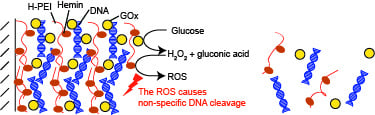
Decomposition of Glucose-Sensitive Layer-by-Layer Films Using Hemin, DNA, and Glucose Oxidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
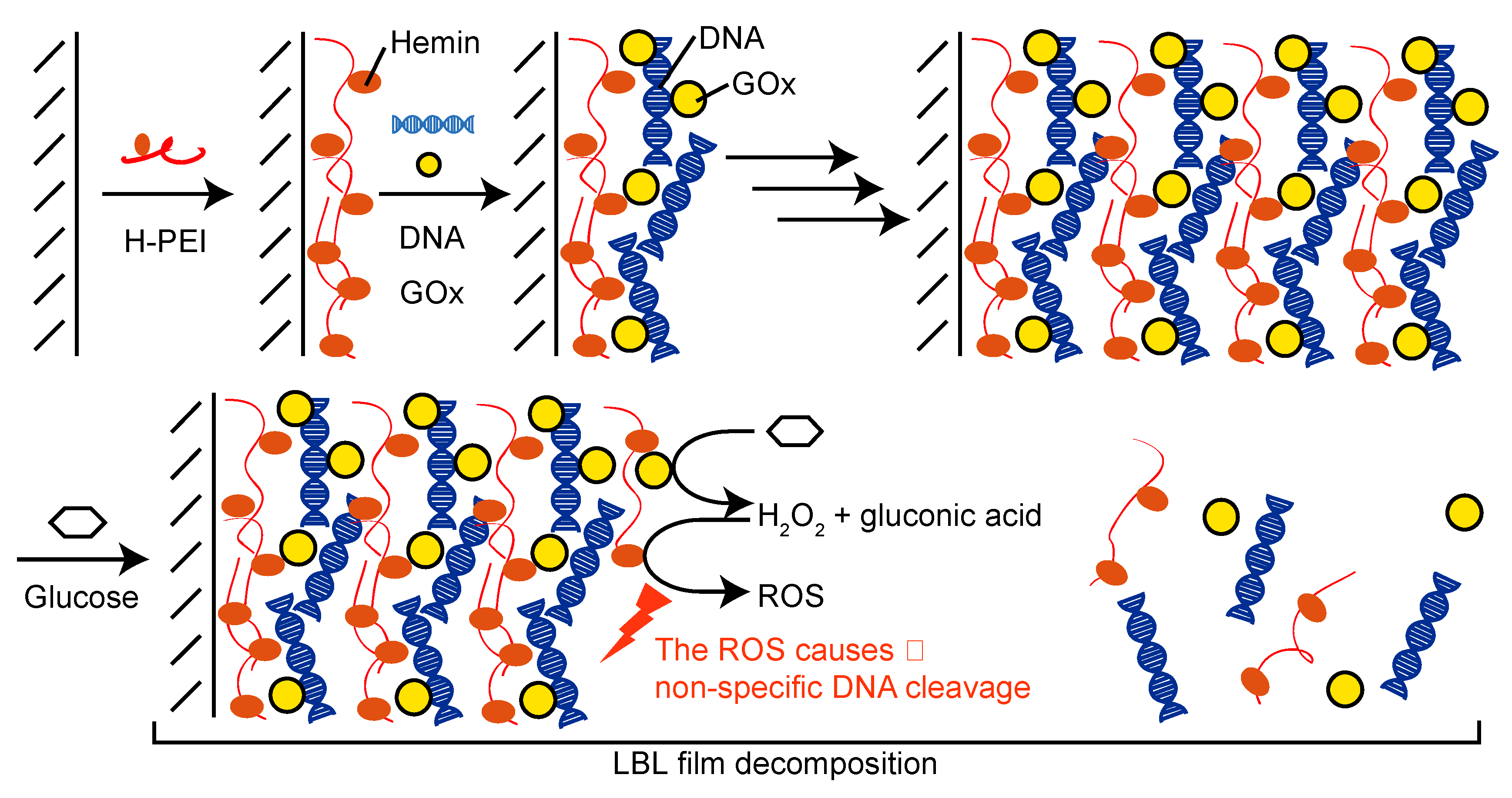
2.3. Preparation of LbL Films
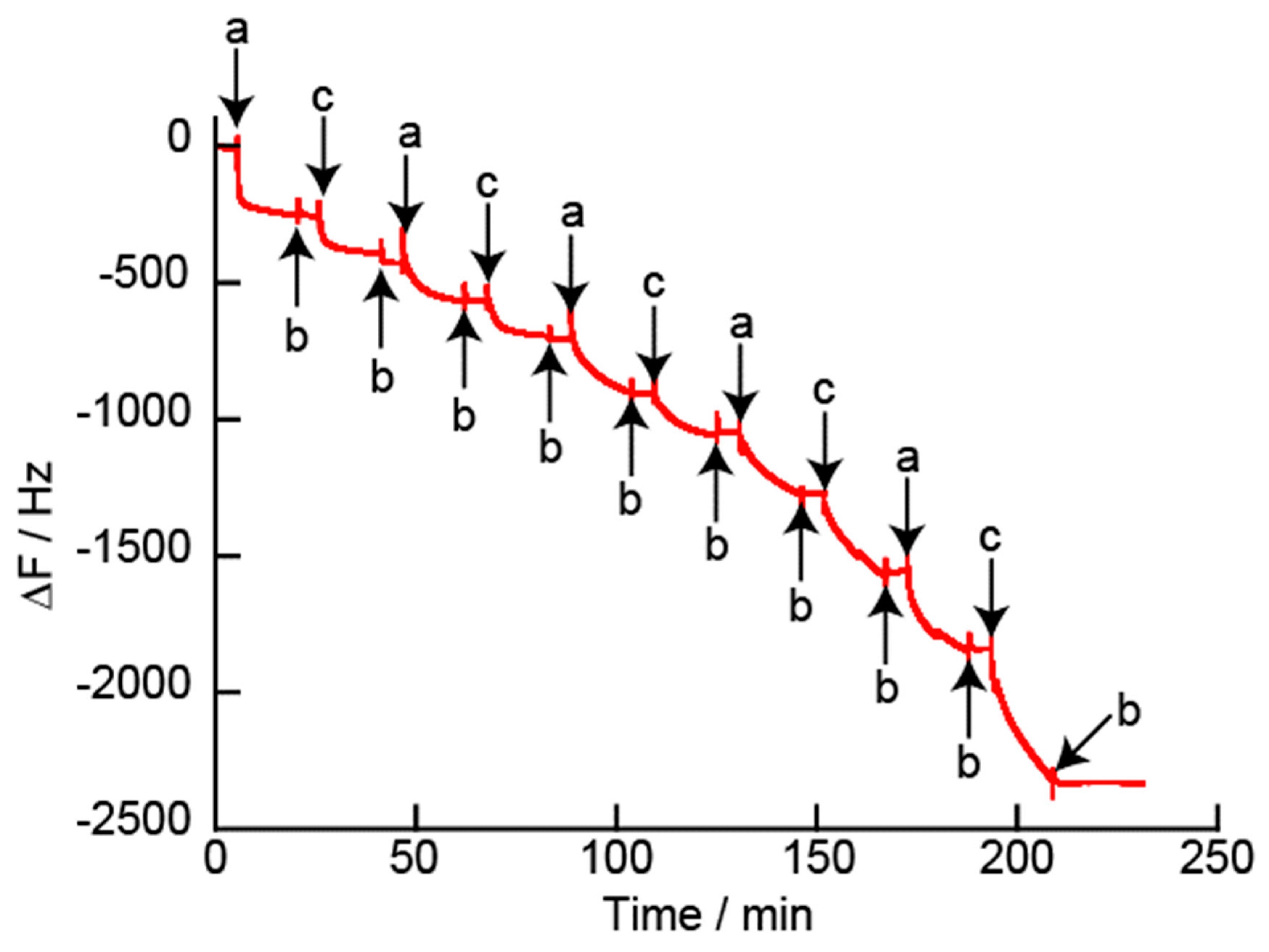
2.4. Decomposition of LbL Films
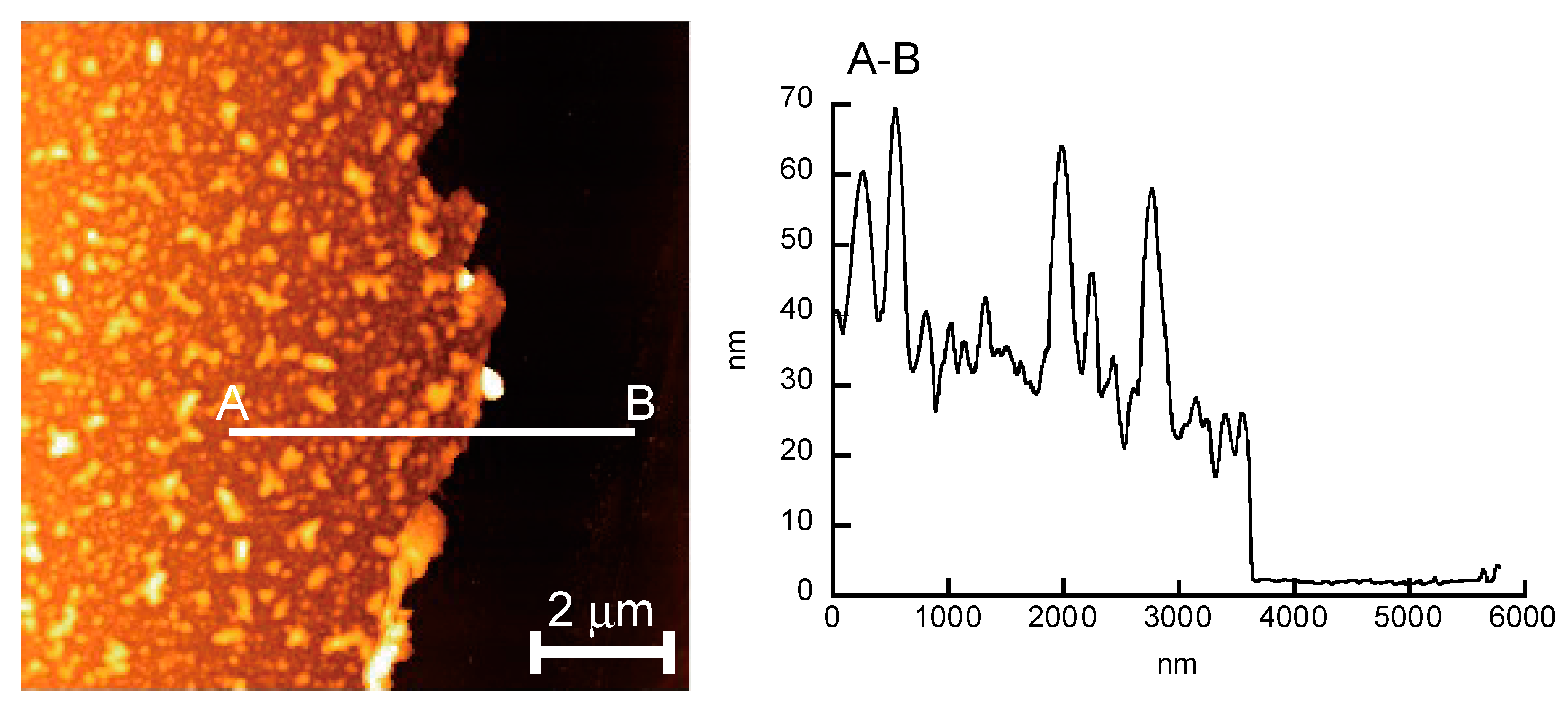
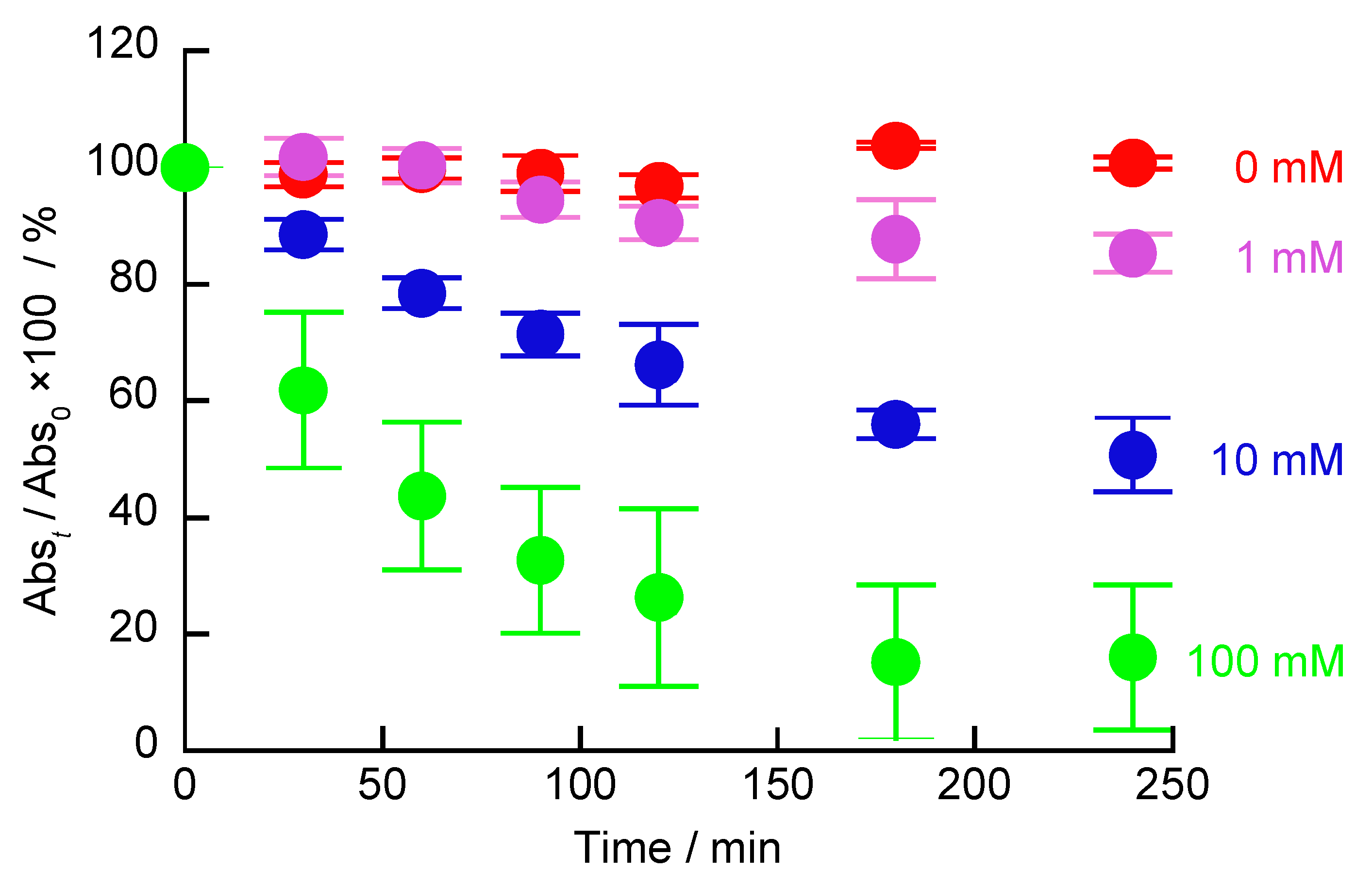
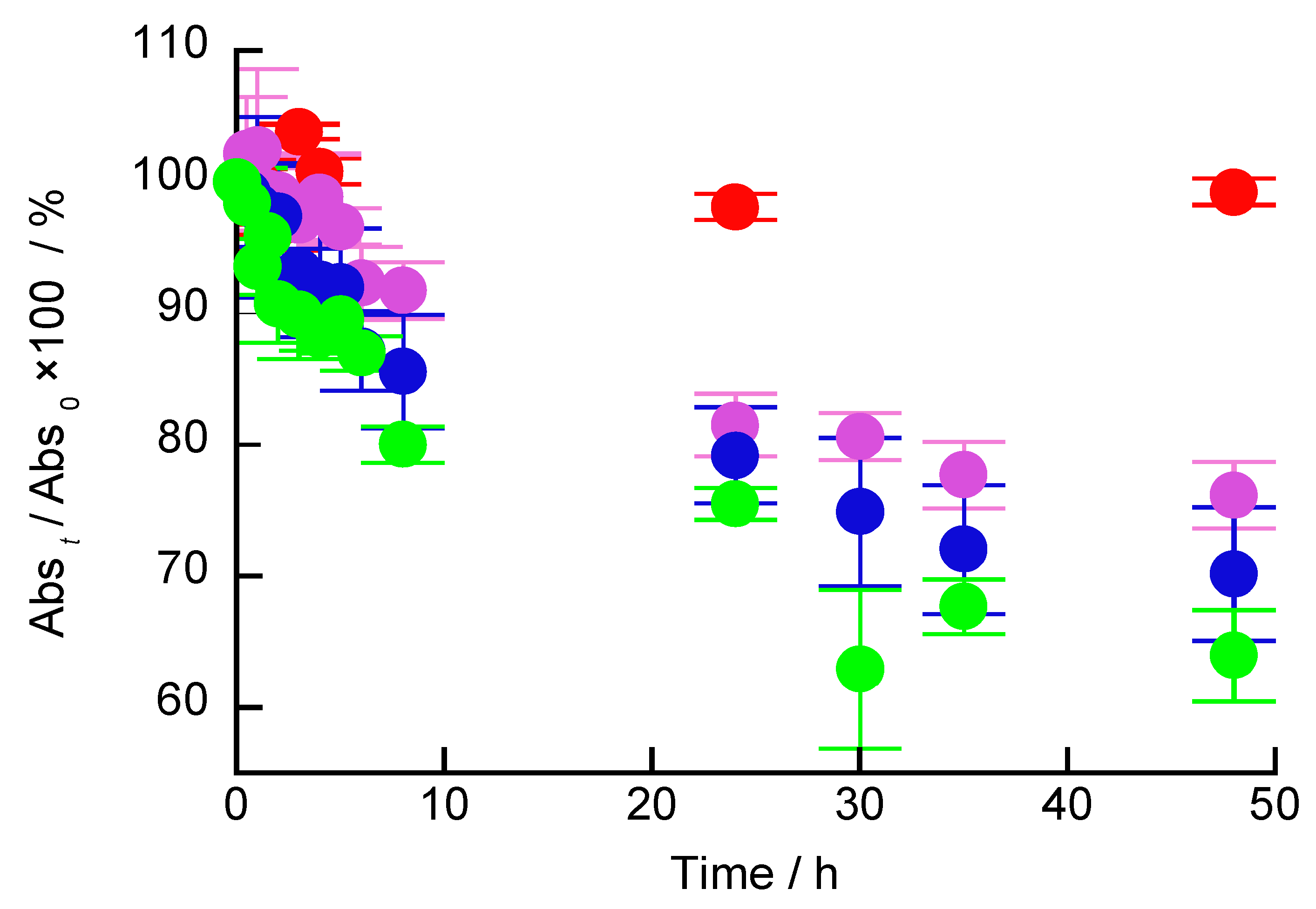
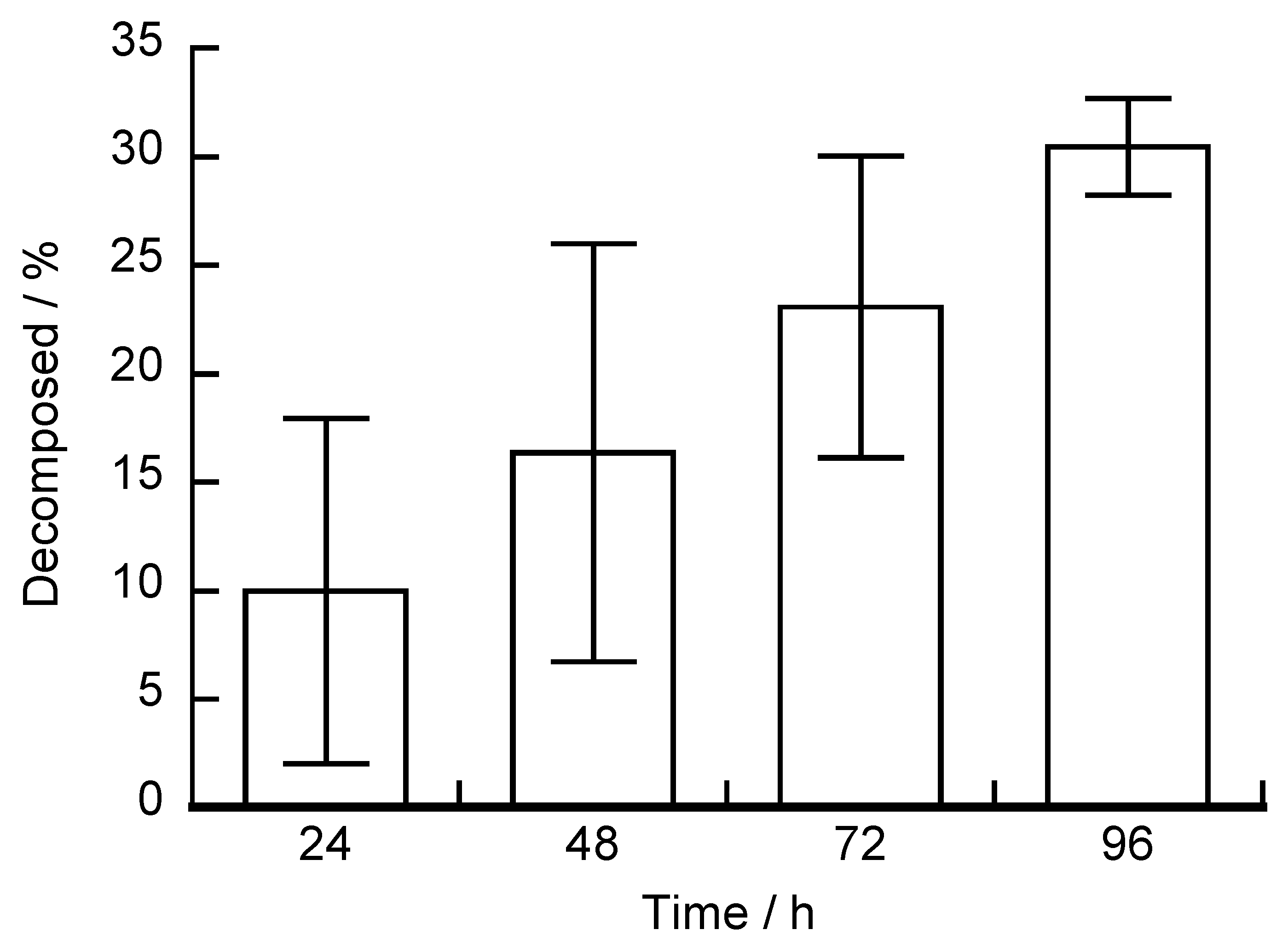
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Izquierdo, A.; Ono, S.S.; Voegel, J.C.; Schaaf, P.; Decher, G. Dipping versus spraying: Exploring the deposition conditions for speeding up layer-by-layer assembly. Langmuir 2005, 21, 7558–7567. [Google Scholar] [CrossRef]
- Yoshida, K.; Sato, K.; Ono, T.; Dairaku, T.; Kashiwagi, Y. Preparation of nafion/polycation layer-by-layer films for adsorption and release of insulin. Polymers 2018, 10, 812. [Google Scholar] [CrossRef] [PubMed]
- Erel, I.; Schlaad, H.; Demirel, A.L. Effect of structural isomerism and polymer end group on the pH-stability of hydrogen-bonded multilayers. J. Colloid Interface Sci. 2011, 361, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, Y.; Miyagishima, T.; Tomida, K.; Wang, B.; Takahashi, S.; Sato, K.; Anzai, J.-I. Dual pH-sensitive layer-by-layer films containing amphoteric poly(diallylamine-co-maleic acid). J. Colloid Interface Sci. 2013, 399, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Sato, K.; Anzai, J.