Highly Crosslinked Polybenzoxazines from Monobenzoxazines: The Effect of Meta-Substitution in the Phenol Ring
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Monomer and Polymer Characterization
2.2.1. General Procedure for the Preparation of Benzoxazine Monomers
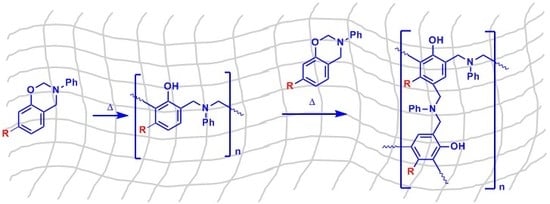
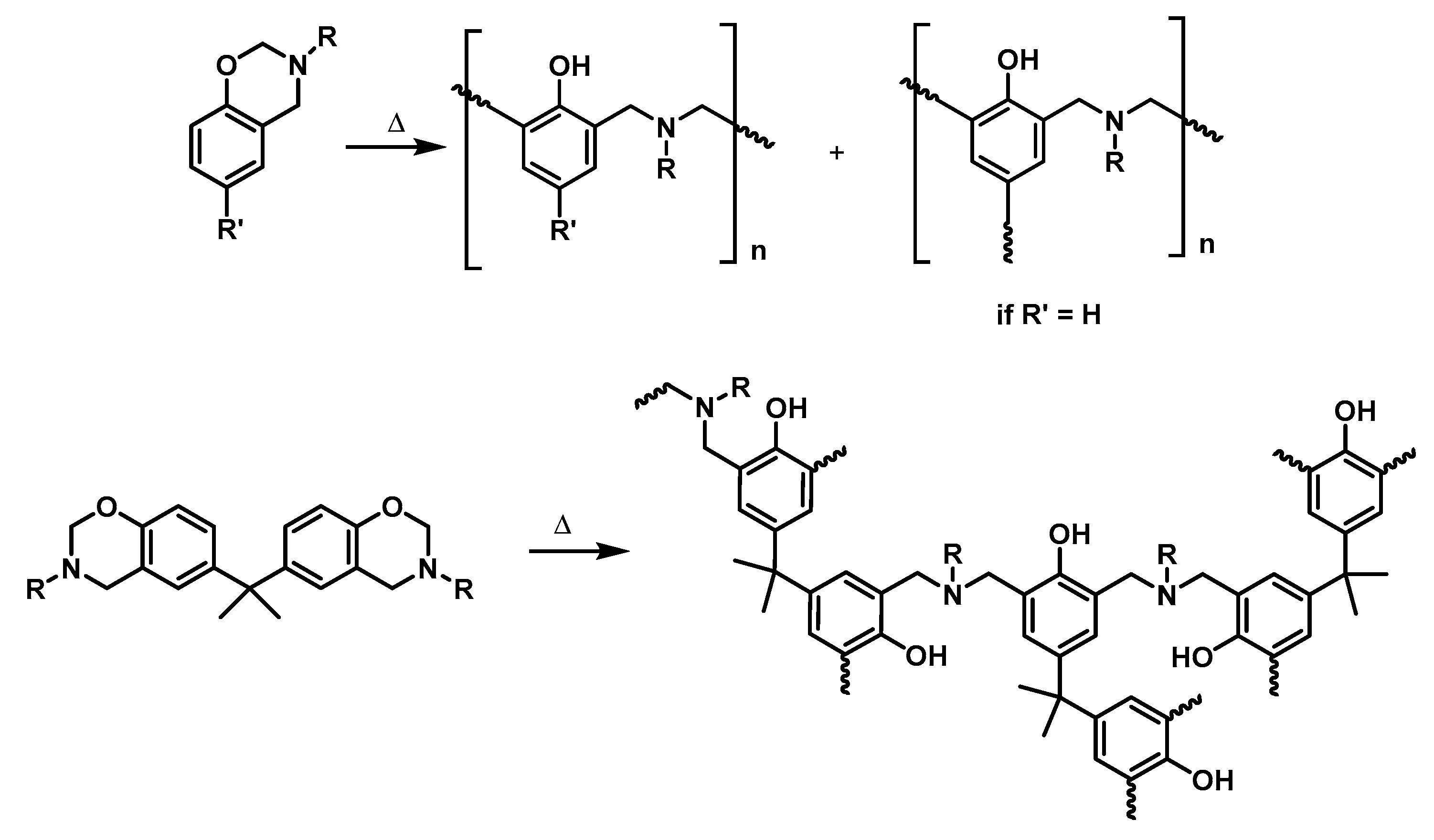
2.2.2. General Procedure for the Polymerization of Benzoxazines
3. Results
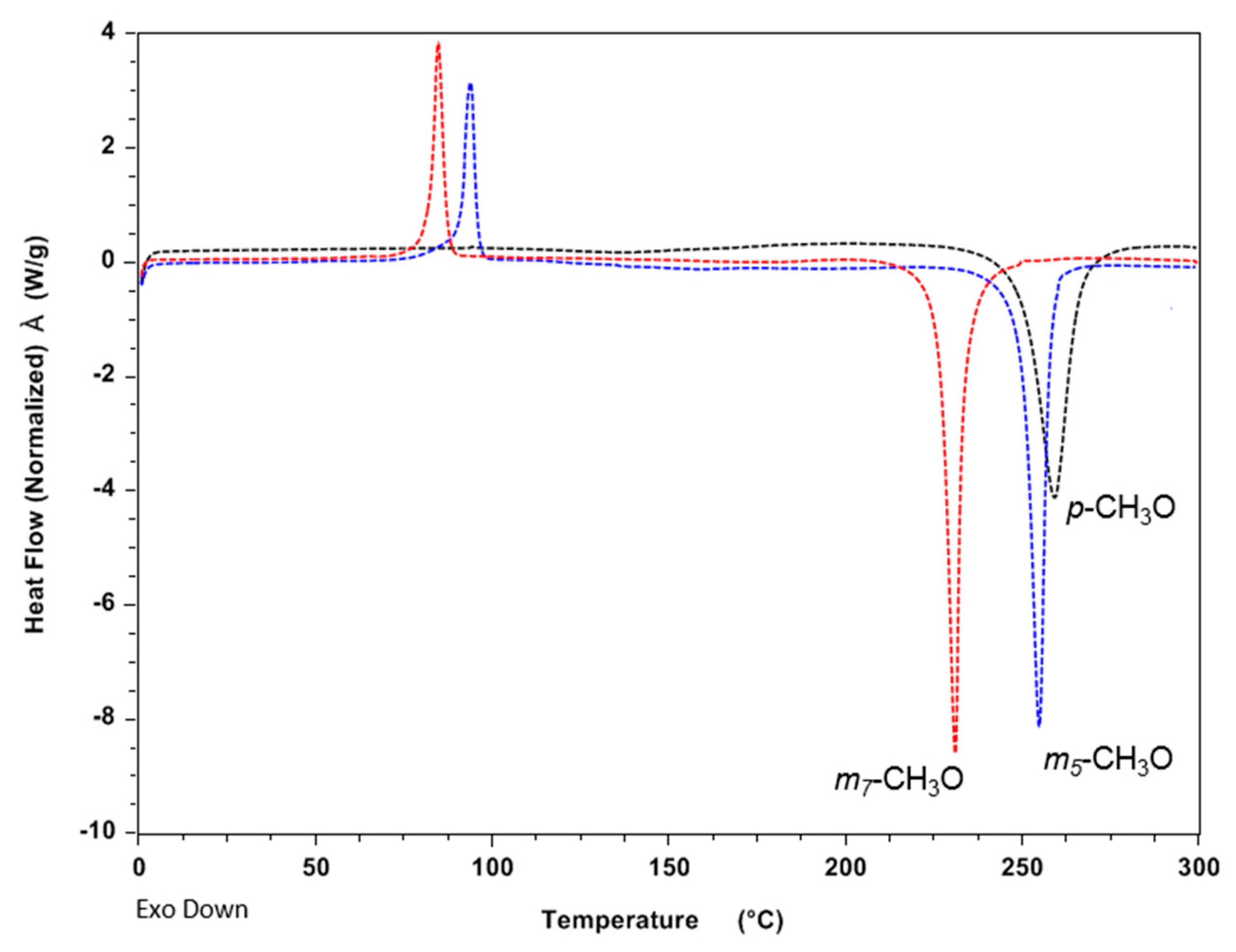
3.1. Differential Scanning Calorimetry (DSC) Analysis
3.2. Polymerization of Monobenzoxazines
3.3. Thermogravimetric Analysis (TGA)
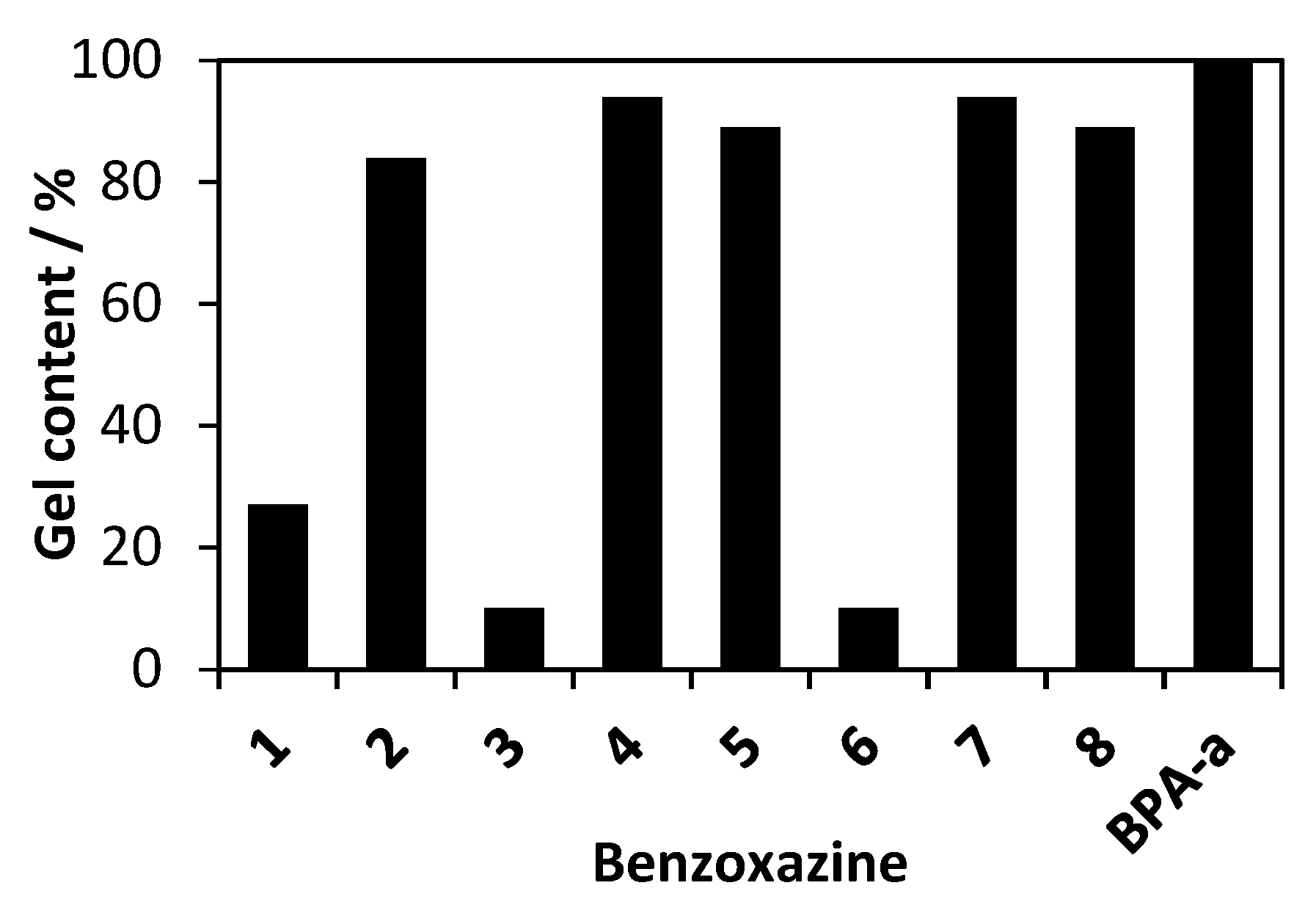
3.4. Study of Gel Content
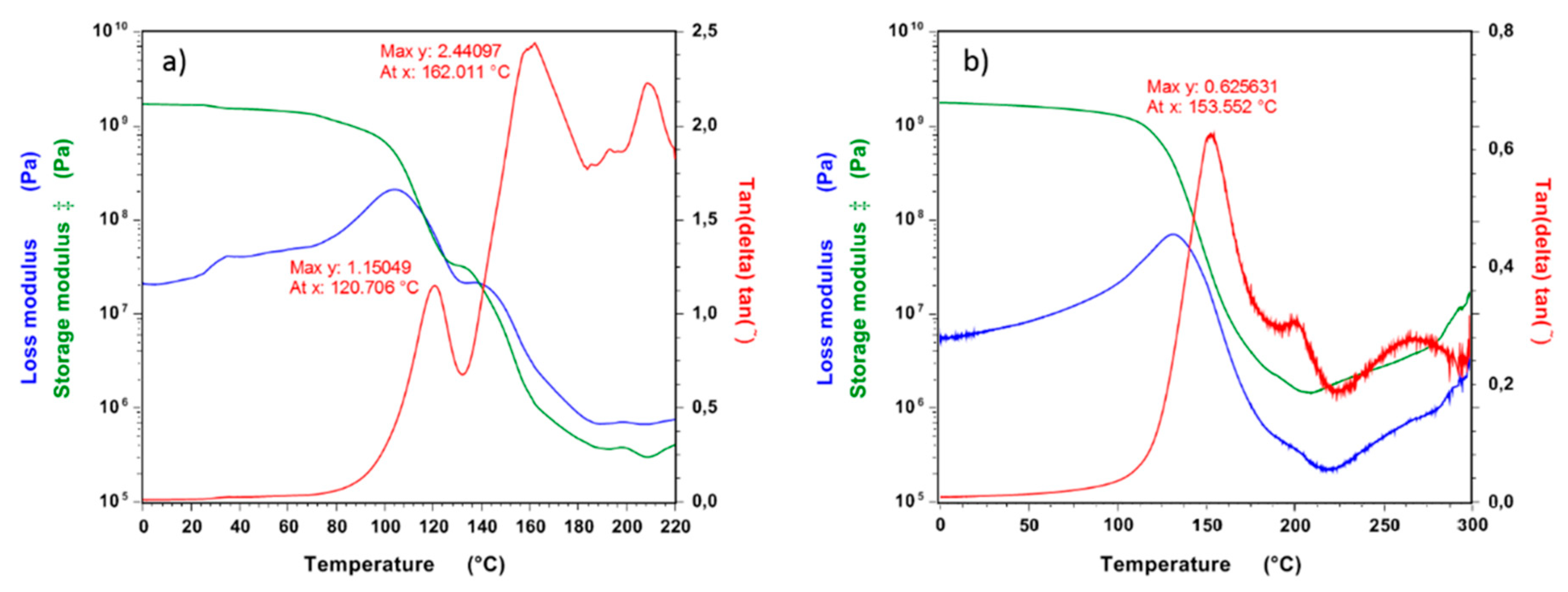
3.5. Dynamic Mechanical Analysis (DMA) and Crosslink Density of Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Asim, M.; Saba, N.; Jawaid, M.; Nasir, M.; Pervaiz, M.; Alothman, O.Y. A Review on Phenolic Resin and its Composites. Curr. Anal. Chem. 2018, 14, 185–197. [Google Scholar] [CrossRef]
- Ishida, H.; Agag, T. Handbook of Benzoxazine Resins; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Ishida, H.; Froimowicz, P. Advanced and Emerging Polybenzoxazine Science and Technology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Liu, C.; Shen, D.; Sebastián, R.M.; Marquet, J.; Schönfeld, R. Catalyst effects on the ring-opening polymerization of 1,3-benzoxazine and on the polymer structure. Polymer 2013, 54, 2873–2878. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, P.; Song, Y.; Zhu, R.Q.; Gu, Y. Effect of hydrogen bonds on the polymerization of benzoxazines: Influence and control. RSC Adv. 2016, 6, 45630–45635. [Google Scholar] [CrossRef]
