Incentives of Using the Hydrodynamic Invariant and Sedimentation Parameter for the Study of Naturally- and Synthetically-Based Macromolecules in Solution
Abstract
:1. Introduction
2. Parametric Considerations
3. The Hydrodynamic Invariant and Sedimentation Parameter
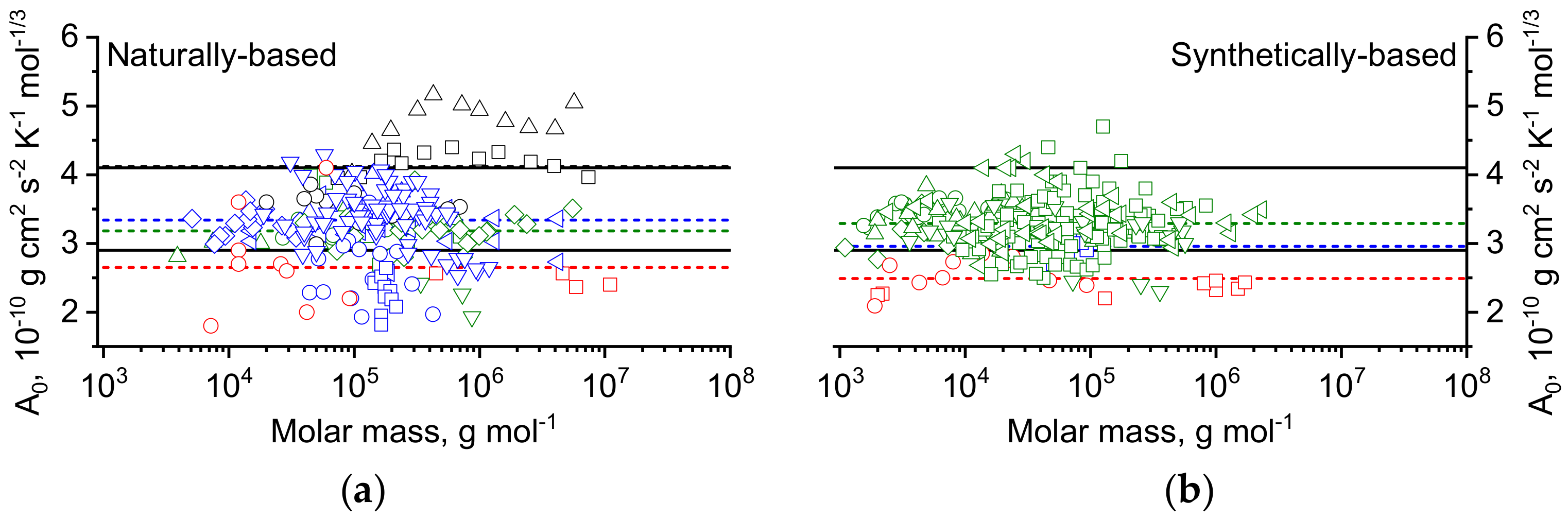
3.1. Recent Values for the Hydrodynamic Invariant,
3.2. Recent Values for the Sedimentation Parameter,
4. Global Discussion
- Dispersity/heterogeneity in macromolecule populations. This aspect is of critical importance since each of the techniques applied for macromolecular characterization may show some bias toward dispersity and existence of subpopulations, i.e., heterogeneity. A classic example of such is the utilization of light scattering on very disperse populations, or disperse small molar mass samples, that may tend to bias average molar mass estimations, particularly if no prefractionation/separation is applied [12,13,79,80]. Care, should therefore, be taken to identify potentially all components of a population by high performance separation and preferably concentration-sensitive detection [13].
- Correct choice of experimental conditions to investigate non-ideality phenomena. A classical and contemporary example is associated to the degree of dilution in hydrodynamic measurements, possible to be defined by the Debye Parameter as the product of intrinsic viscosity, , in units and mass concentration, , in units [12]. Associated effects observed in sedimentation velocity experiments are potentially occurring sedimentation boundary sharpening phenomena [81] and/or the Johnston–Ogston effect [82]. To identify such cases, the measurement of intrinsic viscosities, (Equation (4)) appears necessary, and anomalies in sedimentation profiles be identified by checking sedimentation boundary behavior at different degrees of dilution [12].
