High Efficiency and Low Migration Hyperbranched Silicone Contain Macrophotoinitiators for UV-Cured Transparent Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Hyperbranched Silicone Containing Macrophotoinitiators (HBSMIs)
2.3. Preparation of Ultraviolet (UV)-Cured Coatings
2.4. Characterization
3. Results and Discussion
3.1. Effect of UV-Curing Time on the UV-Cured Coatings Initiated by HBSMI and HMPP
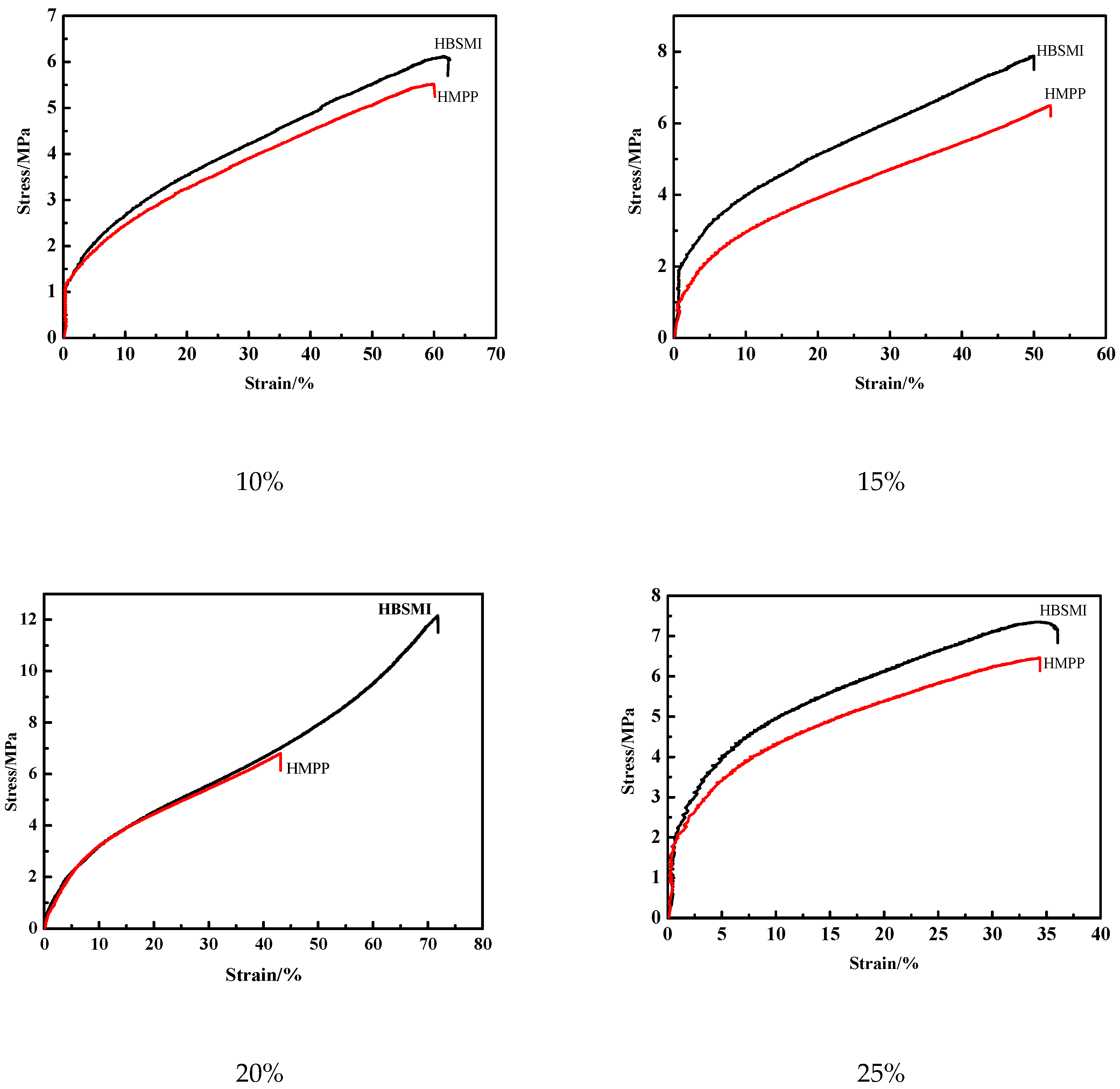
3.2. Mechanical Performance of the UV-Cured Coatings Prepared with Various of PUAs Initiated by HBSMI and HMPP
3.3. The Effect of Different Amount of HBSMI on the UV-Cured Coatings
3.4. The Effect of Different HBSMI on the UV-Cured Coatings
3.5. The Performance of UV-Cured Coatings Initiated by HBSMI
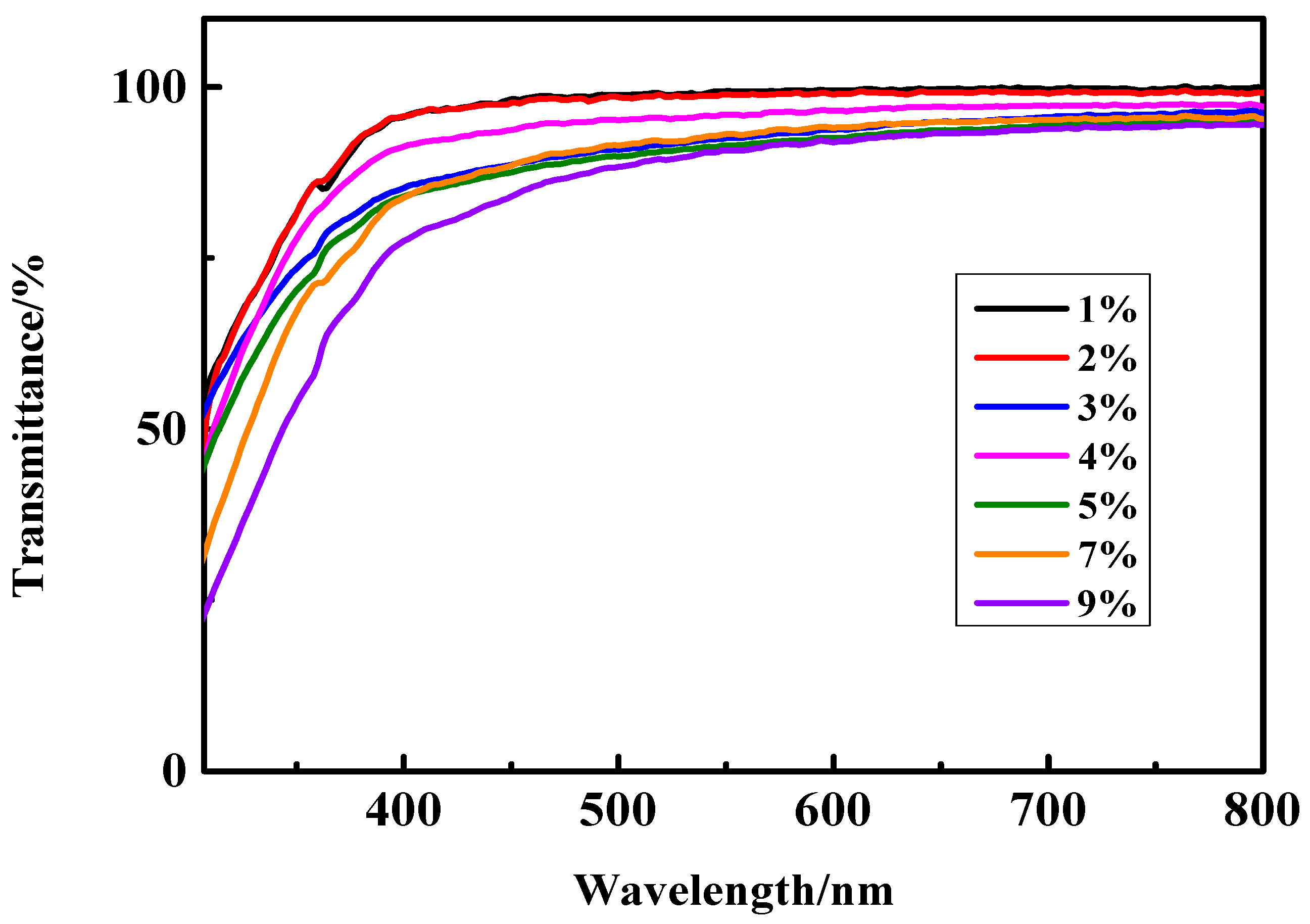
3.5.1. The Transmittance of UV-Cured Coatings
3.5.2. Thermal Stability of UV-Cured Coatings
3.5.3. The UV Resistance of UV-Cured Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Y.; Liu, J.; Cheng, F.; Jiao, X.; Fan, Y.; Lai, G.; Hua, X.; Yang, X. UV cured transparent coatings with good thermal stability prepared from a liquid polyhedral oligomeric silsesquioxane with allyl sulphur−containing carbosilane substitutes. Prog. Org. Coat. 2020, 148, 105886. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, X.; Cheng, F.; Fan, Y.; Wu, Y.; Yang, X. Fabrication and performance of UV cured transparent silicone modified polyurethane–acrylate coatings with high hardness, good thermal stability and adhesion. Prog. Org. Coat. 2020, 144, 105673. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, T.; Cheng, F.; Fan, Y.; Liu, J.; Lai, G.; Wu, Y.; Yang, X. UV-Cured Coatings Prepared with Sulfhydryl-Terminated Branched Polyurethane and Allyl-Terminated Hyperbranched Polycarbosilane. Coatings 2020, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Liu, J.; Cheng, F.; Jiao, X.; Fan, Y.; Lai, G.; Luo, M.; Yang, X. Fabrication of Transparent UV-Cured Coatings with Allyl-Terminated Hyperbranched Polycarbosilanes and Thiol Silicone Resins. ACS Omega 2020, 5, 15311–15316. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Fan, Y.; Zhang, L.; Jiao, X.; Lai, G.; Hua, X.; Yang, X. An Easy Fabrication Method to Prepare Inexpensive UV–Cured Transparent Silicone Modified Polyacrylate Coatings with Good Adhesion and UV Resistance. Coatings 2020, 10, 675. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.P.; Lalevee, J. Structure Design of Naphthalimide Derivatives: Toward Versatile Photoinitiators for Near–UV/Visible LEDs, 3D Printing, and Water–Soluble Photoinitiating Systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Q.; Bao, H.; Liu, J.; Wu, Y.; Lai, G. Preparation and performance of ultraviolet curable silicone resins used for ultraviolet cured coating and ultraviolet-assisted 3D printing materials. OSA Contin. 2018, 1, 542–552. [Google Scholar] [CrossRef]
- Wei, M.; Gao, Y.; Jiang, S.; Nie, J.; Sun, F. Design of photoinitiator-functionalized hydrophilic nanogels with uniform size and excellent biocompatibility. Polym. Chem. 2019, 10, 2812–2821. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, L.; Xiong, Y.; Wu, Q.; Tang, H. A facile method to prepare a polyethylene glycol modified polysilane as a waterborne photoinitiator. J. Photochem. Photobiol. A Chem. 2015, 299, 9–17. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated Polymerization: Advances, Challenges, and Opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Decker, C. Kinetic Study and New Applications of UV Radiation Curing. Macromol. Rapid Commun. 2002, 23, 1067–1093. [Google Scholar] [CrossRef]