- Zeng, K.; Huang, J.Y.; Ren, J.W.; Ran, Q.C. Curing reaction of benzoxazine under high pressure and effect on thermal resistance of polybenzoxazine. Macromol. Chem. Phys. 2019, 220, 1800340. [Google Scholar] [CrossRef]
- Rishwana, S.S.; Pitchaimari, G.; Vijayakumar, C.T. Studies on structurally different diamines and bisphenol benzoxazines: Synthesis and curing kinetics. High Perform. Polym. 2016, 28, 466–478. [Google Scholar] [CrossRef]
- Jin, L.; Agag, T.; Ishida, H. Bis(benzoxazine-maleimide)s as a novel class of high performance resin: Synthesis and properties. Eur. Polym. J. 2010, 46, 354–363. [Google Scholar] [CrossRef]
- Yan, C.; Fan, X.Y.; Li, J.; Shen, S.Z. Synthesis and characterization of bisphenol a diphthalimide bisbenzoxazine monomers and the properties of their polybenzoxazines. J. Appl. Polym. Sci. 2011, 121, 2778–2787. [Google Scholar] [CrossRef]
- Shan, F.; Ohashi, S.; Erlichman, A.; Ishida, H. Non-flammable thiazole-functional monobenzoxazines: Synthesis, polymerization, thermal and thermomechanical properties, and flammability studies. Polymer 2018, 157, 38–49. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Kou, K.C.; Li, Z.Y.; Wu, G.L.; Zhang, Y.; Feng, A.L. Synthesis, characterization, and thermal properties of benzoxazine monomers containing allyl groups. High Perform. Polym. 