- Polyelectrolytes are inherently difficult to study. The sufficient compensation of macromolecular backbone charge effects, necessary to reduce difficulties in the measurements by sedimentation velocity experiments and the typically observed non-linearity of the Huggins- and Kraemer extrapolation procedures for estimations of the intrinsic viscosity, , are critical [54,55,68]. At present, the detailed hydrodynamic study and interrelation of hydrodynamic characteristics within the framework of the hydrodynamic invariant, (Equation (12)), or sedimentation parameter, (Equation (14)), are still awaiting validation and establishment. Under conditions of sufficient screening of charged moieties in macromolecules, the behavior of polyelectrolytes should follow that of their non-charged analogues. In the presence of varying electrostatic interactions, the conformational properties can vary [12].
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Staudinger, H.; Heuer, W. Über hochpolymere Verbindungen, 33. Mitteilung: Beziehungen zwischen Viskosität und Molekulargewicht bei Polystyrolen. Ber. Dtsch. Chem. Ges. 1930, 63, 222–234. [Google Scholar] [CrossRef]
- Staudinger, H.; Fritschi, J. Über Isopren und Kautschuk. 5. Mitteilung. Über die Hydrierung des Kautschuks und über seine Konstitution. Helv. Chim. Acta 1922, 5, 785–806. [Google Scholar] [CrossRef] [Green Version]
- Staudinger, H. Über Polymerisation. Ber. Dtsch. Chem. Ges. 1920, 53, 1073–1085. [Google Scholar] [CrossRef]
- Mülhaupt, R. Hermann Staudinger and the origin of macromolecular chemistry. Angew. Chem. Int. Ed. 2004, 43, 1054–1063. [Google Scholar] [CrossRef]
- Svedberg, T. Zentrifugierung, Diffusion und Sedimentationsgleichgewicht von Kolloiden und hochmolekularen Stoffen. Kolloid Z. 1925, 36, 53–64. [Google Scholar] [CrossRef]
- Svedberg, T.; Rinde, H. The ultra-centrifuge, a new instrument for the determination of size and distribution of size of particle in amicroscopic colloids. J. Am. Chem. Soc. 1924, 46, 2677–2693. [Google Scholar] [CrossRef]
- Svedberg, T.; Fåhraeus, R. A new method for the determination of the molecular weight of the proteins. J. Am. Chem. Soc. 1926, 48, 430–438. [Google Scholar] [CrossRef]
- Lamm, O. Die Differentialgleichung der Ultrazentrifugierung; Almqvist & Wiksell: Stockholm, Sweden, 1929. [Google Scholar]
- Lamm, O.; Polson, A. The determination of diffusion constants of proteins by a refractometric method. Biochem. J. 1936, 30, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, P.J. Light scattering and the absolute characterization of macromolecules. Anal. Chim. Acta 1993, 272, 1–40. [Google Scholar] [CrossRef]
- Grube, M.; Perevyazko, I.; Heinze, T.; Schubert, U.S.; Nischang, I. Revisiting very disperse macromolecule populations in hydrodynamic and light scattering studies of sodium carboxymethyl celluloses. Carbohydr. Polym. 2020, 229, 115452. [Google Scholar] [CrossRef]
- Grube, M.; Leiske, M.N.; Schubert, U.S.; Nischang, I. POx as an alternative to PEG? A hydrodynamic and light scattering study. Macromolecules 2018, 51, 1905–1916. [Google Scholar] [CrossRef]
- Nischang, I.; Perevyazko, I.; Majdanski, T.; Vitz, J.; Festag, G.; Schubert, U.S. Hydrodynamic analysis resolves the pharmaceutically-relevant absolute molar mass and solution properties of synthetic poly(ethylene glycol)s created by varying initiation sites. Anal. Chem. 2017, 89, 1185–1193. [Google Scholar] [CrossRef]
- Mandelkern, L.; Flory, P.J. The frictional coefficient for flexible chain molecules in dilute solution. J. Chem. Phys. 1952, 20, 212–214. [Google Scholar] [CrossRef]
