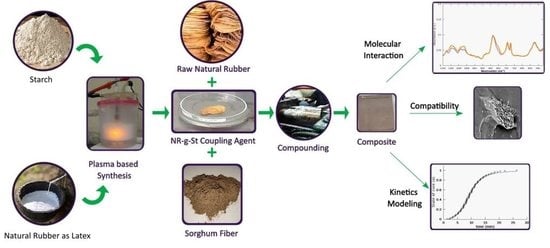
Experimental and Modelling Study of the Effect of Adding Starch-Modified Natural Rubber Hybrid to the Vulcanization of Sorghum Fibers-Filled Natural Rubber
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Methods
2.2.1. Graft Copolymerization
2.2.2. Composite Production
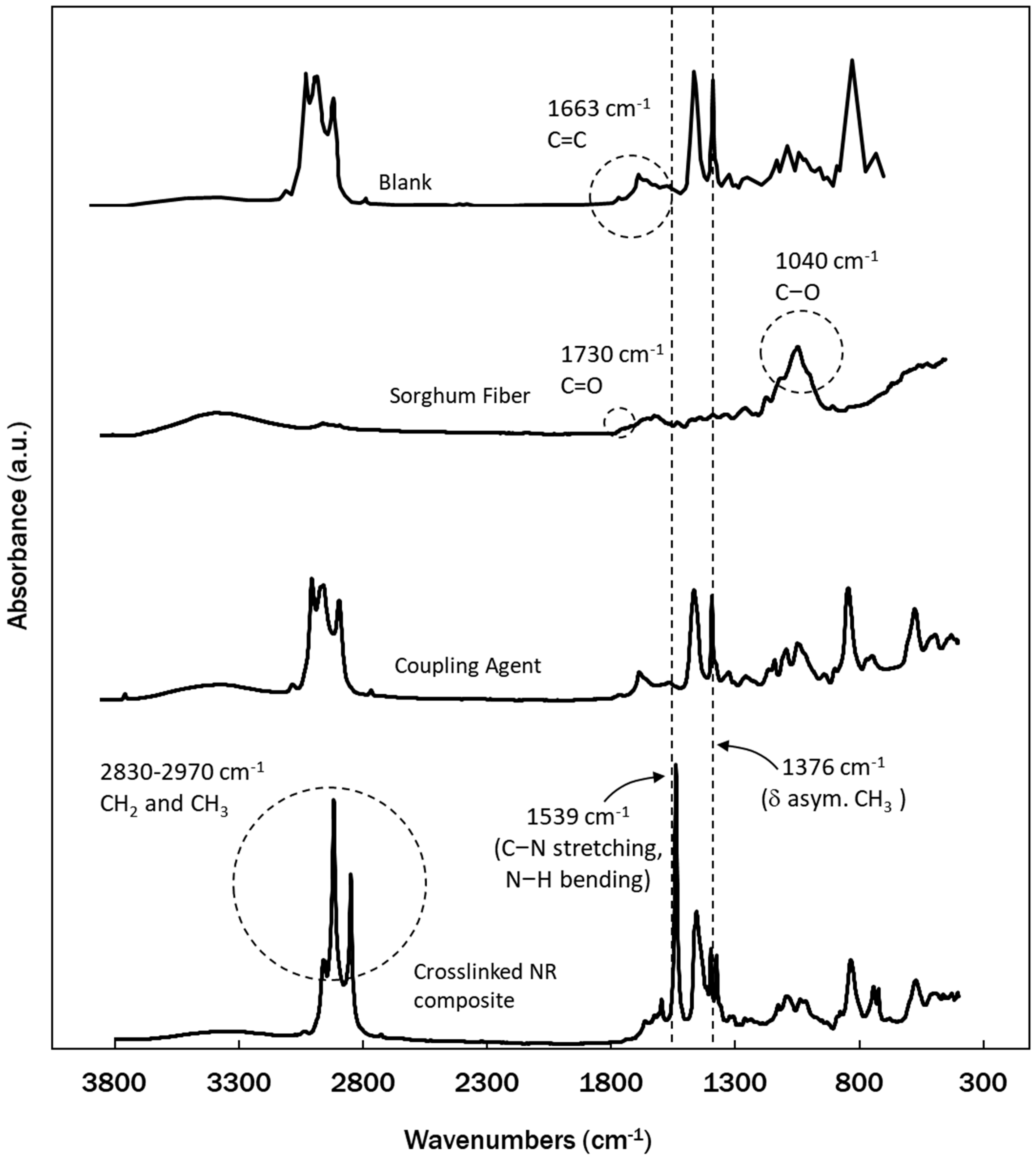
2.2.3. Fourier Transform Infrared (FTIR) Analysis
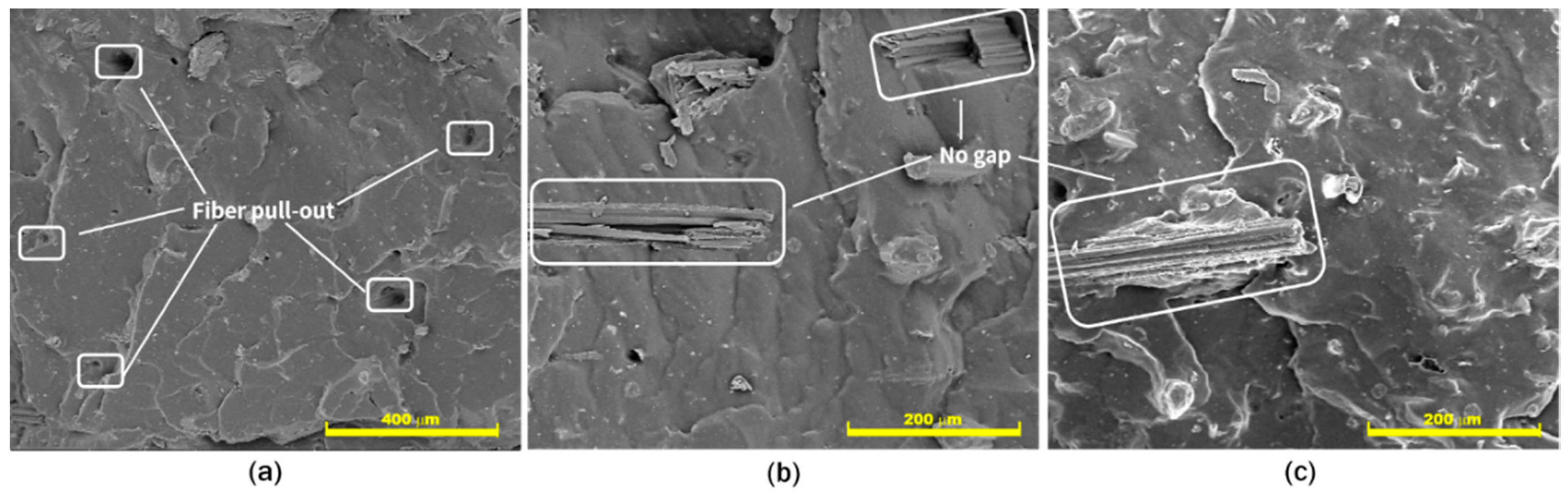
2.2.4. Field Emission—Scanning Electron Microscope (FE-SEM) Observation
2.2.5. Rheometer Test
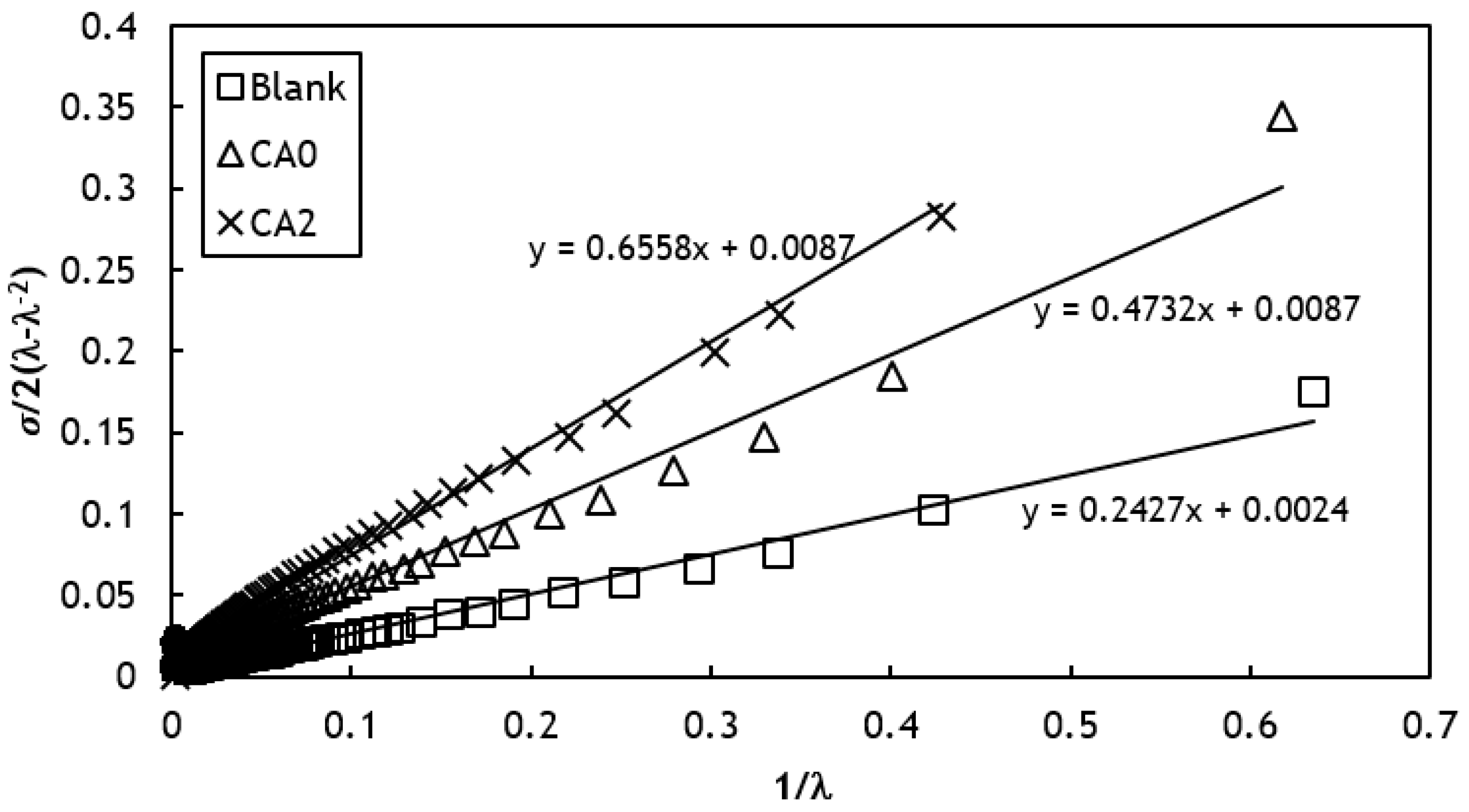
2.2.6. Crosslink Density
3. Results and Discussions
3.1. Molecular Interaction
3.2. Compatibility Study
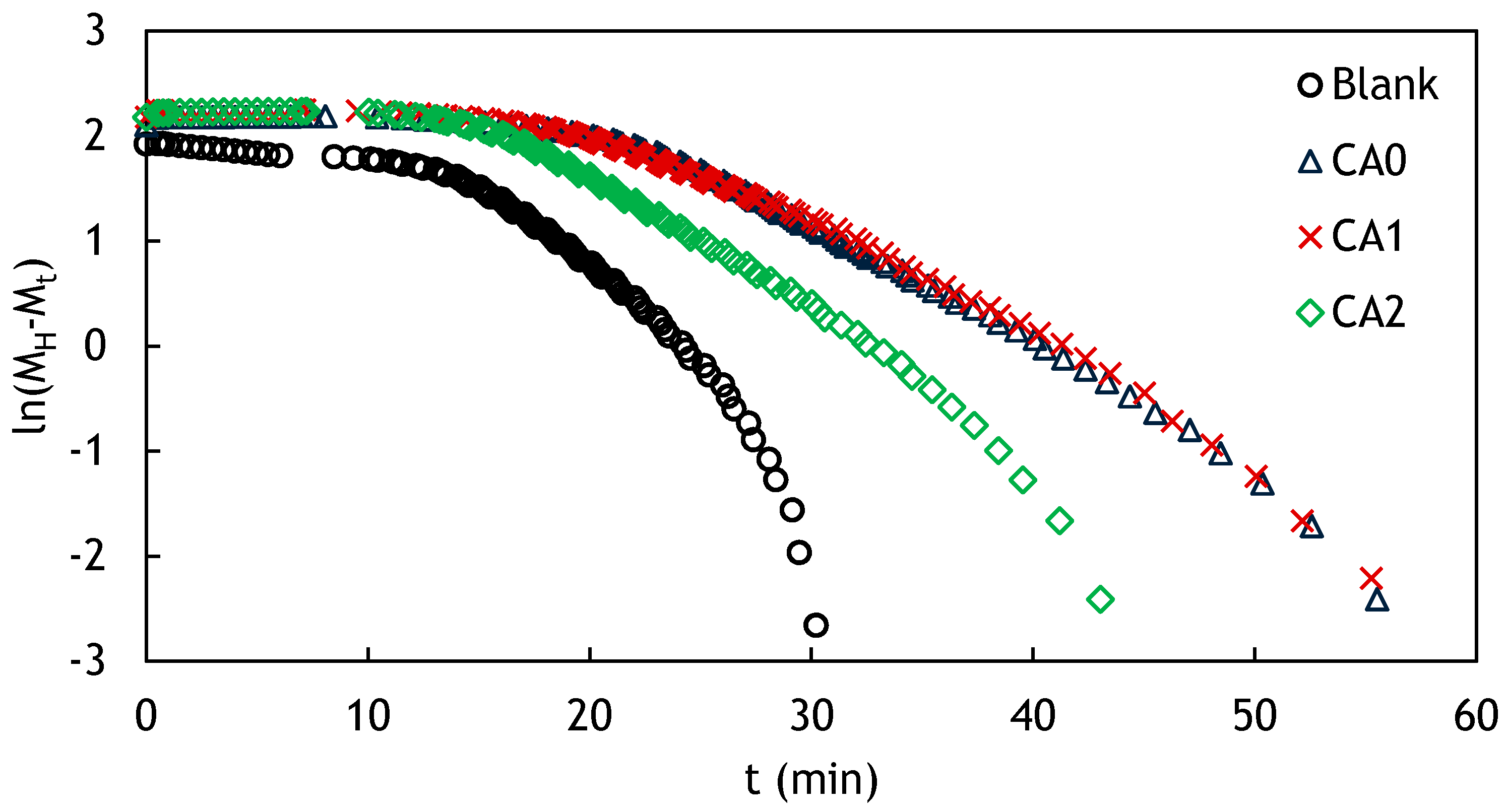
3.3. Rheological Behaviors
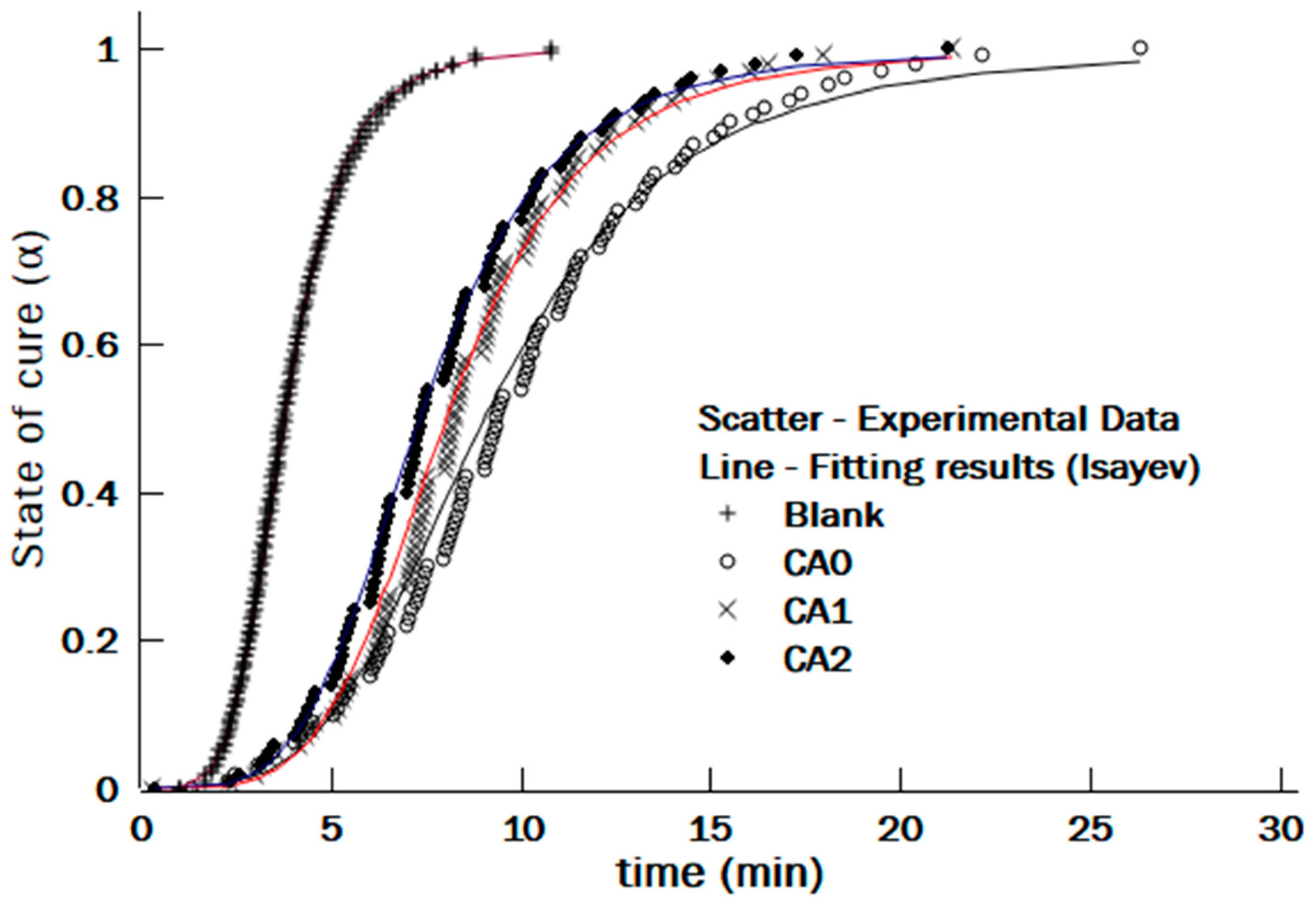
3.4. Modeling of Curing Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Feldman, D. Natural rubber nanocomposites. J. Macromol. Sci. Part A Pure Appl. Chem. 2017, 54, 629–634. [Google Scholar] [CrossRef]
- Bokobza, L. Natural Rubber Nanocomposites: A Review. Nanomaterials 2018, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Ismojo; Yuanita, E.; Rosa, E.M.; Calvin, L.; Chalid, M. Effect of time alkali treatment on chemical composition and tensile strength properties of kenaf single fibers. AIP Conf. Proc. 2019, 2175, 020059. [Google Scholar]
- Kustiyah, E.; Setiaji, D.A.; Nursan, I.A.; Syahidah, W.N.; Chalid, M. Comparison study on morphology and mechanical properties of starch, lignin, cellulose–based polyurethane foam. AIP Conf. Proc. 2019, 2175, 020061. [Google Scholar]
