Review on the Impact of Polyols on the Properties of Bio-Based Polyesters
Abstract
1. Introduction
2. Polyols
2.1. Glycerol
2.2. Erythritol and Threitol
2.3. Xylitol
2.4. Sorbitol, Mannitol, and Galactitol
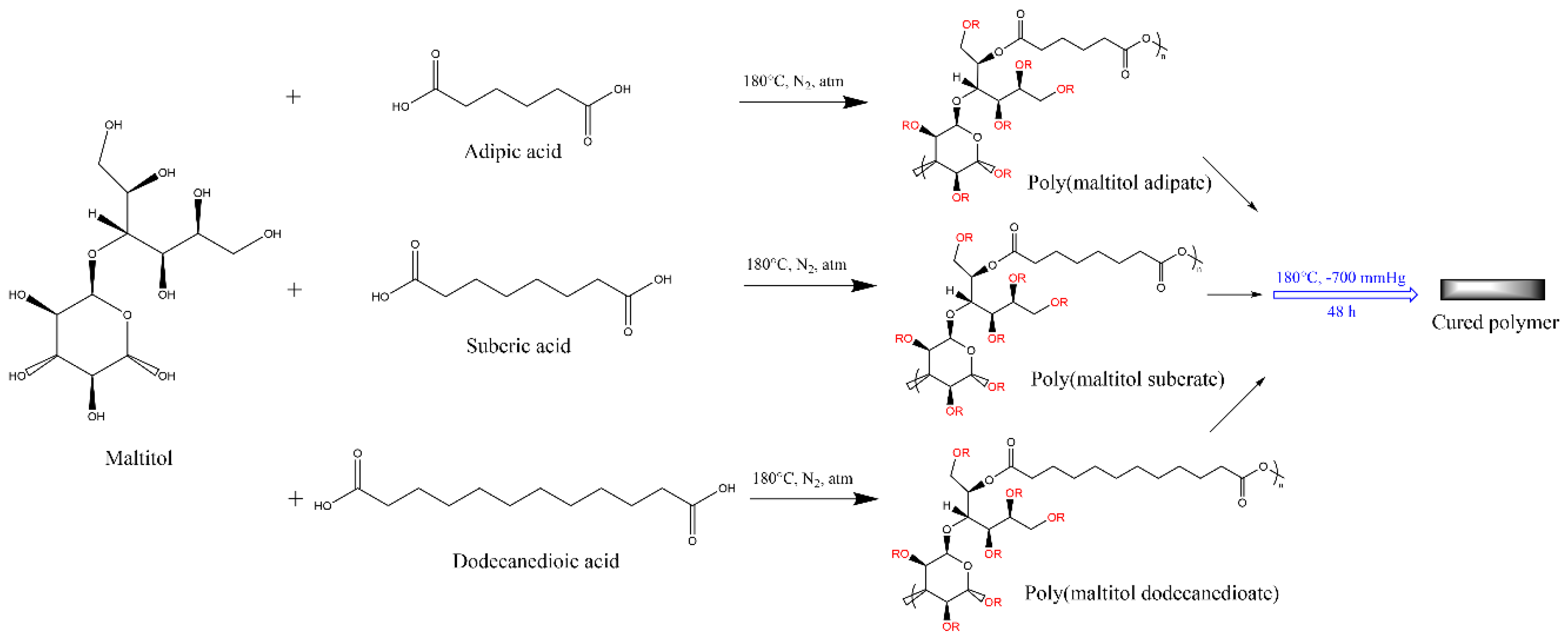
2.5. Maltitol
3. Polyol Polyesters from Glycerol and Sugar Alcohols (Alditols)
3.1. Glycerol-Based Polyesters
3.2. Erythritol-Based Polyesters
3.3. Xylitol-Based Polyesters
3.4. Sorbitol-Based and Mannitol-Based Polyesters
3.5. Maltitol-Based Polyesters
3.6. Cross-Linking Polyol polyesters
4. Conclusions
4.1. Physicochemical Properties
4.2. Mechanical Properties
4.3. Degradation Bbehavior
4.4. Biocompatibility
4.5. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Doppalapudi, S.; Jain, A.; Domb, A.J.; Khan, W. Biodegradable polymers for targeted delivery of anti-cancer drugs. Expert Opin. Drug Deliv. 2016, 13, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.V.; Vasava, D.V. A glimpse of biodegradable polymers and their biomedical applications. e-Polymers 2019, 19, 385–410. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells, Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Pavlath, A.E. Biodegradable polymers: Why, what, how? Phys. Sci. Rev. 2020, 1. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, K.; Kai, D.; Li, Z.; Loh, X.J. Polyester elastomers for soft tissue engineering. Chem. Soc. Rev. 2018, 47, 4545–4580. [Google Scholar] [CrossRef] [PubMed]
- Tham, W.H.; Wahit, M.U.; Kadir, M.R.A.; Wong, T.W.; Hassan, O. Polyol-based biodegradable polyesters: A short review. Rev. Chem. Eng. 2016, 32, 201–221. [Google Scholar] [CrossRef]
- Bîrcă, A.; Gherasim, O.; Grumezescu, V.; Grumezescu, A.M. Introduction in thermoplastic and thermosetting polymers. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–28. [Google Scholar]
- Grumezescu, V.; Grumezescu, A. Thermoset and Thermoplastic Polymers. In Materials for Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Vengatesan, M.; Varghese, A.; Mittal, V. Thermal properties of thermoset polymers. In Thermosets; Elsevier: Amsterdam, The Netherlands, 2018; pp. 69–114. [Google Scholar]
- Flory, P.J. Molecular size distribution in three dimensional polymers. VI. Branched polymers containing A—R—Bf-1 type units. J. Am. Chem. Soc. 1952, 74, 2718–2723. [Google Scholar] [CrossRef]
- Linko, Y.-Y.; Wang, Z.-L.; Seppälä, J. Lipase-catalyzed linear aliphatic polyester synthesis in organic solvent. Enzym. Microb. Technol. 1995, 17, 506–511. [Google Scholar] [CrossRef]
- Lin, Q.; Long, T.E. Polymerization of A2 with B3 monomers: A facile approach to hyperbranched poly (aryl ester) s. Macromolecules 2003, 36, 9809–9816. [Google Scholar] [CrossRef]
- Stumbé, J.F.; Bruchmann, B. Hyperbranched polyesters based on adipic acid and glycerol. Macromol. Rapid Commun. 2004, 25, 921–924. [Google Scholar] [CrossRef]
- Jikei, M.; Kakimoto, M.-A. Hyperbranched aromatic polyamides prepared by direct polycondensation. High Perform. Polym. 2001, 13, S33–S44. [Google Scholar] [CrossRef]
- Fang, J.; Kita, H.; Okamoto, K.-i. Hyperbranched polyimides for gas separation applications. 1. Synthesis and characterization. Macromolecules 2000, 33, 4639–4646. [Google Scholar] [CrossRef]