- Tsvetkov, V.N.; Klenin, S.I. The diffusion of polystyrene fractions in dichloroethane. Dokl. Akad. Nauk. SSSR 1953, 88, 49–52. [Google Scholar]
- Tsvetkov, V.N.; Lavrenko, P.N.; Bushin, S.V. Hydrodynamic invariant of polymer molecules. J. Polym. Sci. Polym. Chem. Ed. 1984, 22, 3447–3486. [Google Scholar] [CrossRef]
- Pavlov, G.; Frenkel, S. Sedimentation parameter of linear polymers. Progr. Colloid Polym. Sci. 1995, 99, 101–108. [Google Scholar]
- Pavlov, G.M.; Frenkel, S.Y. The sedimentation parameter of linear polymer molecules in absence of excluded volume effects. Acta Polym. 1988, 39, 107–111. [Google Scholar] [CrossRef]
- Huggins, M.L. The viscosity of dilute solutions of long-chain molecules. Iv. Dependence on concentration. J. Am. Chem. Soc. 1942, 64, 2716–2718. [Google Scholar] [CrossRef]
- Kraemer, E.O. Molecular weights of celluloses and cellulose derivates. Ind. Eng. Chem. 1938, 30, 1200–1203. [Google Scholar] [CrossRef]
- Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Gralén, N. Sedimentation and Diffusion Measurements on Cellulose and Cellulose Derivatives; Almqvist Och. Wiksell: Uppsala, Sweden, 1944. [Google Scholar]
- Perevyazko, I.; Seiwert, J.; Schömer, M.; Frey, H.; Schubert, U.S.; Pavlov, G.M. Hyperbranched poly(ethylene glycol) copolymers: Absolute values of the molar mass, properties in dilute solution, and hydrodynamic homology. Macromolecules 2015, 48, 5887–5898. [Google Scholar] [CrossRef]
- Sato, T.; Norisuye, T.; Fujita, H. Double-stranded helix of xanthan: Dimensional and hydrodynamic properties in 0.1 m aqueous sodium chloride. Macromolecules 1984, 17, 2696–2700. [Google Scholar] [CrossRef]
- Yevlampieva, N.P.; Gorshkova, M.Y.; Volkova, I.F.; Grigoryan, E.S.; Lezov, A.A.; Khurchak, A.P.; Ryumtsev, E.I. Molecular properties of modified chitosan containing a quaternary amino group. Polym. Sci. Ser. A 2011, 53, 124–132. [Google Scholar] [CrossRef]
- Yevlampieva, N.P.; Gubarev, A.S.; Gorshkova, M.Y.; Okrugin, B.M.; Ryumtsev, E.I. Hydrodynamic behavior of quaternized chitosan at acidic and neutral ph. J. Polym. Res. 2015, 22, 166. [Google Scholar] [CrossRef]
- Yanaki, T.; Norisuye, T.; Fujita, H. Triple helix of schizophyllum commune polysaccharide in dilute solution. 3. Hydrodynamic properties in water. Macromolecules 1980, 13, 1462–1466. [Google Scholar] [CrossRef]
- Morris, G.A.; de al Torre, J.G.; Ortega, A.; Castile, J.; Smith, A.; Harding, S.E. Molecular flexibility of citrus pectins by combined sedimentation and viscosity analysis. Food Hydrocoll. 2008, 22, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.A.; Foster, T.J.; Harding, S.E. The effect of the degree of esterification on the hydrodynamic properties of citrus pectin. Food Hydrocoll. 2000, 14, 227–235. [Google Scholar] [CrossRef]
- Cölfen, H.; Berth, G.; Dautzenberg, H. Hydrodynamic studies on chitosans in aqueous solution. Carbohydr. Polym. 2001, 45, 373–383. [Google Scholar] [CrossRef]
- Fee, M.; Errington, N.; Jumel, K.; Illum, L.; Smith, A.; Harding, S.E. Correlation of sec/malls with ultracentrifuge and viscometric data for chitosans. Eur. Biophys. J. 2003, 32, 457–464. [Google Scholar] [CrossRef]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. Macromolecular conformation of chitosan in dilute solution: A new global hydrodynamic approach. Carbohydr. Polym. 2009, 76, 616–621. [Google Scholar] [CrossRef] [Green Version]