- Husnil, Y.A.; Handayani, A.S.; Setiaji, D.; Chalid, M. The effect of alkalization and bleaching treatment of Sorghum fibre on the crystallinity index of PP composite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012016. [Google Scholar] [CrossRef]
- Yuanita, E.; Pratama, A.Y.; Kurnia, H.; Kustiyah, E.; Chalid, M. Effect of alkalinization-bleaching and acid hydrolysis treatment stalk sweet sorghum waste on compatibilities in polypropylene matrix. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012080. [Google Scholar] [CrossRef]
- Roy, K.; Debnath, S.; Tzounis, L.; Pongwisuthiruchte, A.; Potiyaraj, P. Effect of Various Surface Treatments on the Performance of Jute Fibers Filled Natural Rubber (NR) Composites. Polymers 2020, 12, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Tu, Q.; Shen, X.; Yin, Q.; Pan, M.; Jiang, C.; Hu, C. Enhancing the Interfacial Adhesion with Rubber Matrix by Grafting Polydopamine-Carbon Nanotubes onto Poly(p-phenylene terephthalamide) Fibers. Polymers 2019, 11, 1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stelescu, M.; Manaila, E.; Craciun, G.; Chirila, C. Development and Characterization of Polymer Eco-Composites Based on Natural Rubber Reinforced with Natural Fibers. Materials 2017, 10, 787. [Google Scholar] [CrossRef] [PubMed]
- Moonart, U.; Utara, S. Effect of surface treatments and filler loading on the properties of hemp fiber/natural rubber composites. Cellulose 2019, 26, 7271–7295. [Google Scholar] [CrossRef]
- Ismojo, I.; Ammar, A.; Ramahdita, G.; Zulfia, A.; Chalid, M. Influence of Chemical Treatments Sequence on Morphology and Crystallinity of Sorghum Fibers. Indones. J. Chem. 2018, 18, 349–353. [Google Scholar] [CrossRef] [Green Version]