2016, 28, 1235–1245. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, C.H.; Dai, S.A. Strategy to prepare 4-hydroxylphenyl propargyl ether-based benzoxazine from bisphenol A. RSC Adv. 2015, 5, 74874–74880. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, S.W. Ortho-Imide and Allyl Groups Effect on Highly Thermally Stable Polybenzoxazine/Double-Decker-Shaped Polyhedral Silsesquioxane Hybrids. Macromolecules 2018, 51, 9602–9612. [Google Scholar] [CrossRef]
- Gunasekaran, S.G.; Arivalagan, V.; Stephen, L.D.; Dharmendirakumar, M. Triaryl Pendant Pyridine Core Hydroxyl Terminal Benzoxazine Based Polybenzoxazine-Silica (PBZ-SiO2) Hybrid Nanocomposites. J. Nanosci. Nanotechnol. 2017, 17, 5271–5283. [Google Scholar] [CrossRef]
- Choi, S.W.; Park, J.O.; Pak, C.; Choi, K.H.; Lee, J.C.; Chang, H. Design and Synthesis of Cross-Linked Copolymer Membranes Based on Poly(benzoxazine) and Polybenzimidazole and Their Application to an Electrolyte Membrane for a High-Temperature PEM Fuel Cell. Polymers 2013, 5, 77–111. [Google Scholar] [CrossRef]
- Ipek, H.; Hacaloglu, J. The effect of aromatic diboronic acid on characteristics of polybenzoxazine based on phenol and 4-aminomethylbenzoate. J. Polym. Res. 2018, 25, 263. [Google Scholar] [CrossRef]
- Russell, V.M.; Koenig, J.L.; Low, H.Y.; Ishida, H. Study of the characterization and curing of benzoxazines using 13C solid-state nuclear magnetic resonance. J. Appl. Polym. Sci. 1998, 70, 1413–1425. [Google Scholar] [CrossRef]