- Almutairi, F.M.; Erten, T.; Adams, G.G.; Hayes, M.; McLoughlin, P.; Kok, M.S.; Mackie, A.R.; Rowe, A.J.; Harding, S.E. Hydrodynamic characterisation of chitosan and its interaction with two polyanions: DNA and xanthan. Carbohydr. Polym. 2015, 122, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, G.M.; Korneeva, E.V.; Harding, S.E.; Vichoreva, G.A. Dilute solution properties of carboxymethylchitins in high ionic-strength solvent. Polymer 1998, 39, 6951–6961. [Google Scholar] [CrossRef]
- Filipp, B.; Lavrenko, P.N.; Okatova, O.V.; Filippova, T.V.; Mikryukova, O.I.; Petrov, V.R.; Dautzenberg, H.; Shtennikova, I.N.; Tsvetkov, V.N. Diffusion, sedimentation and flow birefringence in solutions of monosubstituted carboxymethylcellulose in cadoxene. Polym. Sci. USSR 1987, 29, 35–43. [Google Scholar] [CrossRef]
- Bartoshevich, S.F.; Nemchinov, I.A.; Molotkov, V.A.; Klenin, S.I. Aspects of the degradation of water-soluble cellulose derivatives in the course of oxidation by trivalent cobalt salts. Polym. Sci. USSR 1991, 33, 1526–1533. [Google Scholar] [CrossRef]
- Lavrenko, P.N.; Okatova, O.V.; Dauttsenberg, K.; Filipp, B. Diffusion and sedimentation of monosubstituted carboxymethyl cellulose in deca-diluted aqueous cadoxene. Polym. Sci. USSR 1991, 33, 937–944. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Michailova, N.; Tarabukina, E.; Korneeva, E.V. Velocity sedimentation of water-soluble methyl cellulose. Progr. Colloid Polym. Sci. 1995, 99, 109–113. [Google Scholar]
- Lavrenko, P.; Okatova, O.; Dautzenberg, H. Inhomogeneity and conformational parameters of low-substituted carboxymethyl cellulose from analytical ultracentrifugation data. Progr. Colloid Polym. Sci. 1999, 113, 192–200. [Google Scholar]
- Patel, T.R.; Morris, G.A.; de la Torre, J.G.; Ortega, A.; Mischnick, P.; Harding, S.E. Molecular flexibility of methylcelluloses of differing degree of substitution by combined sedimentation and viscosity analysis. Macromol. Biosci. 2008, 8, 1108–1115. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Finet, S.; Tatarenko, K.; Korneeva, E.; Ebel, C. Conformation of heparin studied with macromolecular hydrodynamic methods and x-ray scattering. Eur. Biophys. J. 2003, 32, 437–449. [Google Scholar] [CrossRef]
- Lavrenko, P.N.; Mikryukova, O.I.; Didenko, S.A. Hydrodynamic properties and the shape of the molecules of the polysaccharide ficoll-400 in solution. Polym. Sci. USSR 1986, 28, 576–584. [Google Scholar] [CrossRef]
- Patel, T.R.; Morris, G.A.; Ebringerova, A.; Vodenicarova, M.; Velebny, V.; Ortega, A.; de la Torre, J.G.; Harding, S.E. Global conformation analysis of irradiated xyloglucans. Carbohydr. Polym. 2008, 74, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, G.M.; Korneeva, E.V.; Michailova, N.A.; Ananyeva, E.P. Hydrodynamic properties of the fractions of mannan formed by rhodotorula rubra yeast. Carbohydr. Polym. 1992, 19, 243–248. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Yevlampieva, N.P. Hydrodynamic characteristics and equilibrium rigidity of pullulan molecules. Int. J. Biol. Macromol. 1994, 16, 318–323. [Google Scholar] [CrossRef]
- Almutairi, F.M.; Adams, G.G.; Kok, M.S.; Lawson, C.J.; Gahler, R.; Wood, S.; Foster, T.J.; Rowe, A.J.; Harding, S.E. An analytical ultracentrifugation based study on the conformation of lambda carrageenan in aqueous solution. Carbohydr. Polym. 2013, 97, 203–209. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Ivanova, N.P.; Korneeva, E.V.; Mikhailova, N.A.; Panarin, E.F. Molecular characteristics of poly(methacrylamidod-glucose)1. J. Carbohydr. Chem. 1996, 15, 419–433. [Google Scholar] [CrossRef]