- Wongsorat, W.; Suppakarn, N.; Jarukumjorn, K. Effects of compatibilizer type and fiber loading on mechanical properties and cure characteristics of sisal fiber/natural rubber composites. J. Compos. Mater. 2013, 48, 2401–2411. [Google Scholar] [CrossRef]
- Sookprasert, P.; Hinchiranan, N. Morphology, mechanical and thermal properties of poly(lactic acid) (PLA)/natural rubber (NR) blends compatibilized by NR-graft-PLA. J. Mater. Res. 2017, 32, 788–800. [Google Scholar] [CrossRef]
- Nakason, C.; Kaesaman, A.; Supasanthitikul, P. The grafting of maleic anhydride onto natural rubber. Polym. Test. 2004, 23, 35–41. [Google Scholar] [CrossRef]
- Moolsin, S.; Saksayamkul, N.; Na Wichien, A. Natural rubber grafted poly(methyl methacrylate) as compatibilizer in 50/50 natural rubber/nitrile rubber blend. J. Elastom. Plast. 2016, 49, 422–439. [Google Scholar] [CrossRef]
- Prasassarakich, P.; Sintoorahat, P.; Wongwisetsirikul, N. Enhanced Graft Copolymerization of Styrene and Acrylonitrile onto Natural Rubber. J. Chem. Eng. Jpn. 2001, 34, 249–253. [Google Scholar] [CrossRef]
- Jaimuang, S.; Vatanatham, T.; Limtrakul, S.; Prapainainar, P. Kinetic studies of styrene-grafted natural rubber emulsion copolymerization using transmission electron microscope and thermal gravimetric analysis. Polymer 2015, 67, 249–257. [Google Scholar] [CrossRef]
- Arayapranee, W.; Rempel, G. Effects of Polarity on the Filler-Rubber Interaction and Properties of Silica Filled Grafted Natural Rubber Composites. J. Polym. 2013, 2013, 279529. [Google Scholar] [CrossRef] [Green Version]