- Zhou, R.; Jin, M.; Malval, J.; Pan, H.; Wan, D. Bicarbazole-based oxalates as photoinitiating systems for photopolymerization under UV–Vis LEDs. J. Appl. Polym. Sci. 2020, 58, 1079–1091. [Google Scholar] [CrossRef]
- Wu, Q.; Tang, K.; Xiong, Y.; Wang, X.; Yang, J.; Tang, H. High-Performance and Low Migration One-Component Thioxanthone Visible Light Photoinitiators. Macromol. Chem. Phys. 2017, 218. [Google Scholar] [CrossRef]
- Aparicio, J.L.; Elizalde, M. Migration of Photoinitiators in Food Packaging: A Review. Packag. Technol. Sci. 2015, 28, 181–203. [Google Scholar] [CrossRef]
- Yang, J.; Liao, W.; Xiong, Y.; Wu, Q.; Wang, X.; Li, Z.; Tang, H. Naphthalimide dyes: Polymerizable one-component visible light initiators. Dye. Pigment. 2018, 148, 16–24. [Google Scholar] [CrossRef]
- Wang, K.; Lu, Y.; Chen, P.; Shi, J.; Wang, H.; Yu, Q. Novel one-component polymeric benzophenone photoinitiator containing poly (ethylene glycol) as hydrogen donor. Mater. Chem. Phys. 2014, 143, 1391–1395. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, N.; Nie, J.; Du, H.-G. Control of concentration gradient and initiating gradient photopolymerization of polysiloxane benzophenone photoinitiator. J. Mater. Chem. 2011, 21, 17290–17296. [Google Scholar] [CrossRef]
- Lei, J.; Ulbricht, M. Macroinitiator-mediated photoreactive coating of membrane surfaces with antifouling hydrogel layers. J. Membr. Sci. 2014, 455, 207–218. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Wang, L.; Zhao, J.; Zhao, G. Synthesis and preliminary photopolymerization envaluation of photopolymerizalbe type II photoinitiators BRA and TXRA. Prog. Org. Coat. 2019, 133, 191–197. [Google Scholar] [CrossRef]
- Balta, D.K.; Karahan, Ö.; Avci, D.; Arsu, N. Synthesis, photophysical and photochemical studies of benzophenone based novel monomeric and polymeric photoinitiators. Prog. Org. Coatings 2015, 78, 200–207. [Google Scholar] [CrossRef]
