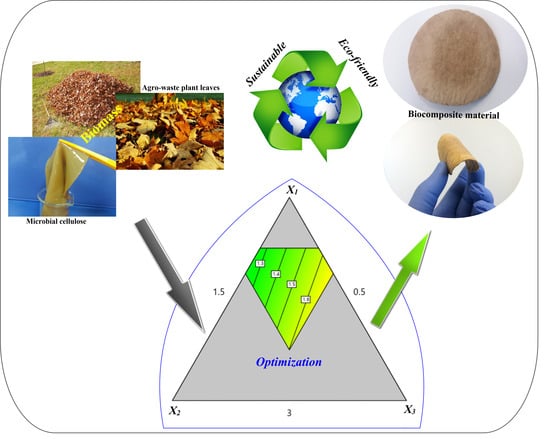
Preparation and Characterization of Nonwoven Fibrous Biocomposites for Footwear Components
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Bio-fillers
2.3. Preparation of Biocomposite Leather
2.4. Material Characterization Techniques
2.4.1. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.2. Mechanical Tests
2.4.3. Morphological Analysis
2.4.4. Surface Wettability Measurements
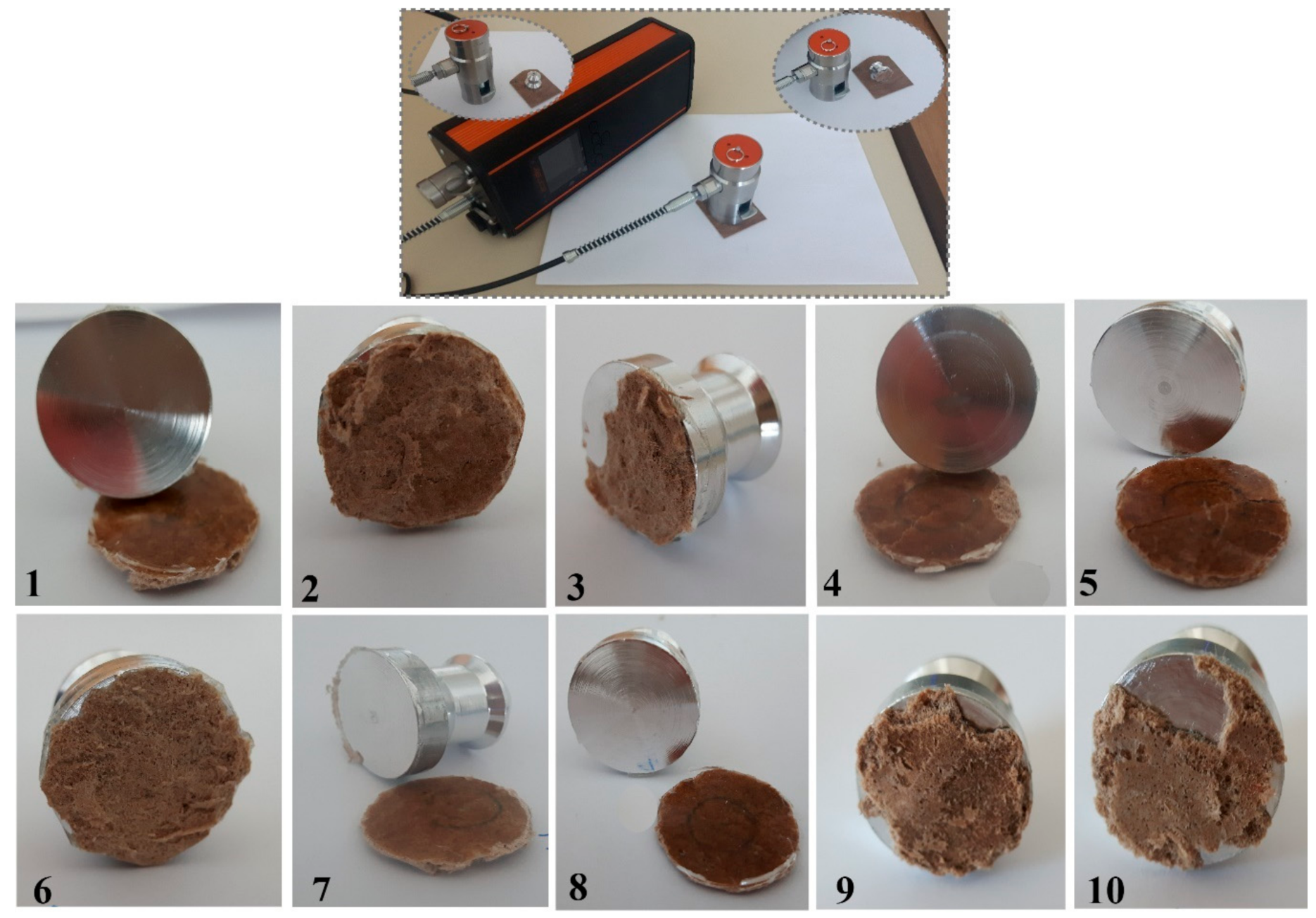
2.4.5. Adhesion Tests
2.4.6. Consumer Properties Measurements
2.5. Experimental Design
2.6. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Biocomposite Leather
3.2. FT-IR Analysis
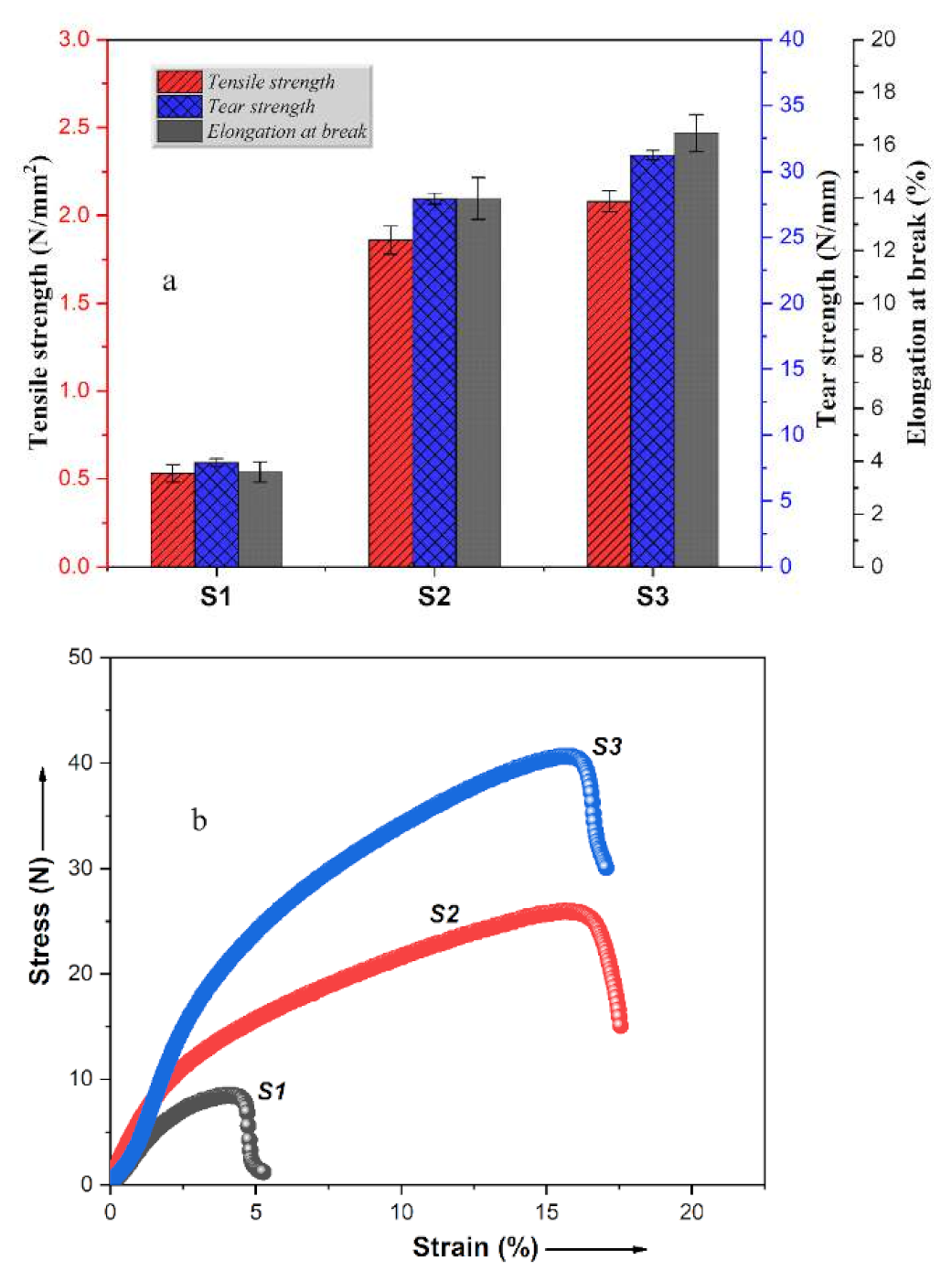
3.3. Mechanical Tests
3.3.1. Tensile Measurements
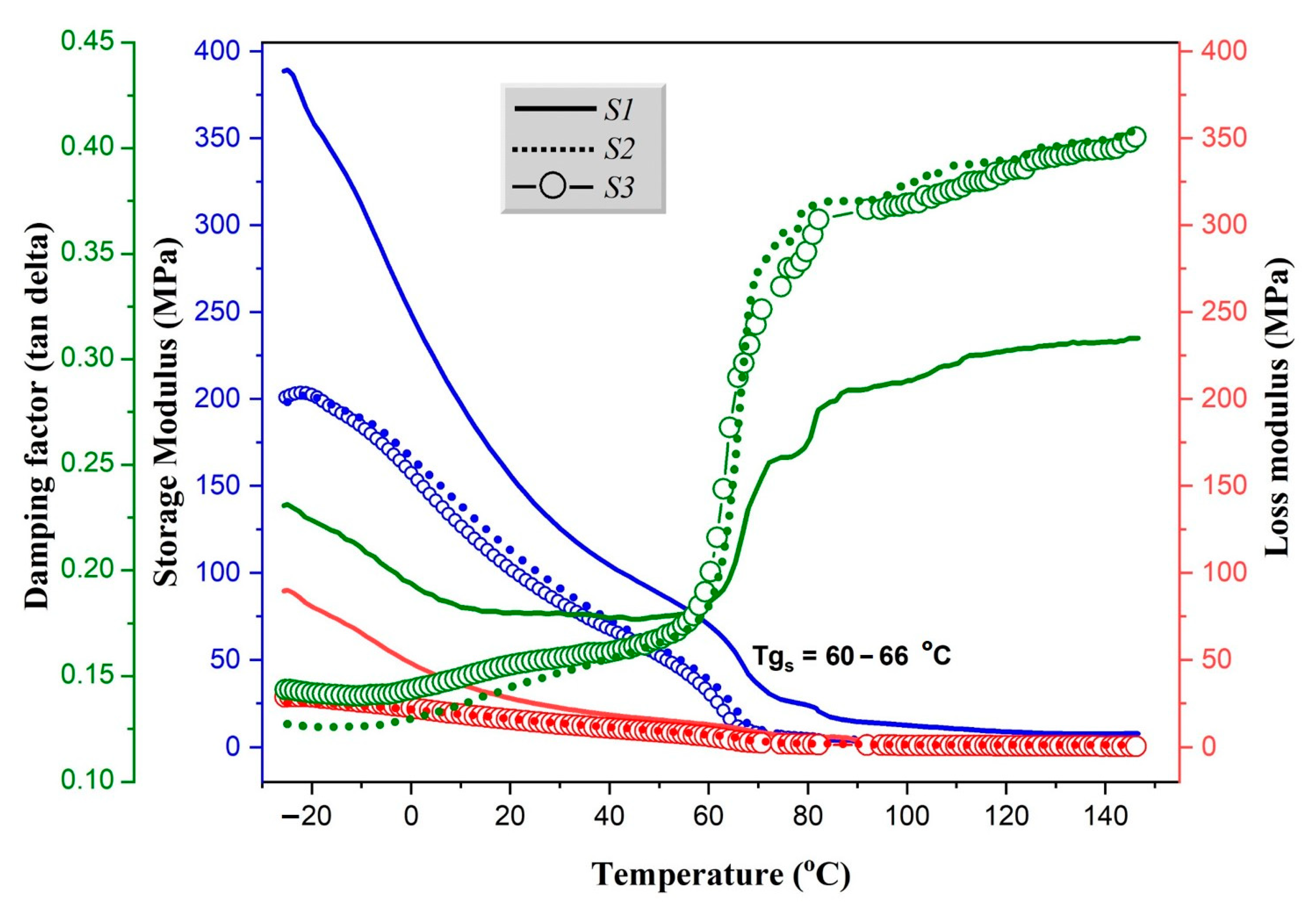
3.3.2. Dynamic Mechanical Analysis
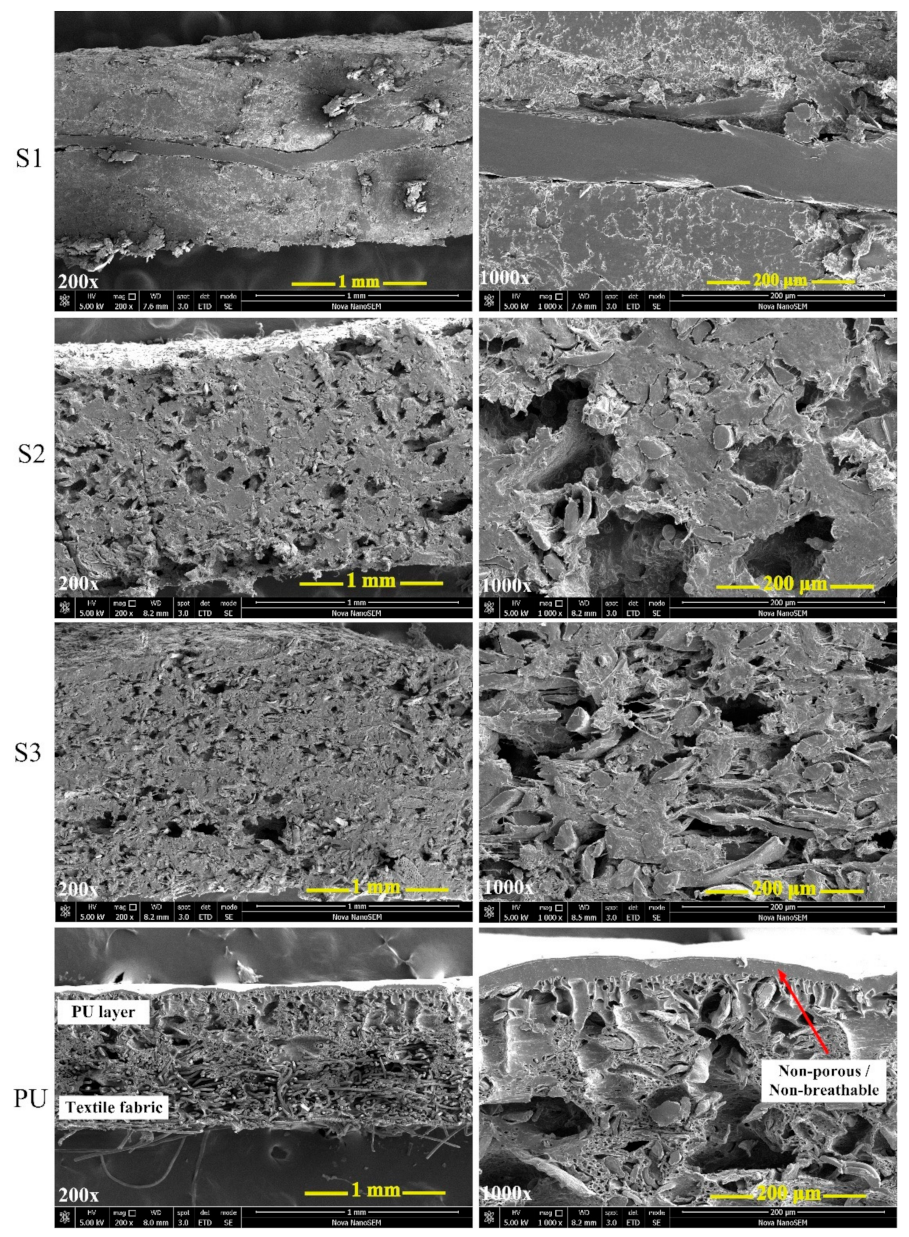
3.4. Surface Morphology Analysis
3.5. Optimization Study
3.5.1. Experimental Design and ANOVA
3.5.2. Optimization and Validation
3.5.3. Adhesion Analysis
3.5.4. Shape Stability, Water Absorption, Contact Angle and Pore Volume Analysis