- Chalid, M.; Putranto, B.; Alfiando, M.; Desfrandanta, J.; Agita, A. Study on grafting of starch on natural rubber latex via GDEP method. AIP Conf. Proc. 2018, 2024, 020066. [Google Scholar]
- Agita, A.; Diviva, B.; Alfiando, M.; Gufran, B.; Chalid, M. Electrolyte Effect to Copolymer Grafting of Starch onto Natural Rubber Using Glow Discharge Electrolysis Plasma (GDEP) Method. Mat. Sci. Forum 2019, 951, 77–82. [Google Scholar] [CrossRef]
- Handayani, A.; Purwaningsih, I.; Chalid, M.; Budianto, E.; Priadi, D. Synthesis of Amylopectin Macro-Initiator for Graft Copolymerization of Amylopectin-g-Poly(Methyl Methacrylate) by ATRP (Atom Transfer Radical Polymerization). Mat. Sci. Forum 2015, 827, 306–310. [Google Scholar] [CrossRef]
- Wang, P.; Tan, K.; Ho, C.; Khew, M.; Kang, E. Surface modification of natural rubber latex films by graft copolymerization. Eur. Polym. J. 2000, 36, 1323–1331. [Google Scholar] [CrossRef]
- Ren, J.; Yao, M.; Yang, W.; Li, Y.; Gao, J. Recent progress in the application of glow-discharge electrolysis plasma. Cent. Eur. J. Chem. 2014, 12, 1213–1221. [Google Scholar] [CrossRef]
- Khang, T.; Ariff, Z. Vulcanization kinetics study of natural rubber compounds having different formulation variables. J. Therm. Anal. Calorim. 2011, 109, 1545–1553. [Google Scholar] [CrossRef]
- Wu, J.; Xing, W.; Huang, G.; Li, H.; Tang, M.; Wu, S.; Liu, Y. Vulcanization kinetics of graphene/natural rubber nanocomposites. Polymer 2013, 54, 3314–3323. [Google Scholar] [CrossRef]
- Hariwongsanupab, N. Development of green natural rubber composites: Effect of nitrile rubber, fiber surface treatment and carbon black on properties of pineapple leaf fiber reinforced natural rubber composites. Ph.D. Thesis, Université de Haute Alsace, Mulhouse, France, 5 May 2017. [Google Scholar]