- Xie, Y.Z.; Huang, H. Preparation and Characterization of An Amphiphilic Macrophotoinitiator Based on 2–Hydroxy–2–methyl–1–phenyl propanone. J. Appl. Polym. Sci. 2016, 133, 43910. [Google Scholar] [CrossRef]
- Han, W.; Lin, B.; Zhou, Y.; Song, J. Synthesis and properties of UV-curable hyperbranched polyurethane acrylate oligomers containing photoinitiator. Polym. Bull. 2012, 68, 729–743. [Google Scholar] [CrossRef]
- Cheng, J.; Jiang, S.; Gao, Y.; Wang, J.; Sun, F. Tuning gradient property and initiating gradient photopolymerization of acrylamide aqueous solution of a hydrosoluble photocleavage polysiloxane-based photoinitiator. Polym. Adv. Technol. 2014, 25, 1412–1418. [Google Scholar] [CrossRef]
- Sun, F.; Li, Y.; Zhang, N.; Nie, J. Initiating gradient photopolymerization and migration of a novel polymerizable polysiloxane α-hydroxy alkylphenones photoinitiator. Polymer 2014, 55, 3656–3665. [Google Scholar] [CrossRef]
- Zhang, G.W.; Cao, Y.; Yu, J.; Sun, F. Photoinitiability of A Water–Borne Polysiloxane–Modifed Benzophenone Macromolecular Photoinitiator. J. Adhes. Sci. Technol. 2016, 30, 2289–2300. [Google Scholar] [CrossRef]
- Si, Q.-F.; Fan, X.-D.; Liu, Y.-Y.; Kong, J.; Wang, S.-J.; Qiao, W.-Q. Synthesis and characterization of hyperbranched-poly(siloxysilane)-based polymeric photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3261–3270. [Google Scholar] [CrossRef]
- Hou, H.; Gan, Y.; Yin, J.; Jiang, X. Multifunctional POSS-Based Nano-Photo-Initiator for Overcoming the Oxygen Inhibition of Photo-Polymerization and for Creating Self-Wrinkled Patterns. Adv. Mater. Interfaces 2014, 1, 1400385–1400390. [Google Scholar] [CrossRef]