- Shukla, S.; Lochab, B. Role of higher aromatic content in modulating properties of cardanol based benzoxazines. Polymer 2016, 99, 684–694. [Google Scholar] [CrossRef]
- Soto, M.; Hiller, M.; Oschkinat, H.; Koschek, K. Multifunctional Benzoxazines Feature Low Polymerization Temperature and Diverse Polymer Structures. Polymers 2016, 8, 278. [Google Scholar] [CrossRef]
- Martos, A.; Sebastián, R.M.; Marquet, J. Studies on the ring-opening polymerization of benzoxazines: Understanding the effect of the substituents. Eur. Polym. J. 2018, 108, 20–27. [Google Scholar] [CrossRef]
- Brunovska, Z.; Liu, J.P.; Ishida, H. 1,3,5-Triphenylhexahydro-1,3,5-triazine-active intermediate and precursor in the novel synthesis of benzoxazine monomers and oligomers. Macromol. Chem. Phys. 1999, 200, 1745–1752. [Google Scholar] [CrossRef]
- Moloney, G.P.; Craik, D.J.; Iskander, M.N. Qualitative Analysis of the Stability of the Oxazine Ring of Various Benzoxazine and Pyridooxazine Derivatives with Proton Nuclear Magnetic Resonance Spectroscopy. J. Pharm. Sci. 1992, 81, 692–697. [Google Scholar] [CrossRef]
- Takeichi, T.; Nakamura, K.; Agag, T.; Muto, H. Synthesis of cresol-based benzoxazine monomers containing allyl groups and the properties of the polymers therefrom. Des. Monomers Polym. 2004, 7, 727–740. [Google Scholar] [CrossRef]
- Andreu, R.; Reina, J.A.; Ronda, J.C. Studies on the thermal polymerization of substituted benzoxazine monomers: Electronic effects. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3353–3366. [Google Scholar] [CrossRef]