-I. Disintegration of layer-by-layer assemblies composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules 2005, 6, 27–29. [Google Scholar] [CrossRef]
- Sato, K.; Imoto, Y.; Sugama, J.; Seki, S.; Inoue, H.; Odagiri, T.; Hoshi, T.; Anzai, J.-I. Sugar-induced disintegration of layer-by-layer assemblies composed of concanavalin A and glycogen. Langmuir 2005, 21, 797–799. [Google Scholar] [CrossRef]
- Decher, G.; Hong, J.D.; Schmitt, J. Buildup of ultrathin multilayer films by a self-assembly process:III. Consecutively alternating adsorption of anionic and cationic polyelectrolytes on charged surfaces. Thin Solid Films 1992, 210–211, 831–835. [Google Scholar] [CrossRef]
- Hammond, P.T. Form and function in multilayer assembly: New applications at the nanoscale. Adv. Mater. 2004, 16, 1271–1293. [Google Scholar] [CrossRef]
- Rochín-Wong, S.; Rosas-Durazo, A.; Zavala-Rivera, P.; Maldonado, A.; Martínez-Barbosa, M.E.; Vélaz, I.; Tánori, J. Drug release properties of diflunisal from layer-by-layer self-assembled κ-carrageenan/chitosan nanocapsules: Effect of Deposited Layers. Polymers 2018, 10, 760. [Google Scholar] [CrossRef]