- Yang, J.; Liao, W.; Xiong, Y.; Wang, X.; Li, Z.; Tang, H. A multifunctionalized macromolecular silicone-naphthalimide visible photoinitiator for free radical polymerization. Prog. Org. Coat. 2018, 115, 151–158. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, Y.; Wang, X.; Li, Z.; Tang, H. Naphthalimide-and Methacrylate-Functionalized Polysiloxanes: Visible-Light Photoinitiators, Modifiers for Polyurethane Acrylate and Photocurable Coatings. ChemPhotoChem 2018, 2, 818–824. [Google Scholar] [CrossRef]
- Wu, Q.; Liao, W.; Xiong, Y.; Yang, J.; Li, Z.; Tang, H. Silicone-Thioxanthone: A Multifunctionalized Visible Light Photoinitiator with an Ability to Modify the Cured Polymers. Polymers 2019, 11, 695. [Google Scholar] [CrossRef] [Green Version]
- Lai, X.-H.; Sun, F.; Yin, R.-X.; Ma, G.-P.; Nie, J. Synthesis of gradient polymer by using polysiloxane-based photoinitiator. Polym. Adv. Technol. 2011, 23, 1246–1251. [Google Scholar] [CrossRef]
- Vaicekauskaite, J.; Skov, A.L.; Vudayagiri, S.; Skov, A.L. Mapping the mechanical and electrical properties of commercial silicone elastomer formulations for stretchable transducers. J. Mater. Chem. C 2020, 8, 1273–1279. [Google Scholar] [CrossRef]
- Yang, X.; Shao, Q.; Yang, L.; Zhu, X.; Hua, X.; Zheng, Q.; Song, G.; Lai, G. Preparation and performance of high refractive index silicone resin-type materials for the packaging of light-emitting diodes. J. Appl. Polym. Sci. 2013, 127, 1717–1724. [Google Scholar] [CrossRef]
- Cicala, G.; Recca, G.; Carciotto, S.; Restuccia, C.L. Development of epoxy/hyperbranched blends for resin transfer molding and vacuum assisted resin transfer molding applications: Effect of a reactive diluent. Polym. Eng. Sci. 2009, 49, 577–584. [Google Scholar] [CrossRef]
- Khan, A.; Malkoch, M.; Montague, M.F.; Hawker, C.J. Synthesis and characterization of hyperbranched polymers with increased chemical versatility for imprint lithographic resists. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6238–6254. [Google Scholar] [CrossRef]