- Huntsman Selector Guide, Araldite®, Benzoxazine Thermoset Resins. Available online: https://www.maxepoxi.com.br/pdf/sortimento_aradlite_benzoxazines.pdf (accessed on 21 January 2020).
- Kobzar, Y.L.; Tkachenko, I.M.; Bliznyuk, V.N.; Shevchenko, V.V. Fluorinated polybenzoxazines as advanced phenolic resins for leading-edge applications. React. Funct. Polym. 2018, 133, 71–92. [Google Scholar] [CrossRef]
- Parveen, A.S.; Thirukumaran, P.; Sarojadevi, M. Low dielectric materials from fluorinated polybenzoxazines. Polym. Adv. Technol. 2014, 25, 1538–1545. [Google Scholar] [CrossRef]
- Dayo, A.Q.; Wang, A.R.; Derradji, M.; Kiran, S.; Zegaoui, A.; Wang, J.; Liu, W.B. Copolymerization of mono and difunctional benzoxazine monomers with bio-based phthalonitrile monomer: Curing behaviour, thermal, and mechanical properties. React. Funct. Polym. 2018, 131, 156–163. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Hoftyzer, P.J. Properties of Polymers, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Li, J.J.; Sun, J.; Xie, Y.X.; Zhao, C.; Ma, H.X.; Liu, C.M. A novel star-shaped, cardanol-based bio-prepolymer: Synthesis, UV curing characteristics and properties of cured films. Polym. Degrad. Stab. 2018, 158, 124–135. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Theory of Rubber Elasticity. Polym. J. 1985, 17, 1–12. [Google Scholar] [CrossRef]
- Hill, L.W. Calculation of crosslink density in short chain networks. Prog. Org. Coat. 1997, 31, 235–243. [Google Scholar] [CrossRef]