4. Conclusions and Research Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mittelstaedt, J.D.; Shultz, C.J.; Kilbourne, W.E.; Peterson, M. Sustainability as Megatrend: Two Schools of Macromarketing Thought. J. Macromark. 2014, 34, 253–264. [Google Scholar] [CrossRef]
- Varadarajan, R. Strategic marketing and marketing strategy: Domain, definition, fundamental issues and foundational premises. J. Acad. Mark. Sci. 2010, 38, 119–140. [Google Scholar] [CrossRef]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Nygren, O.; Wahlberg, J.E. Speciation of chromium in tanned leather gloves and relapse of chromium allergy from tanned leather samples. Analyst 1998, 123, 935–937. [Google Scholar] [CrossRef]
- Nam, C.; Lee, Y.A. Multilayered Cellulosic Material as a Leather Alternative in the Footwear Industry. Cloth. Text. Res. J. 2019, 37, 20–34. [Google Scholar] [CrossRef]
- McNeill, L.; Moore, R. Sustainable fashion consumption and the fast fashion conundrum: Fashionable consumers and attitudes to sustainability in clothing choice. Int. J. Consum. Stud. 2015, 39, 212–222. [Google Scholar] [CrossRef]
- Lundblad, L.; Davies, I.A. The values and motivations behind sustainable fashion consumption. J. Consum. Behav. 2016, 15, 149–162. [Google Scholar] [CrossRef]