- Howse, S.; Porter, C.; Mengistu, T.; Pazur, R. Experimental determination of the quantity and distribution of chemical crosslinks in unaged and aged natural rubber, part 1: Peroxide vulcanization. Polym. Test. 2018, 70, 263–274. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.; Craciun, G.; Ighigeanu, D. Wood Sawdust/Natural Rubber Ecocomposites Cross-Linked by Electron Beam Irradiation. Materials 2016, 9, 503. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres, Fourier Transform—Materials Analysis, Salih Mohammed Salih; IntechOpen: Rijeka, Croatia, 2012; Available online: https://www.intechopen.com/books/fourier-transform-materials-analysis/fourier-transform-infrared-spectroscopy-for-natural-fibres (accessed on 10 March 2020). [CrossRef] [Green Version]
- Rolere, S.; Liengprayoon, S.; Vaysse, L.; Sainte-Beuve, J.; Bonfils, F. Investigating natural rubber composition with Fourier Transform Infrared (FT-IR) spectroscopy: A rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym. Test. 2015, 43, 83–93. [Google Scholar] [CrossRef]
- Nimpaiboon, A.; Sriring, M.; Kumarn, S.; Sakdapipanich, J. Reducing and stabilizing the viscosity of natural rubber by using sugars: Interference of the Maillard reaction between proteins and sugars. J. Appl. Polym. Sci. 2020, 137, 49389. [Google Scholar] [CrossRef]