- Shutava, T.G.; Livanovich, K.S.; Sharamet, A.A. Layer-by-layer films of polysaccharides modified with polyethylene glycol and dextran. Colloids Surf. B Biointerfaces 2019, 173, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 22, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Houska, M.; Brynda, E.; Bohatá, K. The effect of polyelectrolyte chain length on layer-by-layer protein/polyelectrolyte assembly—An experimental study. Colloid Interface Sci. 2004, 273, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hashide, R.; Yoshida, K.; Hasebe, Y.; Seno, M.; Takahashi, S.; Sato, K.; Anzai, J. Poly(lactic acid) microparticles coated with insulin-containing layer-by-layer films and their pH-dependent insulin release. J. Nanosci. Nanotechnol. 2014, 14, 3100–3105. [Google Scholar] [CrossRef] [PubMed]
- Vander Straeten, A.; Bratek-Skicki, A.; Jonas, A.M.; Fustin, C.A.; Dupont-Gillain, C. Integrating proteins in layer-by-layer assemblies independently of their electrical charge. ACS Nano 2018, 12, 8372–8381. [Google Scholar] [CrossRef]
- Lee, L.; Johnston, A.P.; Caruso, F. Programmed degradation of DNA multilayer films. Small 2014, 10, 2902–2909. [Google Scholar] [CrossRef]
- Peng, N.; Yu, H.; Wang, Z.; Zhang, Y.; Deng, K.; Li, J.; Lu, L.; Zou, T.; Liu, Y.; Huang, S. Dendrimer-grafted bioreducible polycation/DNA multilayered films with low cytotoxicity and high transfection ability. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 737–745. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, C.; Van der Bruggen, B. Technology-driven layer-by-layer assembly of a membrane for selective separation of monovalent anions and antifouling. Nanoscale 2019, 11, 2264–2274. [Google Scholar] [CrossRef]