- Chen, B.; Hu, J.; Miller, E.M.; Xie, W.; Cai, M.; Gross, R.A. Candida antarctica lipase B chemically immobilized on epoxy-activated micro- and nanobeads: Catalysts for polyester synthesis. Biomacromolecules 2008, 9, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, A.; Kumar, A.; Gross, R.A. Mild, Solvent-Free ω-Hydroxy Acid Polycondensations Catalyzed by Candida antarctica Lipase B. Biomacromolecules 2004, 5, 62–68. [Google Scholar] [CrossRef]
- Kumar, A.; Kulshrestha, A.S.; Gao, W.; Gross, R.A. Versatile route to polyol polyesters by lipase catalysis. Macromolecules 2003, 36, 8219–8221. [Google Scholar] [CrossRef]
- Dubé, M.A.; Salehpour, S. Applying the Principles of Green Chemistry to Polymer Production Technology. Macromol. React. Eng. 2014, 8, 7–28. [Google Scholar] [CrossRef]
- Gross, R.A.; Ganesh, M.; Lu, W. Enzyme-catalysis breathes new life into polyester condensation polymerizations. Trends Biotechnol. 2010, 28, 435–443. [Google Scholar] [CrossRef]
- Mahapatro, A.; Kalra, B.; Kumar, A.; Gross, R.A. Lipase-Catalyzed Polycondensations: Effect of Substrates and Solvent on Chain Formation, Dispersity, and End-Group Structure. Biomacromolecules 2003, 4, 544–551. [Google Scholar] [CrossRef]
- Lide, D.R. 5. Compounds 21600-27580, Pho-Zir. In Handbook of Data on Organic Compounds; CRC Press: London, UK; Tokyo, Japan, 1994. [Google Scholar]
- Rai, R.; Tallawi, M.; Grigore, A.; Boccaccini, A.R. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012, 37, 1051–1078. [Google Scholar] [CrossRef]
- Hagopian, K.; Ramsey, J.; Weindruch, R. Enzymes of glycerol and glyceraldehyde metabolism in mouse liver: Effects of caloric restriction and age on activities. Biosci. Rep. 2008, 28, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Cryoprotectants and their usage in cryopreservation process. Cryopreserv. Biotechnol. Biomed Biol. Sci. 2018, 7. [Google Scholar] [CrossRef]
- Brandner, J.; Birkmeier, R. Relative esterifiability of the primary and secondary hydroxyl groups of glycerol. J. Am. Oil Chem. Soc. 1960, 37, 390–396. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Dumitrascu, A.; Martin, S.J.; Smith, P.B. Synthesis and characterization of glycerol-adipic acid hyperbranched polyesters. Polymer 2014, 55, 5065–5072. [Google Scholar] [CrossRef]
- Regnat, K.; Mach, R.L.; Mach-Aigner, A.R. Erythritol as sweetener—Wherefrom and whereto? Appl. Microbiol. Biotechnol. 2018, 102, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H. Sweeteners and Sugar Alternatives in Food Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Natural Sweeteners: The Relevance of Food Naturalness for Consumers, Food Security Aspects, Sustainability and Health Impacts. Int. J. Environ. Res. Public Health 2020, 17, 6285. [Google Scholar] [CrossRef] [PubMed]
- Granström, T.B.; Izumori, K.; Leisola, M. A rare sugar xylitol. Part II: Biotechnological production and future applications of xylitol. Appl. Microbiol. Biotechnol. 2007, 74, 273. [Google Scholar] [CrossRef]
- Mayer, G.; Kulbe, K.D.; Nidetzky, B. Utilization of xylitol dehydrogenase in a combined microbial/enzymatic process for production of xylitol from D-glucose. In Biotechnology for Fuels and Chemicals; Humana Press: Towota, NJ, USA, 2002; pp. 577–589. [Google Scholar]
- Ortiz, M.E.; Bleckwedel, J.; Raya, R.R.; Mozzi, F. Biotechnological and in situ food production of polyols by lactic acid bacteria. Appl. Microbiol. Biotechnol. 2013, 97, 4713–4726. [Google Scholar] [CrossRef]