- Niu, S.; Yan, H.; Chen, Z.; Li, S.; Xu, P.; Zhi, X. Unanticipated bright blue fluorescence produced from novel hyperbranched polysiloxanes carrying unconjugated carbon–carbon double bonds and hydroxyl groups. Polym. Chem. 2016, 7, 3747–3755. [Google Scholar] [CrossRef]
- Niu, S.; Yan, H.; Li, S.; Tang, C.; Chen, Z.; Zhi, X.; Xu, P. A multifunctional silicon-containing hyperbranched epoxy: Controlled synthesis, toughening bismaleimide and fluorescent properties. J. Mater. Chem. C 2016, 4, 6881–6893. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Synthesis and photopolymerization of novel UV-curable macro-photoinitiators. Prog. Org. Coat. 2020, 141, 105546. [Google Scholar] [CrossRef]
- Lauer, A.; Fast, D.E.; Kelterer, A.M.; Frick, E.; Neshchadin, D.; Voll, D.; Gescheidt, G.; Barner–Kowollik, C. Systematic Assessment of the Photochemical Stability of Photoinitiator–Derived Macromolecular Chain Termini. Macromolecules 2015, 48, 8451–8460. [Google Scholar] [CrossRef]

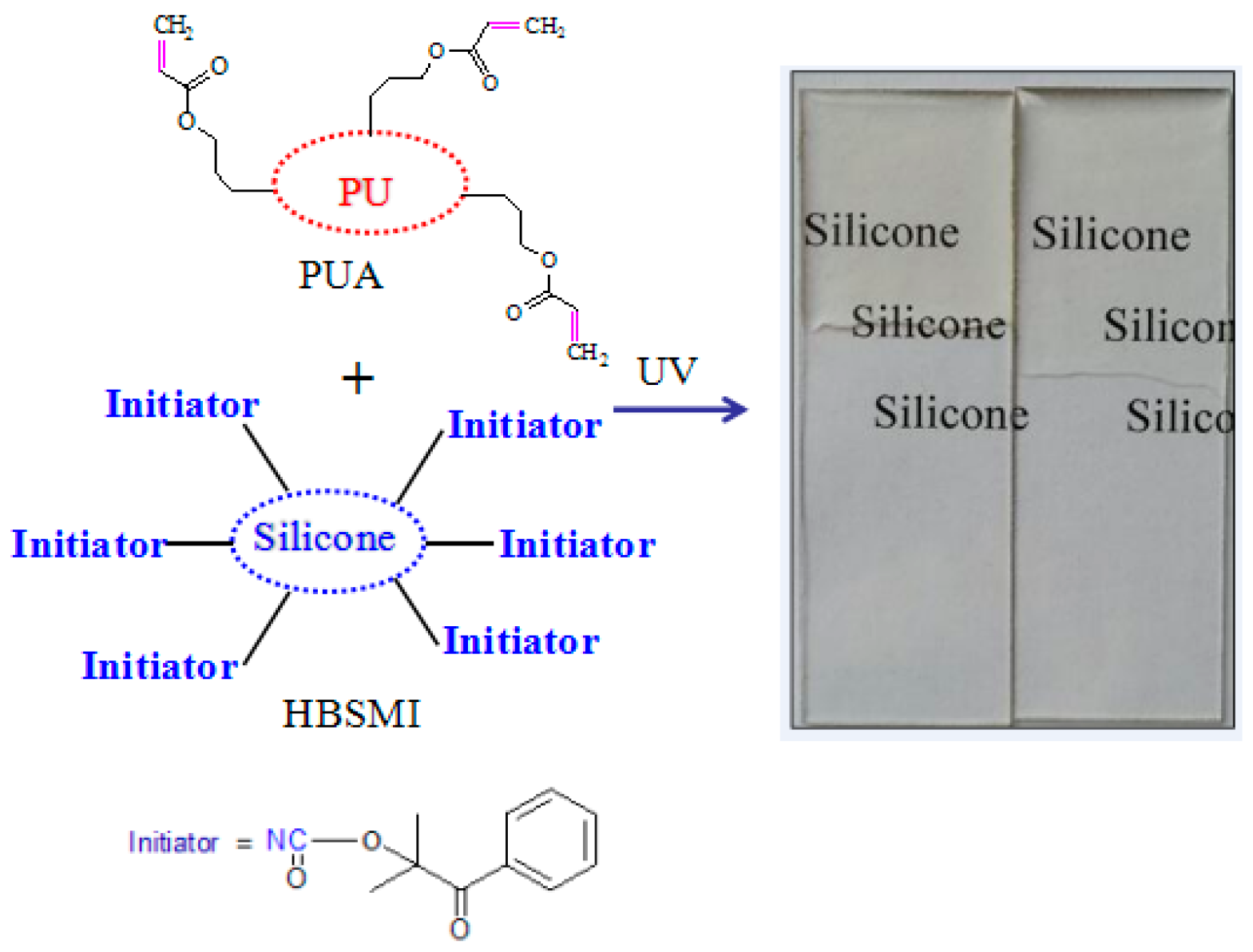



| Entry | Sample Name of PUAs | The Molar Ratio of TMP to PTMG | PTMG/g | TMP/g | IPDI/g | HPA/g |
|---|---|---|---|---|---|---|
| 1 | PUA–1 | 10:100 | 200 | 1.342 | 63.797 | 49.738 |
| 2 | PUA–2 | 15:100 | 200 | 2.013 | 68.910 | 51.901 |
| 3 | PUA–3 | 20:100 | 200 | 2.683 | 72.244 | 56.226 |
| 4 | PUA–4 | 25:100 | 200 | 3.354 | 76.245 | 59.110 |
| Entry | Sample Name of HBSMIs | Molar Ratio of Triethoxysilane with HMPP Substitute | Triethoxysilane with HMPP Substitute/g | NPG/g |
|---|---|---|---|---|
| 1 | HBSMI–1 | 1:1.5 | 40.736 | 15.615 |
| 2 | HBSMI–2 | 1:1.6 | 16.656 | |
| 3 | HBSMI–3 | 1:1.7 | 17.697 | |
| 4 | HBSMI–4 | 1:1.8 | 18.738 | |
| 5 | HBSMI–5 | 1:2.0 | 20.820 | |
| 6 | HBSMI–6 | 1:2.2 | 22.902 |
| Entry | Curing Time/s | Pencil Hardness of Coatings Cured with Different UV Initiators | |
|---|---|---|---|
| HBSMI | HMPP | ||
| 1 | 10 | 4H | 6B |