- Rathinamoorthy, R. Consumer’s Awareness on Sustainable Fashion. In Sustainable Fashion: Consumer Awareness and Education; Muthu, S.S., Ed.; Springer: Singapore, 2019; pp. 1–36. [Google Scholar] [CrossRef]
- Debnath, S. Natural Fibres for Sustainable Development in Fashion Industry. In Sustainable Fibres for Fashion Industry: Volume 1; Muthu, S.S., Gardetti, M.A., Eds.; Springer: Singapore, 2016; pp. 89–108. [Google Scholar] [CrossRef]
- Kanagaraj, J.; Senthilvelan, T.; Panda, R.; Kavitha, S. Eco-friendly waste management strategies for greener environment towards sustainable development in leather industry: A comprehensive review. J. Clean. Prod. 2015, 89, 1–17. [Google Scholar] [CrossRef]
- Zhang, Q.; Khan, M.U.; Lin, X.; Yi, W.; Lei, H. Green-composites produced from waste residue in pulp and paper industry: A sustainable way to manage industrial wastes. J. Clean. Prod. 2020, 121251. [Google Scholar] [CrossRef]
- Samanta, K.K.; Basak, S.; Chattopadhyay, S.K. Recycled Fibrous and Nonfibrous Biomass for Value-Added Textile and Nontextile Applications. In Environmental Implications of Recycling and Recycled Products; Muthu, S.S., Ed.; Springer: Singapore, 2015; pp. 167–212. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Ashori, A. Wood plastic composites from agro-waste materials: Analysis of mechanical properties. Bioresour. Technol. 2010, 101, 2525–2528. [Google Scholar] [CrossRef]