- Barbieri, G.; Barone, C.; Bhagat, A.; Caruso, G.; Conley, Z.R.; Parisi, S. Sweet compounds in foods: Sugar alcohols. In The Influence of Chemistry on New Foods and Traditional Products; Springer: Berlin/Heidelberg, Germany, 2014; pp. 51–59. [Google Scholar]
- Kusserow, B.; Schimpf, S.; Claus, P. Hydrogenation of glucose to sorbitol over nickel and ruthenium catalysts. Adv. Synth. Catal. 2003, 345, 289–299. [Google Scholar] [CrossRef]
- Jonas, R.; Silveira, M.M. Sorbitol can be produced not only chemically but also biotechnologically. Appl. Biochem. Biotechnol. 2004, 118, 321–336. [Google Scholar] [CrossRef]
- Ghoreishi, S.; Shahrestani, R.G. Innovative strategies for engineering mannitol production. Trends Food Sci. Technol. 2009, 20, 263–270. [Google Scholar] [CrossRef]
- Gaspar, P.; Neves, A.R.; Ramos, A.; Gasson, M.J.; Shearman, C.A.; Santos, H. Engineering Lactococcus lactis for production of mannitol: High yields from food-grade strains deficient in lactate dehydrogenase and the mannitol transport system. Appl. Environ. Microbiol. 2004, 70, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Vieille, C. Recent advances in the biological production of mannitol. Appl. Microbiol. Biotechnol. 2009, 84, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.; Movva, S.; Madras, G.; Chatterjee, K. Biodegradable galactitol based crosslinked polyesters for controlled release and bone tissue engineering. Mater. Sci. Eng. C 2017, 77, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Maltitol: Analytical determination methods, applications in the food industry, metabolism and health impacts. Int. J. Environ. Res. Public Health 2020, 17, 5227. [Google Scholar] [CrossRef]
- Hadjikinova, R.; Marudova, M. Thermal behaviour of confectionary sweeteners’ blends. Bulg. Chem. Commun 2016, 48, 446–450. [Google Scholar]
- You, Z.; Cao, H.; Gao, J.; Shin, P.H.; Day, B.W.; Wang, Y. A functionalizable polyester with free hydroxyl groups and tunable physiochemical and biological properties. Biomaterials 2010, 31, 3129–3138. [Google Scholar] [CrossRef]
- You, Z.; Bi, X.; Wang, Y. Fine control of polyester properties via epoxide ROP using monomers carrying diverse functional groups. Macromol. Biosci. 2012, 12, 822–829. [Google Scholar] [CrossRef]
- Slavko, E.; Taylor, M.S. Catalyst-controlled polycondensation of glycerol with diacyl chlorides: Linear polyesters from a trifunctional monomer. Chem. Sci. 2017, 8, 7106–7111. [Google Scholar] [CrossRef]
- Kline, B.J.; Beckman, E.J.; Russell, A.J. One-step biocatalytic synthesis of linear polyesters with pendant hydroxyl groups. J. Am. Chem. Soc. 1998, 120, 9475–9480. [Google Scholar] [CrossRef]
- Iglesias, L.E.; Fukuyama, Y.; Nonami, H.; Erra-Balsells, R.; Baldessari, A. A simple enzymatic procedure for the synthesis of a hydroxylated polyester from glycerol and adipic acid. Biotechnol. Tech. 1999, 13, 923–926. [Google Scholar] [CrossRef]
- Rao, Z.K.; Ni, H.L.; Li, Y.; Zhu, H.Y.; Liu, Y.; Hao, J.Y. Macroscopic Scaffold Control for Lipase-Catalyzed Dendritic Polyol-Polyesters. Macromol. Chem. Phys. 2019, 220, 1900048. [Google Scholar] [CrossRef]
- Zeng, F.; Yang, X.; Li, D.; Dai, L.; Zhang, X.; Lv, Y.; Wei, Z. Functionalized polyesters derived from glycerol: Selective polycondensation methods toward glycerol-based polyesters by different catalysts. J. Appl. Polym. Sci. 2020, 137, 48574. [Google Scholar] [CrossRef]
