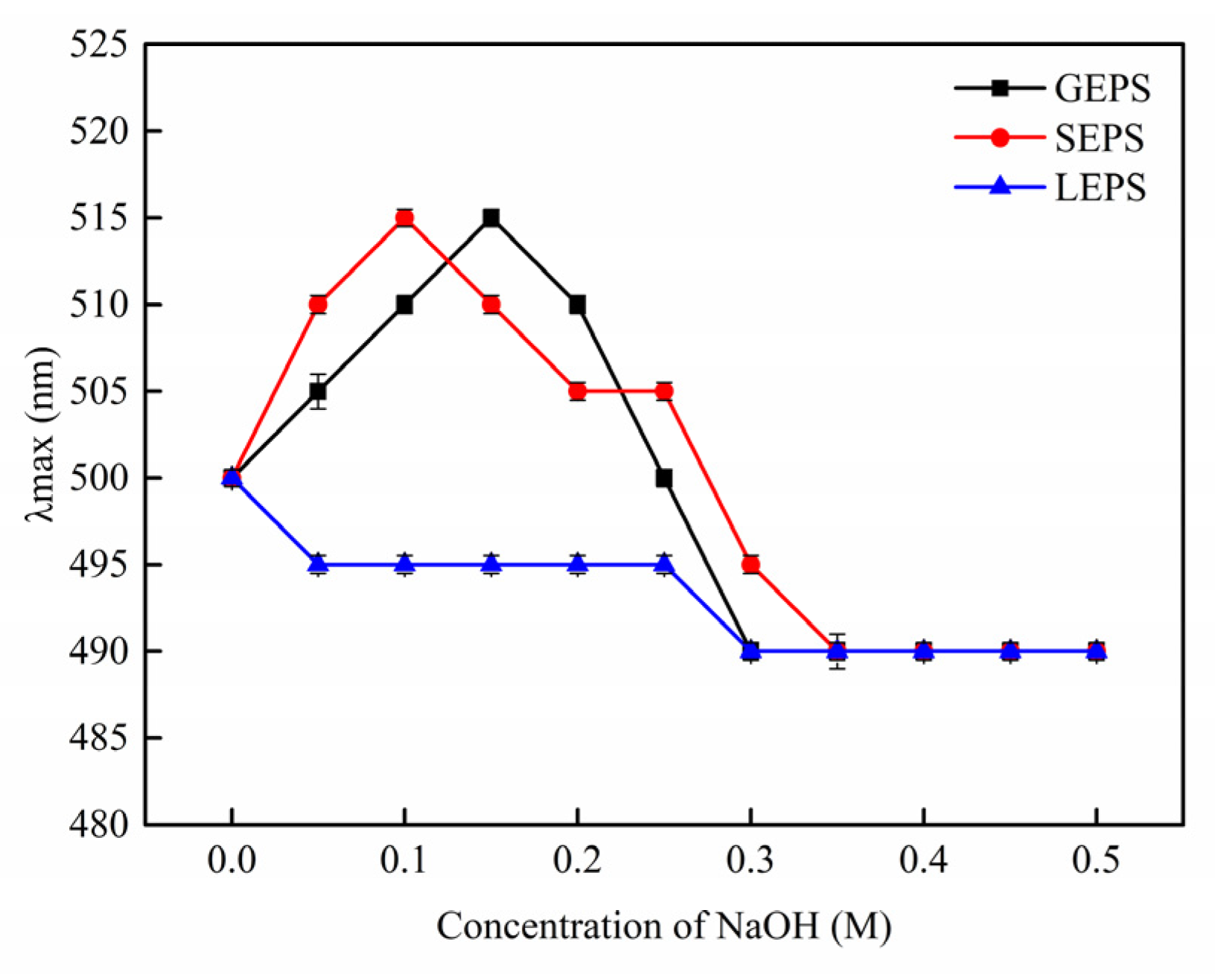
3.3. Physicochemical Characterization of EPS
3.4. Fourier Transform Infrared Spectra Analysis
3.5. Molecular Weight Distribution
3.6. Monosaccharide Composition
3.7. Comparative Analysis of Metabolic Pathways
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.T. Study on the Ability of Lignocellulose Degradation and the Pathway of Scleroglucan Biosynthesis in Sclerotium rolfsii. Master’s Thesis, Southwest University, Chongqing, China, 2017. [Google Scholar]
- Bejarano-Mendoza, C.A.; Zapata, M.; Bosques, A.; Riveraamador, E.; Liu, L.J. Sclerotium rolfsii as a component in the pathological complex that causes dry root rot in tanier (Xanthosoma sagittifolium) in Puerto Rico. J. Agric. Univ. Puerto Rico 1998, 82, 85–95. [Google Scholar]
- Haltrich, D.; Laussamayer, B.; Steiner, W. Xylanase formation by Sclerotium rolfsii: Effect of growth substrates and development of a culture medium using statistically designed experiments. Appl. Microbiol. Biotechnol. 1994, 42, 522–530. [Google Scholar] [CrossRef]
- Vinarta, S.C.; Molina, O.E.; Figueroa, L.I.C.; Fariña, J.I. A further insight into the practical applications of exopolysaccharides from Sclerotium rolfsii. Food Hydrocoll. 2006, 20, 619–6290. [Google Scholar] [CrossRef]
- Han, M. Structure and properties of scleroglucan. Oilfield Chem. 1993, 10, 375–379. [Google Scholar]
- Noïk, C.; Lecourtier, J. Studies on scleroglucan conformation by rheological measurements versus temperature up to 150 °C. Polymer 1993, 34, 150–157. [Google Scholar] [CrossRef]
- Viñarta, S.C.; Delgado, O.D.; Figueroa, L.I.C.; Fariña, J.I. Effects of thermal, alkaline and ultrasonic treatments on scleroglucan stability and flow behavior. Carbohydr. Polym. 2013, 94, 496–504. [Google Scholar] [CrossRef]
- Fariña, J.I.; Siñeriz, F.; Molina, O.E.; Perotti, N.I. High scleroglucan production by Sclerotium rolfsii: Influence of medium composition. Biotechnol. Lett. 1998, 20, 825–831. [Google Scholar] [CrossRef]
- Fariña, J.I.; Viñarta, S.C.; Cattaneo, M.; Figueroa, L.; de Figueroa, L.I.C. Structural stability of Sclerotium rolfsii ATCC 201126 β-glucan with fermentation time: A chemical, infrared spectroscopic and enzymatic approach. J. Appl. Microbiol. 2009, 106, 221–232. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Wang, W.; Zhang, Y.M.; Dong, X.Q. Medium optimization of scleroglucan production by Sclerotium rolfsii SCL2010. China Brew. 2017, 11, 49–53. [Google Scholar]
- Schilling, B.M.; Henning, A.; Rau, U. Repression of oxalic acid biosynthesis in the unsterile scleroglucan production process with Sclerotium rolfsii ATCC 15205. Bioprocess Eng. 2000, 22, 51–55. [Google Scholar] [CrossRef]