- Zou, Y.; Xie, L.; Carroll, S.; Muniz, M.; Gibson, H.; Wei, W.Z.; Liu, H.; Mao, G. Layer-by-layer films with bioreducible and nonbioreducible polycations for sequential DNA release. Biomacromolecules 2014, 15, 3965–3975. [Google Scholar] [CrossRef]
- Locke, A.K.; Means, A.K.; Dong, P.; Nichols, T.J.; Coté, G.L.; Grunlan, M.A. A Layer-by-layer approach to retain a fluorescent glucose sensing assay within the cavity of a hydrogel membrane. ACS Appl. Bio Mater. 2018, 1, 1319–1327. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, H.; Zhang, Y.; Dong, F.; Li, Z. Cellulose acetate nanofibers coated layer-by-layer with polyethylenimine and graphene oxide on a quartz crystal microbalance for use as a highly sensitive ammonia sensor. Colloids Surf. B Biointerfaces 2016, 148, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef] [PubMed]
- Liu, P. Stabilization of layer-by-layer engineered multilayered hollow microspheres. Adv. Colloid Interface Sci. 2014, 207, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.A.; da Silva Abreu, A.; Tedesco, A.C.; Junior, M.B.; Simioni, A.R. Functionalized photosensitive gelatin nanoparticles for drug delivery application. J. Biomater. Sci. Polym. Ed. 2019, 30, 508–525. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Ono, T.; Dairaku, T.; Kashiwagi, Y.; Sato, K. Preparation of hydrogen peroxide sensitive nanofilms by a layer-by-layer technique. Nanomaterials 2018, 8, 941. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yao, Y.; Xie, R.; Dai, D.; Lu, W.; Chen, W.; Zang, L. Enhanced generation of reactive oxygen species for efficient pollutant elimination catalyzed by hemin based on persistent free radicals. Appl. Catal. B Environ. 2016, 183, 291–297. [Google Scholar] [CrossRef]
- Ohbuchi, A.; Kono, M.; Kitagawa, K.; Takenokuchi, M.; Imoto, S.; Saigo, K. Quantitative analysis of hemin-induced neutrophil extracellular trap formation and effects of hydrogen peroxide on this phenomenon. Biochem. Biophys. Rep. 2017, 11, 147–153. [Google Scholar] [CrossRef]
- Travascio, P.; Li, Y.S.D. DNA-enhanced peroxidase activity of a DNA-aptamer-hemin complex. Chem. Biol. 1998, 5, 505–517. [Google Scholar] [CrossRef]
- Song, M.; Zeng, L.; Hong, X.; Meng, Z.; Yin, J.; Wang, H.; Liang, Y.; Jiang, G. Polyvinyl pyrrolidone promotes DNA cleavage by a ROS-independent and depurination mechanism. Environ. Sci. Technol. 2013, 47, 2886–2891. [Google Scholar] [CrossRef]
- Brabec, V.; Pracharova, J.; Stepankova, J.; Sadler, P.J.; Kasparkova, J. Photo-induced DNA cleavage and cytotoxicity of a ruthenium (II) arene anticancer complex. J. Inorg. Biochem. 2016, 160, 149–155. [Google Scholar] [CrossRef]