- Yevlampieva, N.P.; Okrugin, B.M.; Levit, M.L.; Gubarev, A.S.; Nazarova, O.V.; Ryumstev, E.I.; Panarin, E.F. Conformational and hydrodynamic properties of the homopolymer of 2-deoxy-2-methacrylamido-d-glucose and its copolymers with acrylic acid and methacrylic acid. Polym. Sci. Ser. A 2014, 56, 414–421. [Google Scholar] [CrossRef]
- Morris, G.A.; Ang, S.; Hill, S.E.; Lewis, S.; Schäfer, B.; Nobbmann, U.; Harding, S.E. Molar mass and solution conformation of branched α(1→4), α(1→6) glucans. Part i: Glycogens in water. Carbohydr. Polym. 2008, 71, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Shpyrkov, A.A.; Tarasenko, I.I.; Pankova, G.A.; Il’ina, I.E.; Tarasova, E.V.; Tarabukina, E.B.; Vlasov, G.P.; Filippov, A.P. Molecular mass characteristics and hydrodynamic and conformational properties of hyperbranched poly-l-lysines. Polym. Sci. Ser. A 2009, 51, 250–258. [Google Scholar] [CrossRef]
- Yevlampieva, N.P.; Vezo, O.; Simonova, Y.; Timofeeva, L. Protonated member of poly(diallylammonium) family: Hydrodynamic and conformational properties. Int. J. Polym. Anal. Charact. 2018, 23, 403–414. [Google Scholar] [CrossRef]
- Perevyazko, I.; Lezov, A.A.; Gubarev, A.S.; Lebedeva, E.; Festag, G.; Guerrero-Sanchez, C.; Tsvetkov, N.V.; Schubert, U.S. Structure-property relationships via complementary hydrodynamic approaches: Poly(2-(dimethylamino)ethyl methacrylate)s. Polymer 2019, 182, 121828. [Google Scholar] [CrossRef]
- Perevyazko, I.; Trützschler, A.-K.; Gubarev, A.S.; Lebedeva, E.; Traeger, A.; Schubert, U.S.; Tsvetkov, N.V. Molecular and structural analysis via hydrodynamic methods: Cationic poly(2-aminoethyl-methacrylate)s. Polymer 2017, 131, 252–262. [Google Scholar] [CrossRef]
- Perevyazko, I.; Trützschler, A.-K.; Gubarev, A.S.; Lebedeva, E.; Traeger, A.; Tsvetkov, N.V.; Schubert, U.S. Absolute characteristics and conformation of cationic polymers by hydrodynamic approaches: Poly(aema-co-maema-co-dmaema) copolymers. Eur. Polym. J. 2017, 97, 347–355. [Google Scholar] [CrossRef]
- Nekrasova, T.N.; Andreeva, L.N.; Nazarova, O.V.; Bezrukova, M.A.; Zolotova, Y.I.; Imanbaev, R.T.; Skorbunova, O.V.; Pautov, V.D.; Panarin, E.F. Structural and dynamic characteristics of thermo- and ph-sensitive copolymers of 2-(diethylamino)ethyl methacrylate and 2-deoxy-2-methacrylamido-d-glucose. Polymer 2015, 77, 246–253. [Google Scholar] [CrossRef]
- Andreeva, L.N.; Bezrukova, M.A.; Bushin, S.V.; Nekrasova, T.N.; Imanbaev, R.T.; Pautov, V.D.; Zolotova, Y.I.; Nazarova, O.V.; Panarin, E.F. Conformational and dynamic characteristics of copolymers of n,n-dimethylaminoethyl methacrylate and 2-deoxy-2-methacrylamido-d-glucose. Polym. Sci. Ser. A 2014, 56, 405–413. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Panarin, E.F.; Korneeva, E.V.; Gavrilova, I.I.; Tarasova, N.N. Molecular properties and electrostatic interactions of linear poly(allylamine hydrochloride) chains. Progr. Colloid Polym. Sci. 2006, 131, 134–140. [Google Scholar]
- Okatova, O.V.; Gavrilova, I.I.; Ul’yanova, N.N.; Panarin, E.F.; Pavlov, G.M. Hydrodynamic, molecular, and conformational characteristics of macromolecules of a random copolymer of n-methyl-n-vinylacetamide and n-methyl-n-vinylamine hydrochloride. Russ. J. Appl. Chem. 2012, 85, 1239–1246. [Google Scholar] [CrossRef]
- Dommes, O.A.; Okatova, O.V.; Kostina, A.A.; Gavrilova, I.I.; Panarin, E.F.; Pavlov, G.M. Dimensions and conformations of macromolecules of n-methyl-n-vinylacetamide and n-methyl-n-vinylamine hydrochloride in solutions in a wide interval of ionic strength. Polym. Sci. Ser. C 2017, 59, 125–132. [Google Scholar] [CrossRef]