- Vaisanen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Schettini, E.; Santagata, G.; Malinconico, M.; Immirzi, B.; Scarascia Mugnozza, G.; Vox, G. Recycled wastes of tomato and hemp fibres for biodegradable pots: Physico-chemical characterization and field performance. Resour. Conserv. Recycl. 2013, 70, 9–19. [Google Scholar] [CrossRef]
- Shahid-ul-Islam, S.M.; Mohammad, F. Perspectives for natural product based agents derived from industrial plants in textile applications—A review. J. Clean. Prod. 2013, 57, 2–18. [Google Scholar] [CrossRef]
- Sen, T.; Reddy, H.J. Various industrial applications of hemp, kinaf, flax and ramie natural fibres. Int. J. Innov. Manag. Technol. 2011, 2, 192. [Google Scholar]
- Cao, H.; Wool, R.P.; Bonanno, P.; Dan, Q.; Kramer, J.; Lipschitz, S. Development and evaluation of apparel and footwear made from renewable bio-based materials. Int. J. Fash. Des. Technol. Educ. 2014, 7, 21–30. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Yildiz, U. Nature-derived fibrous nanomaterial toward biomedicine and environmental remediation: Today’s state and future prospects. J. Appl. Polym. Sci. 2019, 136, 47878. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues–Wheat straw and soy hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A.J. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Gallegos, A.M.A.; Carrera, S.H.; Parra, R.; Keshavarz, T.; Iqbal, H.M. Bacterial cellulose: A sustainable source to develop value-added products—A review. BioResources 2016, 11, 5641–5655. [Google Scholar] [CrossRef]
- Karim, Z.; Afrin, S. Bacterial cellulose: Preparation and characterization. In Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Abdul Khalil, H.P.S., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 327–340. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef]
- García, C.; Prieto, M.A. Bacterial cellulose as a potential bioleather substitute for the footwear industry. Microb. Biotechnol. 2018, 12, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Eluxe Magazine. Eco-Friendly Vegan Leathers. Available online: https://eluxemagazine.com/fashion/5-truly-eco-friendly-vegan-leathers/ (accessed on 22 May 2019).
- Material District. Malai Biocomposite. Available online: https://materialdistrict.com/material/malai/ (accessed on 8 March 2019).