- Gao, R.W.; Zhou, J.W.; Xu, G.Q. Strategies for feeding control in microbial production of scleroglucan by Sclerotium rolfsii WSHG01. Food Ferment Ind. 2018, 44, 51–56. [Google Scholar]
- Schmid, J.; Müller-Hagen, D.; Bekel, T.; Funk, L.; Stahl, U.; Sieber, V.; Meyer, V. Transcriptome sequencing and comparative transcriptome analysis of the scleroglucan producer Sclerotium rolfsii. BMC Genom. 2010, 11, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Yi, P.; Li, N.S.; Wan, J.B.; Zhang, D.Z.; Li, M.Y.; Yan, C.Y. Structural characterization and antioxidant activity of a heteropolysaccharide from Ganoderma capense. Carbohydr. Polym. 2015, 121, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Li, Y.L.; Li, S.W.; Li, Q.Y.; Fan, W.G.; Kiatoukosin, L.; Chen, J.X. Extracellular polysaccharides of endophytic fungus Alternaria tenuissima F1 from Angelica sinensis: Production conditions, purification, and antioxidant properties. Int. J. Biol. Macromol. 2019, 133, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.S.; He, X.H.; Lin, H.; Liang, R.H.; Liang, L.; Chen, J.; Liu, C.M. Solubility difference between pectic fractions from creeping fig Seeds. Polymers 2019, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.X. Isolation, Purification, Structure Elucidationand Biological Activities of Polysaccharides from the Fruiting Bodies of Pholiota adiposa. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 2011. [Google Scholar]
- Ma, C.M.; Sun, Z.; Chen, C.B.; Zhang, L.L.; Zhu, S.H. Simultaneous separation and determination of fructose, sorbitol, glucose and sucrose in fruits by HPLC–ELSD. Food Chem. 2014, 145, 784–788. [Google Scholar] [CrossRef]
- Fariña, J.I.; Siñeriz, F.; Molina, O.E.; Perotti, N.I. Isolation and physicochemical characterization of soluble scleroglucan from Sclerotium rolfsii. rheological properties, molecular weight and conformational characteristics. Carbohydr. Polym. 2001, 44, 41–50. [Google Scholar] [CrossRef]
- Schmid, J.; Meyer, V.; Sieber, V. Scleroglucan: Biosynthesis, production and application of a versatile hydrocolloid. Appl. Microbiol. Biotechnol. 2011, 91, 937–947. [Google Scholar] [CrossRef]
- He, Y.L.; Ye, M.; Du, Z.Z.; Wang, H.Y.; Wu, Y.N.; Yang, L. Purification, characterization and promoting effect on wound healing of an exopolysaccharide from Lachnum YM405. Carbohydr. Polym. 2014, 105, 169–176. [Google Scholar] [CrossRef]
- Chien, R.C.; Yen, M.T.; Tseng, Y.H.; Mau, J.L. Chemical characteristics and anti-proliferation activities of Ganoderma tsugae polysaccharides. Carbohydr Polym. 2015, 128, 90–98. [Google Scholar] [CrossRef]