- Salvo-Comino, C.; García-Hernández, C.; García-Cabezón, C.; Rodríguez-Méndez, M.L. Discrimination of milks with a multisensor system based on layer-by-layer films. Sensors 2018, 18, 2716. [Google Scholar] [CrossRef] [PubMed]
- Pallarola, D.; von Bildering, C.; Pietrasanta, L.; Queralto, N.; Knoll, W.; Battaglini, F.; Azzaroni, O. Recognition-driven layer-by-layer construction of multiprotein assemblies on surfaces: A biomolecular toolkit for building up chemoresponsive bioelectrochemical interfaces. Phys. Chem. Chem. Phys. 2012, 14, 11027–11039. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Ran, M.; Zhang, L.; Huang, H.; Li, X.; Chen, M.; Akashi, M. Fabrication of biobased polyelectrolyte capsules and their application for glucose-triggered insulin delivery. ACS Appl. Mater. Interfaces 2016, 8, 13688–13697. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Awaji, K.; Shimizu, S.; Iwasaki, M.; Oide, Y.; Ito, M.; Dairaku, T.; Ono, T.; Kashiwagi, Y.; Sato, K. Preparation of microparticles capable of glucose-induced insulin release under physiological conditions. Polymers 2018, 10, 1164. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Ritacco, H.; Rubio, J.E.F.; Rubio, R.G.; Ortega, F. Salt-induced changes in the growth of polyelectrolyte layers of poly(diallyldimethylammoniumchloride) and poly(4-styrene sulfonate of sodium). Soft Matter 2009, 5, 2130–2142. [Google Scholar] [CrossRef]