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Tepe, O.; Dursun, A.Y. Exo-pectinase production by Bacillus pumilus using different agricultural wastes and optimizing of medium components using response surface methodology. Environ. Sci. Pollut. Res. 2014, 21, 9911–9920. [Google Scholar] [CrossRef]
- Durakovic, B. Design of experiments application, concepts, examples: State of the art. Period. Eng. Nat. Sci. 2017, 5, 421–439. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Saha, N.; Brodnjak, U.V.; Saha, P. Bacterial cellulose based greener packaging material: A bioadhesive polymeric film. Mater. Res. Express 2018, 5, 115405. [Google Scholar] [CrossRef]
- Chen, H.; Yu, Y.; Zhong, T.; Wu, Y.; Li, Y.; Wu, Z.; Fei, B. Effect of alkali treatment on microstructure and mechanical properties of individual bamboo fibers. Cellulose 2017, 24, 333–347. [Google Scholar] [CrossRef]
- BASF. Pocket Book for Leather Technologist, 4th ed.; Aktiengesellschaft: Ludwigshafen, Germany, 2007; p. 454. [Google Scholar]
- Yin, H.; Chen, Z.; Gu, Z.; Han, Y. Optimization of natural fermentative medium for selenium-enriched yeast by d-optimal mixture design. LWT Food Sci. Technol. 2009, 42, 327–331. [Google Scholar] [CrossRef]
- Jeirani, Z.; Jan, B.M.; Si Ali, B.; Noor, I.M.; Chun Hwa, S.; Saphanuchart, W. The optimal mixture design of experiments: Alternative method in optimizing the aqueous phase composition of a microemulsion. Chemom. Intell. Lab. Syst. 2012, 112, 1–7. [Google Scholar] [CrossRef]
- Mura, P.; Furlanetto, S.; Cirri, M.; Maestrelli, F.; Marras, A.M.; Pinzauti, S. Optimization of glibenclamide tablet composition through the combined use of differential scanning calorimetry and d-optimal mixture experimental design. J. Pharm. Biomed. Anal. 2005, 37, 65–71. [Google Scholar] [CrossRef]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Prithivirajan, R.; Jayabal, S.; Bharathiraja, G. Bio-based composites from waste agricultural residues: Mechanical and morphological properties. Cellul. Chem. Technol. 2015, 49, 65–68. [Google Scholar]
- Quiles-Carrillo, L.; Montanes, N.; Pineiro, F.; Jorda-Vilaplana, A.; Torres-Giner, S. Ductility and Toughness Improvement of Injection-Molded Compostable Pieces of Polylactide by Melt Blending with Poly(ε-caprolactone) and Thermoplastic Starch. Materials 2018, 11, 2138. [Google Scholar] [CrossRef]
- Akos, N.I.; Wahit, M.U.; Mohamed, R.; Yussuf, A.A. Preparation, characterization, and mechanical properties of poly (ε-caprolactone)/polylactic acid blend composites. Polym. Compos. 2013, 34, 763–768. [Google Scholar] [CrossRef]
- Reddy, K.O.; Uma Maheswari, C.; Muzenda, E.; Shukla, M.; Rajulu, A.V. Extraction and characterization of cellulose from pretreated ficus (peepal tree) leaf fibers. J. Nat. Fibers 2016, 13, 54–64. [Google Scholar] [CrossRef]
- Laaziz, S.A.; Raji, M.; Hilali, E.; Essabir, H.; Rodrigue, D.; Bouhfid, R. Bio-composites based on polylactic acid and argan nut shell: Production and properties. Int. J. Biol. Macromol. 2017, 104, 30–42. [Google Scholar] [CrossRef]
- Saba, N.; Mohammad, F.; Pervaiz, M.; Jawaid, M.; Alothman, O.Y.; Sain, M. Mechanical: Morphological and structural properties of cellulose nanofibers reinforced epoxy composites. Int. J. Biol. Macromol. 2017, 97, 190–200. [Google Scholar] [CrossRef]