- Montha, S.; Suwandittakul, P.; Poonsrisawat, A.; Oungeun, P.; Kongkaew, C. Maillard Reaction in Natural Rubber Latex: Characterization and Physical Properties of Solid Natural Rubber. Adv. Mater. Sci. Eng. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pongdong, W.; Nakason, C.; Kummerlöwe, C.; Vennemann, N. Influence of Filler from a Renewable Resource and Silane Coupling Agent on the Properties of Epoxidized Natural Rubber Vulcanizates. J. Chem. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Roy, K.; Potiyaraj, P. Exploring the comparative effect of silane coupling agents with different functional groups on the cure, mechanical and thermal properties of nano-alumina (Al2O3)-based natural rubber (NR) compounds. Polym. Bull. 2018, 76, 883–902. [Google Scholar] [CrossRef]
- Ismail, H.; Nordin, R.; Noor, A. The Effect of Filler Loading on Curing and Mechanical Properties of Natural Rubber/recycled Rubber Powder Blends. Int. J. Polym. Mater. Polym. Biomater. 2005, 54, 9–20. [Google Scholar] [CrossRef]
- Musto, S.; Barbera, V.; Cipolletti, V.; Citterio, A.; Galimberti, M. Master curves for the sulphur assisted crosslinking reaction of natural rubber in the presence of nano- and nano-structured sp2 carbon allotropes. Express Polym. Lett. 2017, 11, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasan, D.; Sujith, A.; Rajesh, C. Cure characteristics and mechanical properties of biocomposites of natural rubber reinforced with chicken feather fibre: Effect of fibre loading, alkali treatment, bonding and vulcanizing systems. Mater. Today Commun. 2019, 20, 100555. [Google Scholar] [CrossRef]
- Zachariah, A.; Chandra, A.; Mohammed, P.; Thomas, S. Vulcanization kinetics and mechanical properties of organically modified nanoclay incorporated natural and chlorobutyl rubber nanocomposites. Polym. Test. 2019, 76, 154–165. [Google Scholar] [CrossRef]