| 2 | 20 | 5H | 6B |
| 3 | 30 | 6H | 6B |
| 4 | 40 | 7H | 2H |
| 5 | 50 | 8H | 3H |
| 6 | 60 | 9H | 9H |
| 7 | 70 | 9H | 9H |
| Entry | mHBSMI/mPUA/% | Degree of Curing Content/% | Pencil Hardness |
|---|---|---|---|
| 1 | 1 | 97.7 | 6H |
| 2 | 2 | 97.5 | 7H |
| 3 | 3 | 98.0 | 8H |
| 4 | 4 | 98.6 | 9H |
| 5 | 5 | 96.1 | 9H |
| 6 | 7 | 94.3 | 8H |
| 7 | 9 | 92.5 | 8H |
| Entry | Sample of HBSMI | Degree of Curing Content/% | Migration Rate of HBSMI/% | Pencil Hardness |
|---|---|---|---|---|
| 1 | HBSMI–1 | 97.7 | 7.5 | 5H |
| 2 | HBSMI–2 | 97.7 | 3.3 | 5H |
| 3 | HBSMI–3 | 97.1 | 5.5 | 6H |
| 4 | HBSMI–4 | 97.2 | 7.3 | 8H |
| 5 | HBSMI–5 | 98.6 | 4.0 | 9H |
| 6 | HBSMI–6 | 98.6 | 5.5 | 7H |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Song, Y.; He, N.; Cheng, F.; Jiao, X.; Lai, G.; Hua, X.; Yang, X. High Efficiency and Low Migration Hyperbranched Silicone Contain Macrophotoinitiators for UV-Cured Transparent Coatings. Polymers 2020, 12, 3005. https://doi.org/10.3390/polym12123005
Fan Y, Song Y, He N, Cheng F, Jiao X, Lai G, Hua X, Yang X. High Efficiency and Low Migration Hyperbranched Silicone Contain Macrophotoinitiators for UV-Cured Transparent Coatings. Polymers. 2020; 12(12):3005. https://doi.org/10.3390/polym12123005
Chicago/Turabian StyleFan, Yunxin, Yan Song, Na He, Fei Cheng, Xiaojiao Jiao, Guoqiao Lai, Xilin Hua, and Xiongfa Yang. 2020. "High Efficiency and Low Migration Hyperbranched Silicone Contain Macrophotoinitiators for UV-Cured Transparent Coatings" Polymers 12, no. 12: 3005. https://doi.org/10.3390/polym12123005
APA StyleFan, Y., Song, Y., He, N., Cheng, F., Jiao, X., Lai, G., Hua, X., & Yang, X. (2020). High Efficiency and Low Migration Hyperbranched Silicone Contain Macrophotoinitiators for UV-Cured Transparent Coatings. Polymers, 12(12), 3005. https://doi.org/10.3390/polym12123005