- Thirukumaran, P.; Parveen, A.S.; Sarojadevi, M. Synthesis and Copolymerization of Fully Biobased Benzoxazines from Renewable Resources. ACS Sustain. Chem. Eng. 2014, 2, 2790–2801. [Google Scholar] [CrossRef]
- Defize, T.; Thomassin, J.M.; Alexandre, M.; Gilbert, B.; Riva, R.; Jérôme, C. Comprehensive study of the thermo-reversibility of Diels–Alder based PCL polymer networks. Polymer 2016, 84, 234–242. [Google Scholar] [CrossRef]


| Benzoxazine | DSC Thermogram | |||
|---|---|---|---|---|
| Tm(onset) (°C) a | Tp(onset) (°C) b | Tp(peak) (°C) c | ∆H (kJ·mol−1) d | |
| p-CH3(Bz), 1 | 47.5 | 257 | 265 | −72.8 |
| m-CH3 (Bz), 2 e | oil | 250 | 257 | −76.4 |
| p-OCH3(Bz), 3 | oil | 250 | 259 | −77.6 |
| m5-OCH3(Bz), 4 | 91.2 | 250 | 255 | −68.5 |
| m7-OCH3(Bz), 5 | 82.5 | 227 | 231 | −69.5 |
| p-F(Bz), 6 | 48.2 | 271 | 276 | −72.7 |
| m5-F(Bz), 7 | oil | 226 | 231 | −90.0 |
| m7-F(Bz), 8 | oil | 231 | 237 | −99.6 |
| Polybenzoxazine | T5% a (°C) | T10% b (°C) | CY c (%) | LOI d |
|---|---|---|---|---|
| p-CH3 | 261 | 298 | 32.9 | 30.7 |
| m-CH3 | 328 | 362 | 32.4 | 30.5 |
| p-OCH3 | 317 | 354 | 37.4 | 32.5 |
| m5-OCH3 | 322 | 352 | 41.4 | 34.1 |
| m7-OCH3 | 306 | 334 | 38.3 | 32.8 |
| p-F | 340 | 372 | 41.5 | 34.1 |
| m5-F | 338 | 374 | 45.0 | 35.5 |
| m7-F | 353 | 383 | 45.9 | 35.9 |
| Polybenzoxazine | E’(25 °C) c /GPa | Tga /°C | Trb /°C | E’(Tr) c /MPa | ved /mol·m−3 |
|---|---|---|---|---|---|
| p-CH3(Bz) | 1.67 | 120 | 185 | 0.41 | 36 |