- Saba, N.; Safwan, A.; Sanyang, M.L.; Mohammad, F.; Pervaiz, M.; Jawaid, M.; Alothman, O.Y.; Sain, M. Thermal and dynamic mechanical properties of cellulose nanofibers reinforced epoxy composites. Int. J. Biol. Macromol. 2017, 102, 822–828. [Google Scholar] [CrossRef]
- Kakroodi, A.R.; Kazemi, Y.; Rodrigue, D.; Park, C.B. Facile production of biodegradable PCL/PLA in situ nanofibrillar composites with unprecedented compatibility between the blend components. Chem. Eng. J. 2018, 351, 976–984. [Google Scholar] [CrossRef]
- Jeyapalina, S.; Attenburrow, G.E.; Covington, A.D. Dynamic mechanical thermal analysis (DMTA) of leather part 1: Effect of tanning agent on the glass transition temperature of collagen. J. Soc. Leather Technol. Chem. 2007, 91, 236–242. [Google Scholar]
- Misra, M.; Pandey, J.K.; Mohanty, A. Biocomposites: Design and Mechanical Performance; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- ISO. ISO/TR 20879:2007. Footwear—Performance Requirements for Components for Footwear—Uppers; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 403. [Google Scholar]
- Nguyen, M.H.; Kim, B.S.; Ha, J.R.; Song, J., II. Effect of plasma and NaOH treatment for rice Husk/PP composites. Adv. Compos. Mater. 2011, 20, 435–442. [Google Scholar] [CrossRef]
- Essabir, H.; Ouadi Bensalah, M.; Rodrigue, D.; Bouhfid, R.; Qaiss, A. Biocomposites based on Argan nut shell and a polymer matrix: Effect of filler content and coupling agent. Carbaohydrate Polym. 2016, 143, 70–83. [Google Scholar] [CrossRef] [PubMed]


| Component | Name | Low Limit (% w/w) (Coding Level = –1) | High Limit (% w/w) (Coding Level = +1) | Proportion Constraints (% w/w) |
|---|---|---|---|---|
| X1 | BC | 30 | 40 | 30 ≤ X1 ≤ 40 |
| X2 | ML | 5 | 10 | 5 ≤ X2 ≤ 10 |
| X3 | PCL | 15 | 20 | 15 ≤ X3 ≤ 20 |
| X4 | PLA | 5 | 10 | 5 ≤ X4 ≤ 10 |
| Run | Individual Components | Response 1 | Response 2 | |||||
|---|---|---|---|---|---|---|---|---|
| BC X1 | ML X2 | PCL X3 | PLA X4 | Tensile Strength (N/mm2) | Elastic Modulus (MPa) | |||
| Actual | Predicted | Actual | Predicted | |||||
| 1 | +1 | –1 | +1 | +1 | 2.10 | 1.96 | 146.10 | 143.69 |
| 2 | –1 | +1 | +1 | +1 | 1.80 | 1.77 | 152.30 | 136.52 |
| 3 | –1 | +1 | –1 | –1 | 1.68 | 1.61 | 49.53 | 43.61 |
| 4 | +1 | +1 | –1 | +1 | 0.88 | 0.59 | 84.20 | 98.24 |
| 5 | –1 | +1 | +1 | +1 | 0.89 | 0.96 | 159.20 | 146.26 |
| 6 | +1 | –1 | –1 | +1 | 1.27 | 1.40 | 103.30 | 88.39 |
| 7 | –1 | –1 | +1 | +1 | 1.72 | 1.75 | 73.98 | 99.19 |
| 8 | +1 | +1 | +1 | –1 | 2.00 | 2.09 | 137.30 | 151.11 |
| 9 | +1 | +1 | –1 | +1 | 0.46 | 0.68 | 76.48 | 83.27 |
| 10 | +1 | –1 | +1 | –1 | 1.36 | 1.34 | 79.20 | 71.31 |
| Tensile Strength | ||||||
|---|---|---|---|---|---|---|
| Source | Sum of SQUARES | df | Mean Square | F-Value | p-Value | |
| Model | 4.86 | 6 | 0.8105 | 27.84 | < 0.0001 | Significant |
| Linear Mixtures | 4.86 | 6 | 0.8105 | 27.84 | < 0.0001 | Significant |
| Lack of Fit | 0.3785 | 3 | 0.1262 | |||
| R2 | 0.928 | |||||
| Adjusted R2 | 0.895 | |||||
| Predicted R2 | 0.846 | |||||
| Std. Dev. | 0.171 | |||||
| Storage modulus | ||||||
| Model | 24,073.60 | 6 | 4012.27 | 14.40 | < 0.0001 | Significant |
| Linear Mixtures | 24,073.60 | 6 | 4012.27 | 14.40 | < 0.0001 | Significant |
| Residual | 3621.84 | 13 | 278.60 | |||
| Lack of Fit | 3621.84 | 3 | 1207.28 | |||