- Uyama, H.; Inada, K.; Kobayashi, S. Regioselective polymerization of divinyl sebacate and triols using lipase catalyst. Macromol. Rapid Commun. 1999, 20, 171–174. [Google Scholar] [CrossRef]
- Uyama, H.; Inada, K.; Kobayashi, S. Regioselectivity control in lipase-catalyzed polymerization of divinyl sebacate and triols. Macromol. Biosci. 2001, 1, 40–44. [Google Scholar] [CrossRef]
- Kulshrestha, A.S.; Gao, W.; Gross, R.A. Glycerol copolyesters: Control of branching and molecular weight using a lipase catalyst. Macromolecules 2005, 38, 3193–3204. [Google Scholar] [CrossRef]
- Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; St. Pourçain, C.B.; Garnett, M.C. Novel Functionalized Biodegradable Polymers for Nanoparticle Drug Delivery Systems. Biomacromolecules 2005, 6, 1885–1894. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, W.; Cai, J.; Hou, Y.; Ouyang, S.; Xie, W.; Gross, R.A. Poly (oleic diacid-co-glycerol): Comparison of polymer structure resulting from chemical and lipase catalysis. Macromolecules 2011, 44, 1977–1985. [Google Scholar] [CrossRef]
- Naolou, T.; Weiss, V.; Conrad, D.; Busse, K.; Mäder, K.; Kressler, J. Fatty acid modified poly (glycerol adipate)-Polymeric analogues of glycerides. In Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications; ACS Publications: Washington, WA, USA, 2013; pp. 39–52. [Google Scholar]
- Taresco, V.; Creasey, R.; Kennon, J.; Mantovani, G.; Alexander, C.; Burley, J.C.; Garnett, M.C. Variation in structure and properties of poly (glycerol adipate) via control of chain branching during enzymatic synthesis. Polymer 2016, 89, 41–49. [Google Scholar] [CrossRef]
- Wang, Y.; Ameer, G.A.; Sheppard, B.J.; Langer, R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Bismarck, A.; Hansen, U.; Junaid, S.; Tran, M.Q.; Harding, S.E.; Ali, N.N.; Boccaccini, A.R. Characterisation of a soft elastomer poly (glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008, 29, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Loh, X.J.; Abdul Karim, A.; Owh, C. Poly(glycerol sebacate) biomaterial: Synthesis and biomedical applications. J. Mater. Chem. B 2015, 3, 7641–7652. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, J.M.; Hollister, S.J. Tailoring the mechanical properties of 3D-designed poly(glycerol sebacate) scaffolds for cartilage applications. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater.Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 94A, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, H.; Jiang, C.; He, S. Preparation and characterization of poly (glycerol sebacate)/cellulose nanocrystals elastomeric composites. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, J.; Tan, T.; Zhang, L.; Chen, D.; Tian, W. Preparation, properties and cytotoxicity evaluation of a biodegradable polyester elastomer composite. Polym. Degrad. Stab. 2009, 94, 1427–1435. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Patel, A.; Dolatshahi-Pirouz, A.; Zhang, H.; Rangarajan, K.; Iviglia, G.; Shin, S.-R.; Hussain, M.A.; Khademhosseini, A. Elastomeric nanocomposite scaffolds made from poly(glycerol sebacate) chemically crosslinked with carbon nanotubes. Biomater. Sci. 2015, 3, 46–58. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, Y.; Du, Y.; Chen, X.; Lei, B.; Xue, Y.; Ma, P.X. A highly bioactive and biodegradable poly (glycerol sebacate)–silica glass hybrid elastomer with tailored mechanical properties for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 3222–3233. [Google Scholar] [CrossRef]