- Ai, L.; Guo, Q.; Ding, H.; Guo, B.; Chen, W.; Cui, S.W. Structure characterization of exopolysaccharides from Lactobacillus casei LC2W from skim milk. Food Hydrocoll. 2016, 56, 134–143. [Google Scholar] [CrossRef]
- Adesulu-Dahunsi, A.T.; Sanni, A.I.; Jeyaram, K. Production, characterization and in vitro antioxidant activities of exopolysaccharide from Weissella cibaria GA44. LWT Food Sci. Technol. 2018, 87, 432–442. [Google Scholar] [CrossRef]
- Soni, B.; Hassan, E.B.; Mahmoud, B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr Polym. 2015, 134, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Xu, J.L.; Zhang, J.C.; Liu, Y.; Sun, H.J.; Zha, X. Physicochemical properties and antioxidant activities of polysaccharide from floral mushroom cultivated in Huangshan Mountain. Carbohydr. Polym. 2015, 131, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Gangoiti, M.V.; Puertas, A.I.; Hamet, M.F.; Peruzzo, P.J.; Llamas, M.G.; Medrano, M.; Prieto, A.; Dueñas, M.T.; Abraham, A.G. Lactobacillus plantarum CIDCA 8327: An α-glucan producing-strain isolated from kefir grains. Carbohydr. Polym. 2017, 170, 52–59. [Google Scholar] [CrossRef]
- Han, P.P.; Yao, S.Y.; Guo, R.J.; Shen, S.G.; Yan, R.R.; Tan, Z.L. The relationship between monosaccharide composition of extracellular polysaccharide and activities of related enzymes in Nostoc flagelliforme under different culture conditions. Carbohydr. Polym. 2017, 174, 111–119. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis Maria, A.M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar]
- Ruffing, A.; Chen, R.R. Metabolic engineering of microbes for oligosaccharide and polysaccharide synthesis. Microb. Cell Factories 2006, 5, 25. [Google Scholar] [CrossRef]
- Yoshimi, A.; Miyazawa, K.; Abe, K. Function and Biosynthesis of cell wall α-1, 3-Glucan in Fungi. J. Fungi 2017, 3, 63. [Google Scholar] [CrossRef]
- Jin, K.H.; Li, L.; Sun, X.H.; Xu, Q.Y.; Song, S.Y.; Shen, Y.M.; Deng, X.M. Mycoepoxydiene suppresses HeLa cell growth by inhibiting glycolysis and the pentose phosphate pathway. Appl. Microbiol. Biotechnol. 2017, 101, 4201–4213. [Google Scholar] [CrossRef]
- Van, P.D.A.; Taminiau, B.; Pinheiro, I.; Duysburgh, C.; Jacobs, H.; Pijls, L.; Marzorati, M. Arabinoxylo- oligosaccharides and inulin impact inter-individual variation on microbial metabolism and composition, which immunomodulates human cells. J. Agric. Food Chem. 2018, 66, 1121–1130. [Google Scholar]