| R2 | 0.869 | |||||
| Adjusted R2 | 0.809 | |||||
| Predicted R2 | 0.709 | |||||
| Std. Dev. | 16.69 | |||||
| Parameters | Goal | Experimental Region | Results | Model Desirability | ||||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Optimized | Validation | |||||
| X1: BC | In range | 30 | 40 | 37 | 37 | 0.901 | ||
| X2: ML | In range | 5 | 10 | 5 | 5 | |||
| X3: PCL | In range | 15 | 20 | 18 | 18 | |||
| X4: PLA | In range | 5 | 10 | 5 | 5 | |||
| Y1: Tensile strength | Maximized | 0.46 | 2.10 | 1.94 ± 0.17 | 2.03 ± 0.29 | |||
| Y2: Storage modulus | Minimized | 49.53 | 159.20 | 61.41 ± 1.69 | 63.93 ± 1.63 | |||
| Samples | Responses | |||||||
| Tensile strength (N/mm2) | Elongation at break (%) | Tear strength (N/mm) | Elastic modulus (MPa) | |||||
| Experimental | 2.08 ± 0.06 | 16.45 ± 0.70 | 31.24 ± 0.35 | 72.17 ± 0.94 | ||||
| Optimized | 2.13 ± 0.29 | 19.23 ± 1.09 | 32.93 ± 1.33 | 76.93 ± 1.63 | ||||
| PU Synthetic | 5.28 ± 0.69 | 31.54 ± 2.32 | 79.20 ± 2.45 | 106.10 ± 2.70 | ||||
| Experimental Runs | Adhesion Force (MPa) | Type of Failure |
|---|---|---|
| 1 | 7.28 ± 0.40 | 100% glue failure |
| 2 | 6.37 ± 0.15 | Partly cohesive failure |
| 3 | 6.42 ± 0.23 | Partly cohesive failure |
| 4 | 7.05 ± 0.33 | 100% glue failure |
| 5 | 4.56 ± 0.21 | 100% glue failure |
| 6 | 7.88 ± 0.49 | Partly cohesive failure |
| 7 | 6.35 ± 0.35 | 100% glue failure |
| 8 | 4.71 ± 0.20 | 100% glue failure |
| 9 | 4.86 ± 0.24 | Partly cohesive failure |
| 10 | 4.55 ± 0.22 | Partly cohesive failure |
| Experimental Run | Water Absorption Capacity (%) | Water Contact Angle (°) θdH2O | Pore Volume (mL/min) |
|---|---|---|---|
| 1 | 44.89 ± 2.24 | 65.40 ± 1.99 | 2192 ± 109.60 |
| 2 | 39.37 ± 2.46 | 78.30 ± 1.06 | 1043 ± 47.14 |
| 3 | 37.63 ± 1.88 | 82.80 ± 1.87 | 1440 ± 72.50 |
| 4 | 46.05 ± 2.60 | 76.80 ± 2.09 | 1599 ± 79.95 |
| 5 | 45.17 ± 2.44 | 80.30 ± 1.52 | 1670 ± 83.50 |
| 6 | 52.50 ± 1.63 | 81.50 ± 2.68 | 2935 ± 146.75 |
| 7 | 45.71 ± 2.30 | 80.20 ± 1.96 | 1723 ± 86.15 |
| 8 | 46.17 ± 1.46 | 68.50 ± 1.07 | 1886 ± 94.30 |
| 9 | 54.37 ± 2.70 | 77.40 ± 0.88 | 3000 ± 150.33 |
| 10 | 47.17 ± 2.55 | 69.55 ± 1.23 | 2043 ± 102.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asabuwa Ngwabebhoh, F.; Saha, N.; Nguyen, H.T.; Brodnjak, U.V.; Saha, T.; Lengalova, A.; Saha, P. Preparation and Characterization of Nonwoven Fibrous Biocomposites for Footwear Components. Polymers 2020, 12, 3016. https://doi.org/10.3390/polym12123016
Asabuwa Ngwabebhoh F, Saha N, Nguyen HT, Brodnjak UV, Saha T, Lengalova A, Saha P. Preparation and Characterization of Nonwoven Fibrous Biocomposites for Footwear Components. Polymers. 2020; 12(12):3016. https://doi.org/10.3390/polym12123016
Chicago/Turabian StyleAsabuwa Ngwabebhoh, Fahanwi, Nabanita Saha, Hau Trung Nguyen, Urška Vrabič Brodnjak, Tomas Saha, Anežka Lengalova, and Petr Saha. 2020. "Preparation and Characterization of Nonwoven Fibrous Biocomposites for Footwear Components" Polymers 12, no. 12: 3016. https://doi.org/10.3390/polym12123016
APA StyleAsabuwa Ngwabebhoh, F., Saha, N., Nguyen, H. T., Brodnjak, U. V., Saha, T., Lengalova, A., & Saha, P. (2020). Preparation and Characterization of Nonwoven Fibrous Biocomposites for Footwear Components. Polymers, 12(12), 3016. https://doi.org/10.3390/polym12123016