- Andreeva, L.N.; Bushin, S.V.; Bezrukova, M.A.; Nekrasova, T.N.; Imanbaev, R.T.; Pautov, V.D.; Nazarova, O.V.; Zolotova, Y.I.; Panarin, E.F. Conformation properties of poly(n,n-dimethylaminoethyl methacrylate) macromolecules in various solvents. Russ. J. Appl. Chem. 2012, 85, 417–425. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Dommes, O.A.; Gosteva, A.A.; Okatova, O.V.; Gavrilova, I.I.; Panarin, E.F. Sizes of macromolecules of copolymers of n-methyl-n-vinylacetamide and n-methyl-n-vinylamine hydrochloride with low charge linear density. Polym. Sci. Ser. A 2018, 60, 172–178. [Google Scholar] [CrossRef]
- Solovskii, M.V.; Smirnova, M.Y.; Amirova, A.I.; Tarabukina, E.B. Synthesis of low-molecular-weight n-(2-hydroxypropyl)methacrylamide sulfonated copolymers as carriers of biologically active substances. Russ. J. Appl. Chem. 2012, 85, 426–431. [Google Scholar] [CrossRef]
- Weber, C.; Babiuch, K.; Rogers, S.; Perevyazko, I.; Hoogenboom, R.; Schubert, U.S. Unexpected radical polymerization behavior of oligo(2-ethyl-2-oxazoline) macromonomers. Polym. Chem. 2012, 3, 2976. [Google Scholar] [CrossRef]
- Gubarev, A.S.; Monnery, B.D.; Lezov, A.A.; Sedlacek, O.; Tsvetkov, N.V.; Hoogenboom, R.; Filippov, S.K. Conformational properties of biocompatible poly(2-ethyl-2-oxazoline)s in phosphate buffered saline. Polym. Chem. 2018, 9, 2232–2237. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, G.M.; Korneeva, E.V.; Ivanova, N.P.; Pavlova, O.A.; Panarin, E.F. Synthesis and hydrodynamic and molecular characteristics of n-methacryloylglucosamine n-vinylformamide copolymers. Russ. J. Appl. Chem. 2007, 80, 777–782. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Okatova, O.V.; Mikhailova, A.V.; Ulyanova, N.N.; Gavrilova, I.I.; Panarin, E.F. Conformational parameters of poly(n-methyl-n-vinylacetamide) molecules through the hydrodynamic characteristics studies. Macromol. Biosci. 2010, 10, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Perevyazko, I.; Gubarev, A.S.; Tauhardt, L.; Dobrodumov, A.; Pavlov, G.M.; Schubert, U.S. Linear poly(ethylene imine)s: True molar masses, solution properties and conformation. Polym. Chem. 2017, 8, 7169–7179. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Panarin, E.F.; Korneeva, E.V.; Kurochkin, C.V.; Baikov, V.E.; Ushakova, V.N. Hydrodynamic properties of poly(1-vinyl-2-pyrrolidone) molecules in dilute solution. Makromol. Chem. 1990, 191, 2889–2899. [Google Scholar] [CrossRef]
- Urinov, E.; Abdullayev, F.T.; Mirzayev, U.M. Molecular and conformational parameters of the copolymer of vinylpyrrolidone with crotonic aldehyde and its complexes with 3d-transition metals in dilute solution. Polym. Sci. USSR 1989, 31, 660–666. [Google Scholar] [CrossRef]
- Gorshkov, N.I.; Miroslavov, A.E.; Alekseev, I.E.; Lumpov, A.A.; Murko, A.Y.; Gavrilova, I.I.; Saprykina, N.N.; Bezrukova, M.A.; Kipper, A.I.; Krasikov, V.D.; et al. Study of n-vinylpyrrolidone-n-vinylformamide copolymers labelled with indium-113m. J. Label. Compd. Radiopharm. 2017, 60, 302–311. [Google Scholar] [CrossRef]
- Tarabukina, E.B.; Zakharova, N.V.; Borisenko, M.S.; Solovskii, M.V. Molecular and associative properties of n-vinylpyrrolidone copolymers with n-crotonoylaminocaproic acid in dilute solutions. Polym. Sci. Ser. A 2017, 59, 295–300. [Google Scholar] [CrossRef]
- Pavlov, G.M.; Korneeva, E.V.; Jumel, K.; Harding, S.E.; Meijer, E.W.; Peerlings, H.W.I.; Stoddart, J.F.; Nepogodiev, S.A. Hydrodynamic properties of carbohydrate-coated dendrimers. Carbohydr. Polym. 1999, 38, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, G.M.; Korneeva, E.V.; Roy, R.; Michailova, N.A.; Ortega, P.C.; Perez, M.A. Sedimentation, translational diffusion, and viscosity of lactosylated polyamidoamine dendrimers. Progr. Colloid Polym. Sci. 1999, 113, 150–157. [Google Scholar]