- Salvato, F.; Wilson, R.; Portilla Llerena, J.P.; Kiyota, E.; Lima Reis, K.; Boaretto, L.F.; Balbuena, T.S.; Azevedo, R.A.; Thelen, J.J.; Mazzafera, P. Luxurious nitrogen fertilization of two sugarcane genotypes contrasting for lignin composition causes changes in the stem proteome related to carbon, nitrogen and oxidant metabolism but does not alter lignin content. J. Proteome Res. 2017, 16, 3688–3703. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.S.; Harris, P.J. Xylans of red and green algae: What is known about their structures and how they are synthesised? Polymers 2019, 11, 354. [Google Scholar] [CrossRef] [PubMed]
| Method | GEPS | LEPS | SEPS |
|---|---|---|---|
| Iodination test | − | − | − |
| Fehling’s test | − | − | − |
| Phenol-sulfuric acid test | + | + | + |
| Coomassie brilliant blue test | − | − | − |
| FeCl3 test | − | − | − |
| Samples | Metals (mg/g) | ||||
|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | Fe3+ | |
| GEPS | 0.66 ± 0.12 a,b | 14.68 ± 1.25 a,b | 23.06 ± 2.04 a,b | 1.95 ± 0.31 a,b | 0.89 ± 0.22 a,b |
| LEPS | 52.37 ± 3.65 c | 19.65 ± 2.32 c | 2.03 ± 0.43 | 16.87 ± 2.13 c | 12.74 ± 1.55 c |
| SEPS | 64.57 ± 3.07 | 27.54 ± 2.04 | 1.97 ± 0.42 | 20.48 ± 1.02 | 15.37 ± 1.24 |
| Standard Samples | D-Fucose | D-Xylose | Fructose | Mannose | D-Glucose |
|---|---|---|---|---|---|
| Retention time (min) | 5.679 | 6.767 | 7.231 | 8.264 | 9.157 |
| GEPS | - | - | - | - | + |
| SEPS-A | - | - | - | - | + |
| SEPS-B | - | - | - | - | + |
| LEPS-A | - | - | - | - | + |
| LEPS-B | - | - | - | - | + |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Jia, Y.-X.; Su, Y.; Zhang, X.-Y.; Tu, L.-N.; Nie, Z.-Q.; Zheng, Y.; Wang, M. Initial Analysis on the Characteristics and Synthesis of Exopolysaccharides from Sclerotium rolfsii with Different Sugars as Carbon Sources. Polymers 2020, 12, 348. https://doi.org/10.3390/polym12020348
Song J, Jia Y-X, Su Y, Zhang X-Y, Tu L-N, Nie Z-Q, Zheng Y, Wang M. Initial Analysis on the Characteristics and Synthesis of Exopolysaccharides from Sclerotium rolfsii with Different Sugars as Carbon Sources. Polymers. 2020; 12(2):348. https://doi.org/10.3390/polym12020348
Chicago/Turabian StyleSong, Jia, Yu-Xiang Jia, Yan Su, Xiao-Yu Zhang, Lin-Na Tu, Zhi-Qiang Nie, Yu Zheng, and Min Wang. 2020. "Initial Analysis on the Characteristics and Synthesis of Exopolysaccharides from Sclerotium rolfsii with Different Sugars as Carbon Sources" Polymers 12, no. 2: 348. https://doi.org/10.3390/polym12020348
APA StyleSong, J., Jia, Y.-X., Su, Y., Zhang, X.-Y., Tu, L.-N., Nie, Z.-Q., Zheng, Y., & Wang, M. (2020). Initial Analysis on the Characteristics and Synthesis of Exopolysaccharides from Sclerotium rolfsii with Different Sugars as Carbon Sources. Polymers, 12(2), 348. https://doi.org/10.3390/polym12020348