| m-CH3 (Bz) e | 3.11 | 155 | 210 | 13.1 | 1090 |
| p-OCH3(Bz) | 3.41 | 135 | 210 | 8.09 | 671 |
| m5-OCH3(Bz) | 2.79 | 158 | 210 | 14.1 | 1173 |
| m7-OCH3(Bz) | 2.76 | 153 | 210 | 11.4 | 943 |
| p-F(Bz) | 2.48 | 154 | 210 | 8.67 | 720 |
| m5-F(Bz) | 3.15 | 171 | 240 | 21.1 | 1652 |
| m7-F(Bz) | 2.87 | 172 | 220 | 20.7 | 1681 |
| BPA-a | 3.00 | 150 | 210 | 30.0 | 2490 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martos, A.; Soto, M.; Schäfer, H.; Koschek, K.; Marquet, J.; Sebastián, R.M. Highly Crosslinked Polybenzoxazines from Monobenzoxazines: The Effect of Meta-Substitution in the Phenol Ring. Polymers 2020, 12, 254. https://doi.org/10.3390/polym12020254
Martos A, Soto M, Schäfer H, Koschek K, Marquet J, Sebastián RM. Highly Crosslinked Polybenzoxazines from Monobenzoxazines: The Effect of Meta-Substitution in the Phenol Ring. Polymers. 2020; 12(2):254. https://doi.org/10.3390/polym12020254
Chicago/Turabian StyleMartos, Alba, Marc Soto, Hannes Schäfer, Katharina Koschek, Jordi Marquet, and Rosa M. Sebastián. 2020. "Highly Crosslinked Polybenzoxazines from Monobenzoxazines: The Effect of Meta-Substitution in the Phenol Ring" Polymers 12, no. 2: 254. https://doi.org/10.3390/polym12020254
APA StyleMartos, A., Soto, M., Schäfer, H., Koschek, K., Marquet, J., & Sebastián, R. M. (2020). Highly Crosslinked Polybenzoxazines from Monobenzoxazines: The Effect of Meta-Substitution in the Phenol Ring. Polymers, 12(2), 254. https://doi.org/10.3390/polym12020254