- Pavlov, G.M.; Errington, N.; Harding, S.E.; Korneeva, E.V.; Roy, R. Dilute solution properties of lactosylated polyamidoamine dendrimers and their structural characteristics. Polymer 2001, 42, 3671–3678. [Google Scholar] [CrossRef]
- Wales, M.; Van Holde, K.E. The concentration dependence of the sedimentation constants of flexible macromolecules. J. Polym. Sci. 1954, 14, 81–86. [Google Scholar] [CrossRef]
- Tarabukina, E.B.; Pavlov, G.M.; Frenkel, S.Y. Investigation of Cellulose Derivatives. Method of Obtaining the Whole Molecular Characteristics by Velocity Ultracentrifugation. In Cellulose and Cellulose Derivatives; Elsevier: Amsterdam, The Netherlands, 1995; pp. 239–244. [Google Scholar]
- Gillis, R.B.; Adams, G.G.; Besong, D.T.; Machova, E.; Ebringerova, A.; Rowe, A.J.; Harding, S.E.; Patel, T.R. Application of novel analytical ultracentrifuge analysis to solutions of fungal mannans. Eur. Biophys. J. 2017, 46, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hokputsa, S.; Jumel, K.; Alexander, C.; Harding, S.E. A comparison of molecular mass determination of hyaluronic acid using sec/malls and sedimentation equilibrium. Eur. Biophys. J. 2003, 32, 450–456. [Google Scholar] [CrossRef]
- Sitaramaiah, G.; Goring, D.A.I. Hydrodynamic studies on sodium carboxymethyl cellulose in aqueous solutions. J. Polym. Sci. 1962, 58, 1107–1131. [Google Scholar] [CrossRef]
- Fujita, H. Effects of a concentration dependence of the sedimentation coefficient in velocity ultracentrifugation. J. Chem. Phys. 1956, 24, 1084–1090. [Google Scholar] [CrossRef]
- Johnston, J.P.; Ogston, A.G. A boundary anomaly found in the ultracentrifugal sedimentation of mixtures. Trans. Faraday Soc. 1946, 42, 789–799. [Google Scholar] [CrossRef]


| Macromolecular System | Naturally-Based | Synthetically-Based |
|---|---|---|
| Flexible | 0.37 | |
| Rigid | ||
| (Hyper-)branched |
| Macromolecular System | Naturally-Based | Synthetically-Based |
|---|---|---|
| Flexible | ||
| Rigid | ||
| (Hyper-)branched |
| Macromolecular System | ||||
|---|---|---|---|---|
| Flexible | ||||
| Rigid | 0 | |||
| (Hyper-)branched | - | - | ||
| Sphere | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grube, M.; Cinar, G.; Schubert, U.S.; Nischang, I. Incentives of Using the Hydrodynamic Invariant and Sedimentation Parameter for the Study of Naturally- and Synthetically-Based Macromolecules in Solution. Polymers 2020, 12, 277. https://doi.org/10.3390/polym12020277
Grube M, Cinar G, Schubert US, Nischang I. Incentives of Using the Hydrodynamic Invariant and Sedimentation Parameter for the Study of Naturally- and Synthetically-Based Macromolecules in Solution. Polymers. 2020; 12(2):277. https://doi.org/10.3390/polym12020277
Chicago/Turabian StyleGrube, Mandy, Gizem Cinar, Ulrich S. Schubert, and Ivo Nischang. 2020. "Incentives of Using the Hydrodynamic Invariant and Sedimentation Parameter for the Study of Naturally- and Synthetically-Based Macromolecules in Solution" Polymers 12, no. 2: 277. https://doi.org/10.3390/polym12020277
APA StyleGrube, M., Cinar, G., Schubert, U. S., & Nischang, I. (2020). Incentives of Using the Hydrodynamic Invariant and Sedimentation Parameter for the Study of Naturally- and Synthetically-Based Macromolecules in Solution. Polymers, 12(2), 277. https://doi.org/10.3390/polym12020277