- Zhang, S.; Demoustier-Champagne, S.; Jonas, A.M. Quantitative collection and enzymatic activity of glucose oxidase nanotubes fabricated by templated layer-by-layer assembly. Biomacromolecules 2015, 16, 2382–2393. [Google Scholar] [CrossRef]
- Kakade, S.; Manickam, D.S.; Handa, H.; Mao, G.; Oupický, D. Transfection activity of layer-by-layer plasmid DNA/poly(ethylenimine) films deposited on PLGA microparticles. Int. J. Pharm. 2009, 365, 44–52. [Google Scholar] [CrossRef]
- Dunford, H.B. Free radicals in iron-containing systems. Free Radic. Biol. Med. 1987, 3, 405–421. [Google Scholar] [CrossRef]
- Travascio, P.; Witting, P.K.; Mauk, A.G.; Sen, D. The peroxidase activity of a hemin--DNA oligonucleotide complex: Free radical damage to specific guanine bases of the DNA. J. Am. Chem. Soc. 2001, 123, 1337–1348. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, M.; Ito, M.; Abe, E.; Anzai, J.-I. Glucose-induced decomposition of layer-by-layer films composed of phenylboronic acid-bearing poly(allylamine) and poly(vinyl alcohol) under physiological conditions. J. Mater. Chem. B 2015, 3, 7796–7802. [Google Scholar] [CrossRef]
- Seno, M.; Yoshida, K.; Sato, K.; Anzai, J.-I. pH- and sugar-sensitive multilayer films composed of phenylboronic acid (PBA)-modified poly(allylamine hydrochloride) (PBA-PAH) and poly(vinyl alcohol) (PVA): A significant effect of PBA content on the film stability. Mater. Sci. Eng. C 