- Ghosh, P.; Katare, S.; Patkar, P.; Caruthers, J.; Venkatasubramanian, V.; Walker, K. Sulfur Vulcanization of Natural Rubber for Benzothiazole Accelerated Formulations: From Reaction Mechanisms to a Rational Kinetic Model. Rubber Chem. Technol. 2003, 76, 592–693. [Google Scholar] [CrossRef] [Green Version]
- Aprem, A.; Thomas, S.; Joseph, K.; Barkoula, N.; Kocsis, J. Sulphur Vulcanisation of Styrene Butadiene Rubber Using New Binary Accelerator Systems. J. Elastom. Plast. 2003, 35, 29–55. [Google Scholar] [CrossRef]
- Isayev, A.; Deng, J. Nonisothermal Vulcanization of Rubber Compounds. Rubber Chem. Technol. 1988, 61, 340–361. [Google Scholar] [CrossRef]
- Han, I.; Chung, C.; Lee, J. Optimal Curing of Rubber Compounds with Reversion Type Cure Behavior. Rubber Chem. Technol. 2000, 73, 101–113. [Google Scholar] [CrossRef]
- Leroy, E.; Souid, A.; Sarda, A.; Deterre, R. A knowledge based approach for elastomer cure kinetic parameters estimation. Polym. Test. 2013, 32, 9–14. [Google Scholar] [CrossRef]



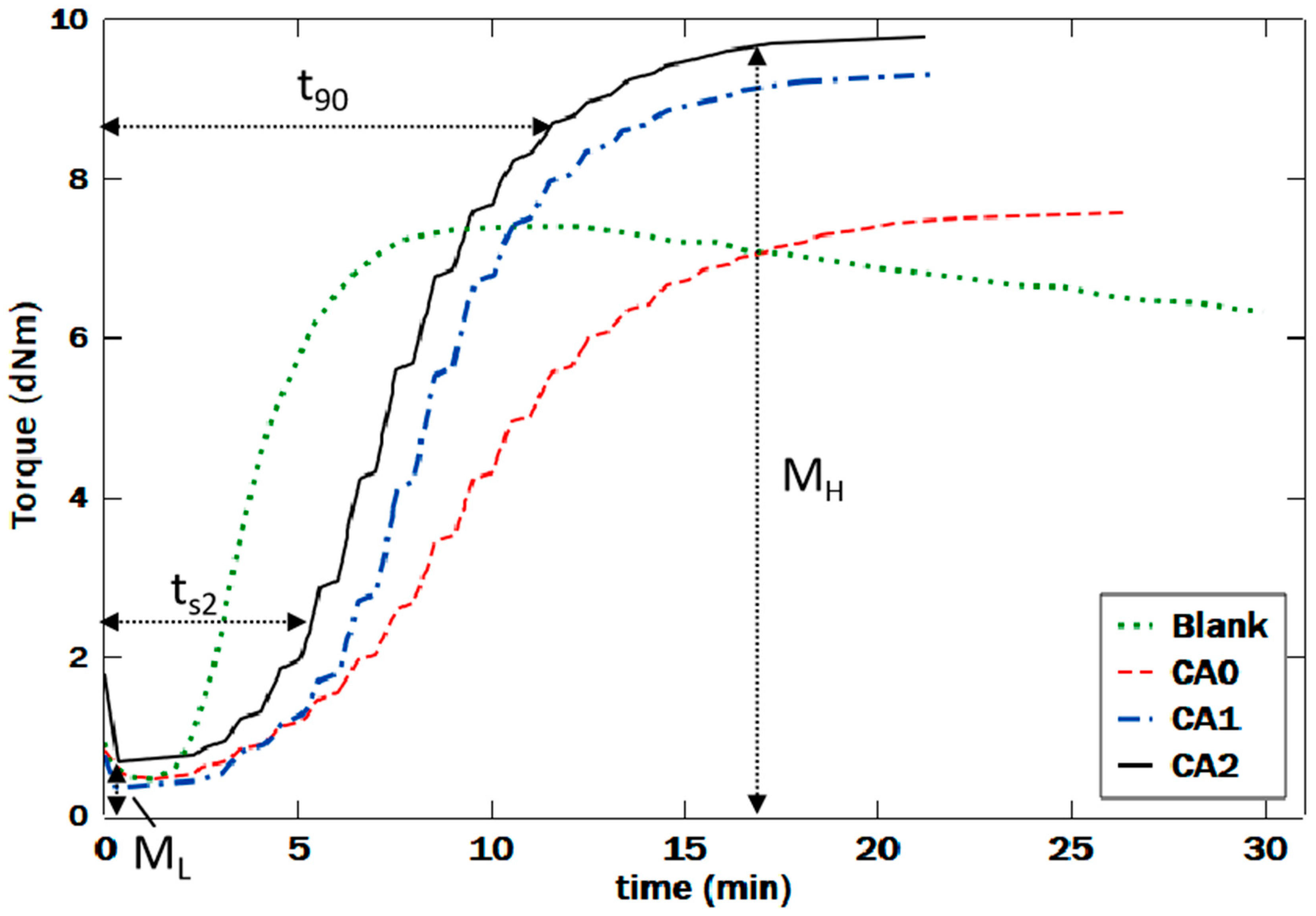



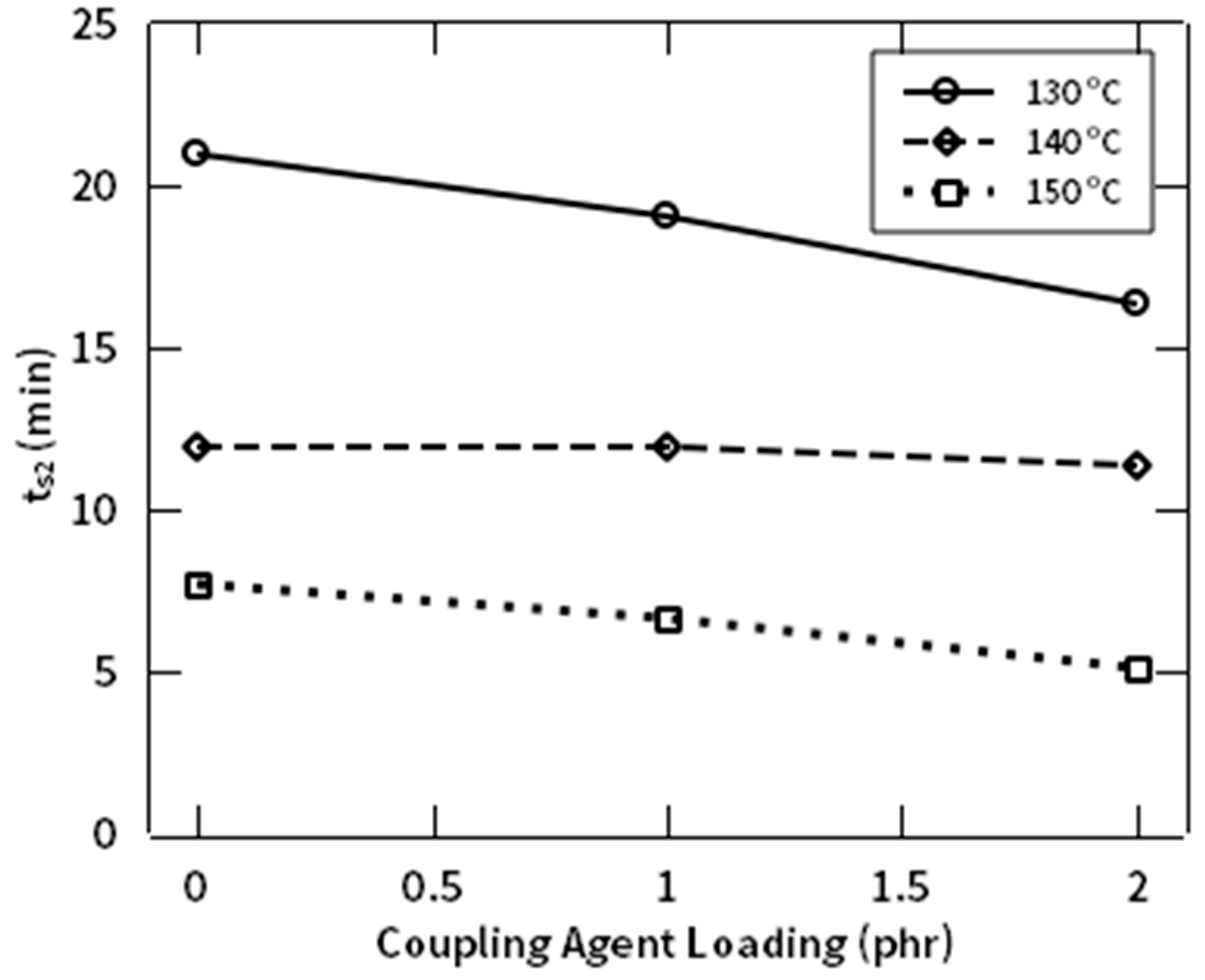
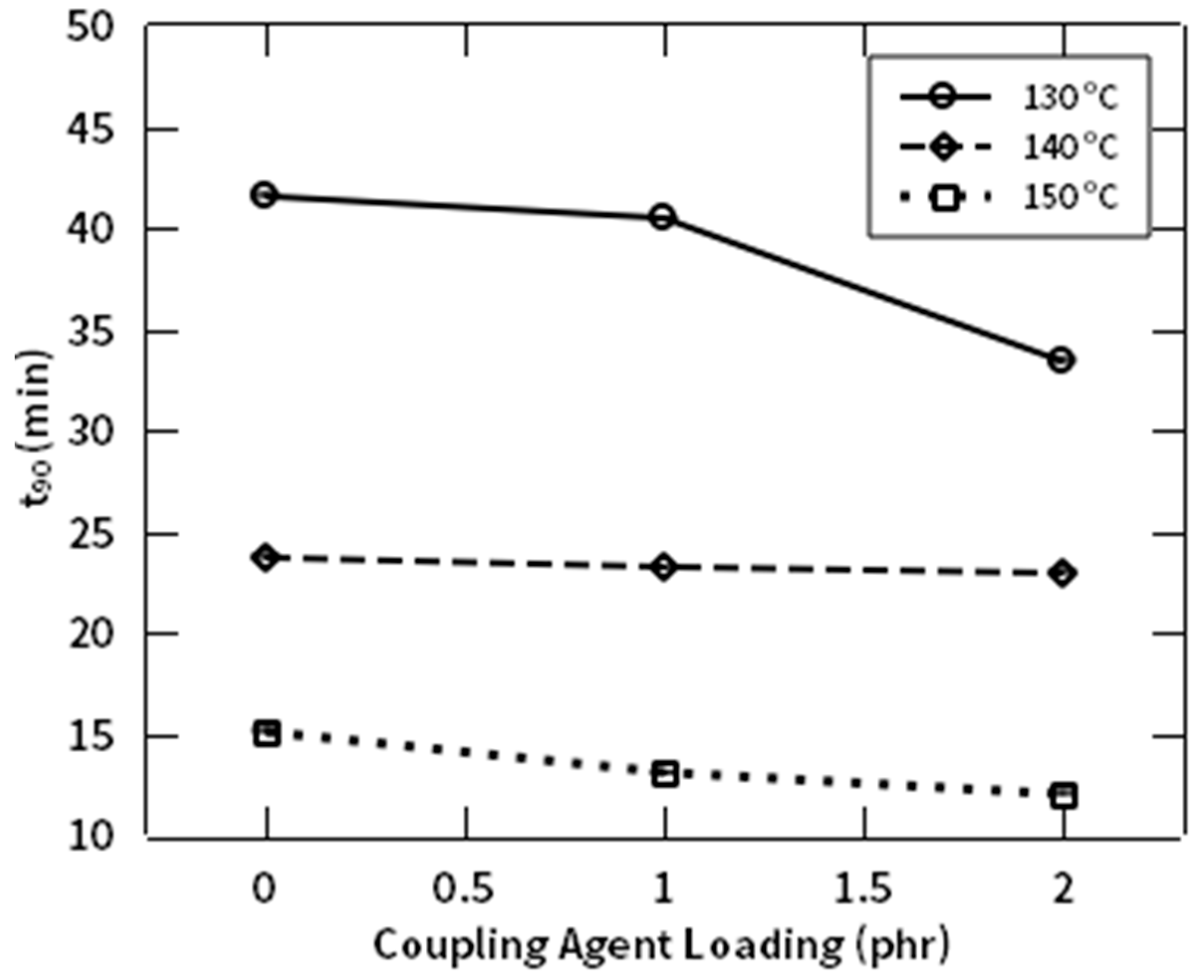
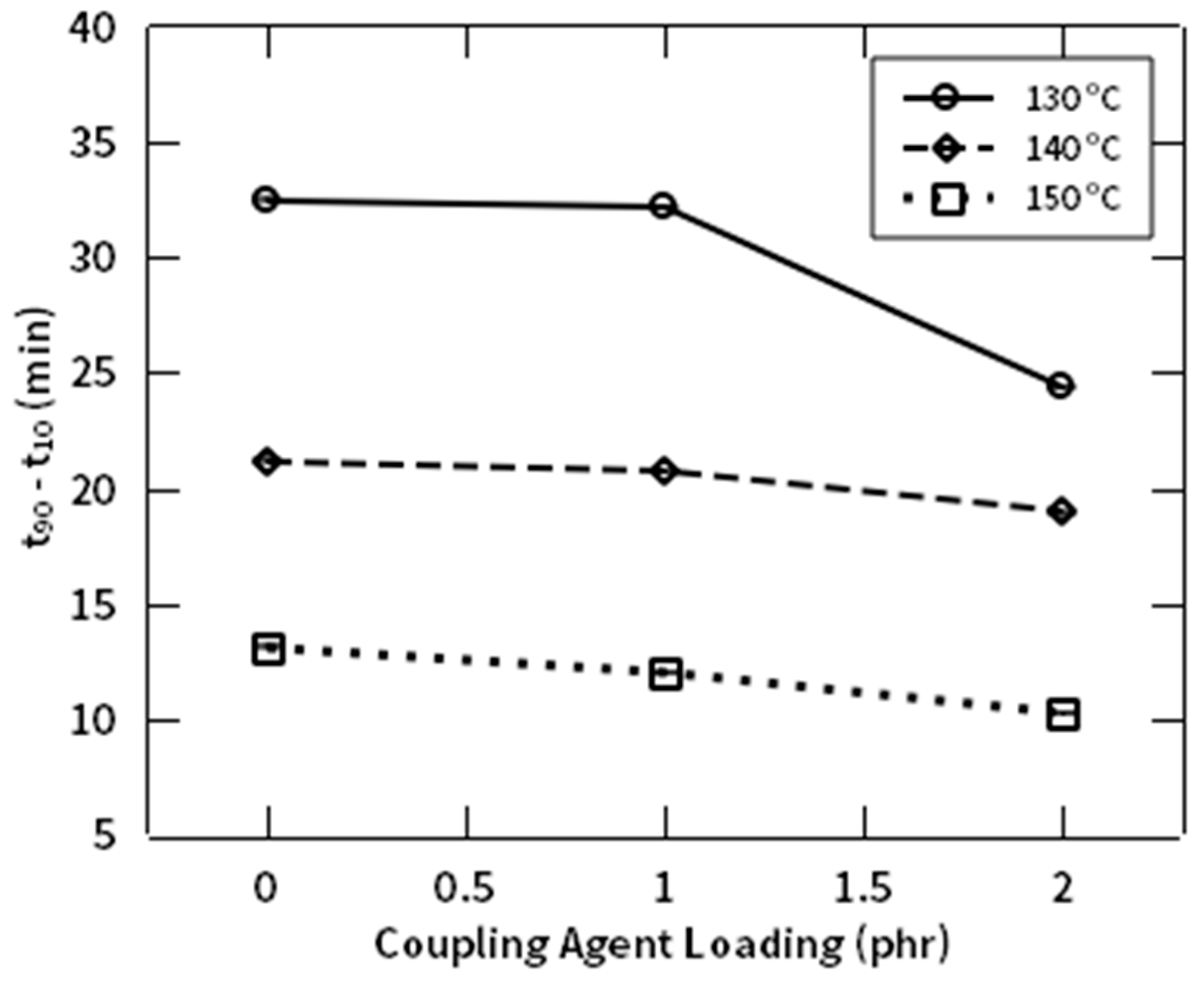
| Natural Rubbers (NR) | Natural Fiber (NF) | NR-g-St | ZnO | Stearic Acid | CBS | Sulfur | |
|---|---|---|---|---|---|---|---|
| Blank | 100 | - | - | 6 | 0.5 | 0.7 | 2.5 |
| CA0 | 100 | 20 | - | 6 | 0.5 | 0.7 | 2.5 |
| CA1 | 100 | 20 | 1 | 6 | 0.5 | 0.7 | 2.5 |
| CA2 | 100 | 20 | 2 | 6 | 0.5 | 0.7 | 2.5 |
| Sample | |||||
|---|---|---|---|---|---|
| Blank | 130 | 5.476 | 1.1 | 0.981 | 7.34 |