2016, 62, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Hashide, R.; Ishii, T.; Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer films composed of poly(allylamine) and insulin for pH-triggered release of insulin. Colloids Surf. B Biointerfaces 2012, 91, 274–279. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Kashimura, Y.; Kamijo, T.; Ono, T.; Dairaku, T.; Sato, T.; Kashiwagi, Y.; Sato, K. Decomposition of Glucose-Sensitive Layer-by-Layer Films Using Hemin, DNA, and Glucose Oxidase. Polymers 2020, 12, 319. https://doi.org/10.3390/polym12020319
Yoshida K, Kashimura Y, Kamijo T, Ono T, Dairaku T, Sato T, Kashiwagi Y, Sato K. Decomposition of Glucose-Sensitive Layer-by-Layer Films Using Hemin, DNA, and Glucose Oxidase. Polymers. 2020; 12(2):319. https://doi.org/10.3390/polym12020319
Chicago/Turabian StyleYoshida, Kentaro, Yu Kashimura, Toshio Kamijo, Tetsuya Ono, Takenori Dairaku, Takaya Sato, Yoshitomo Kashiwagi, and Katsuhiko Sato. 2020. "Decomposition of Glucose-Sensitive Layer-by-Layer Films Using Hemin, DNA, and Glucose Oxidase" Polymers 12, no. 2: 319. https://doi.org/10.3390/polym12020319
APA StyleYoshida, K., Kashimura, Y., Kamijo, T., Ono, T., Dairaku, T., Sato, T., Kashiwagi, Y., & Sato, K. (2020). Decomposition of Glucose-Sensitive Layer-by-Layer Films Using Hemin, DNA, and Glucose Oxidase. Polymers, 12(2), 319. https://doi.org/10.3390/polym12020319