| 140 | 5.121 | 313.99 | 0.983 | ||
| 150 | 4.862 | 34,281.51 | 0.994 | ||
| CA0 | 130 | 5.143 | 0.33 | 0.994 | 10.09 |
| 140 | 4.709 | 125.13 | 0.991 | ||
| 150 | 3.748 | 559,255.37 | 0.973 | ||
| CA1 | 130 | 4.677 | 12.48 | 0.994 | 4.71 |
| 140 | 4.751 | 101.73 | 0.975 | ||
| 150 | 4.474 | 9821.82 | 0.977 | ||
| CA2 | 130 | 5.239 | 0.59 | 0.990 | 8.00 |
| 140 | 4.642 | 250.73 | 0.984 | ||
| 150 | 4.284 | 46,740.28 | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalid, M.; Husnil, Y.A.; Puspitasari, S.; Cifriadi, A. Experimental and Modelling Study of the Effect of Adding Starch-Modified Natural Rubber Hybrid to the Vulcanization of Sorghum Fibers-Filled Natural Rubber. Polymers 2020, 12, 3017. https://doi.org/10.3390/polym12123017
Chalid M, Husnil YA, Puspitasari S, Cifriadi A. Experimental and Modelling Study of the Effect of Adding Starch-Modified Natural Rubber Hybrid to the Vulcanization of Sorghum Fibers-Filled Natural Rubber. Polymers. 2020; 12(12):3017. https://doi.org/10.3390/polym12123017
Chicago/Turabian StyleChalid, Mochamad, Yuli Amalia Husnil, Santi Puspitasari, and Adi Cifriadi. 2020. "Experimental and Modelling Study of the Effect of Adding Starch-Modified Natural Rubber Hybrid to the Vulcanization of Sorghum Fibers-Filled Natural Rubber" Polymers 12, no. 12: 3017. https://doi.org/10.3390/polym12123017
APA StyleChalid, M., Husnil, Y. A., Puspitasari, S., & Cifriadi, A. (2020). Experimental and Modelling Study of the Effect of Adding Starch-Modified Natural Rubber Hybrid to the Vulcanization of Sorghum Fibers-Filled Natural Rubber. Polymers, 12(12), 3017. https://doi.org/10.3390/polym12123017