- Gao, J.; Crapo, P.M.; Wang, Y. Macroporous elastomeric scaffolds with extensive micropores for soft tissue engineering. Tissue Eng. 2006, 12, 917–925. [Google Scholar] [CrossRef]
- Aydin, H.M.; Salimi, K.; Rzayev, Z.M.O.; Pişkin, E. Microwave-assisted rapid synthesis of poly(glycerol-sebacate) elastomers. Biomater. Sci. 2013, 1, 503. [Google Scholar] [CrossRef]
- Lang, K.; Bhattacharya, S.; Ning, Z.; Sánchez-Leija, R.J.; Bramson, M.T.K.; Centore, R.; Corr, D.T.; Linhardt, R.J.; Gross, R.A. Enzymatic Polymerization of Poly(glycerol-1,8-octanediol-sebacate): Versatile Poly(glycerol sebacate) Analogues that Form Monocomponent Biodegradable Fiber Scaffolds. Biomacromolecules 2020. [Google Scholar] [CrossRef]
- Perin, G.B.; Felisberti, M.I. Enzymatic Synthesis of Poly(glycerol sebacate): Kinetics, Chain Growth, and Branching Behavior. Macromolecules 2020. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.R.; Hong, S.-P.; Kong, B.; Choi, I.S. Polycondensation of sebacic acid with primary and secondary hydroxyl groups containing diols catalyzed by Candida antarctica lipase B. Synth. Commun. 2012, 42, 3504–3512. [Google Scholar] [CrossRef]
- Cha, H.-J.; Park, J.-B.; Park, S. Esterification of secondary alcohols and multi-hydroxyl compounds by Candida antarctica lipase B and subtilisin. Biotechnol. Bioprocess Eng. 2019, 24, 41–47. [Google Scholar] [CrossRef]
- Schmid, R.D.; Verger, R. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Li, X.; Hong, A.T.L.; Naskar, N.; Chung, H.-J. Criteria for Quick and Consistent Synthesis of Poly(glycerol sebacate) for Tailored Mechanical Properties. Biomacromolecules 2015, 16, 1525–1533. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, R.; Chen, D.; Zhang, L.; Tian, W. Nanosilica filled poly (glycerol-sebacate-citrate) elastomers with improved mechanical properties, adjustable degradability, and better biocompatibility. J. Appl. Polym. Sci. 2012, 123, 1612–1620. [Google Scholar] [CrossRef]
- Nijst, C.L.E.; Bruggeman, J.P.; Karp, J.M.; Ferreira, L.; Zumbuehl, A.; Bettinger, C.J.; Langer, R. Synthesis and Characterization of Photocurable Elastomers from Poly(glycerol-co-sebacate). Biomacromolecules 2007, 8, 3067–3073. [Google Scholar] [CrossRef]
- Swainson, S.M.; Styliari, I.D.; Taresco, V.; & Garnett, M.C. Poly (glycerol adipate)(PGA), an enzymatically synthesized functionalizable polyester and versatile drug delivery carrier: A literature update. Polymers 2019, 11(10), 1561. [Google Scholar] [CrossRef]
- Naolou, T.; Jbeily, M.; Scholtysek, P.; Kressler, J. Synthesis and characterization of stearoyl modified poly (glycerol adipate) containing ATRP initiator on its backbone. Adv. Mater. Res. 2013, 812, 1–11. [Google Scholar] [CrossRef]
- Navarro, L.; Ceaglio, N.; Rintoul, I. Structure and properties of biocompatible poly (glycerol adipate) elastomers modified with ethylene glycol. Polym. J. 2017, 49, 625–632. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Smith, P.B. Thermal degradation of glycerol/adipic acid hyperbranched poly (ester) s containing either hydroxyl or carboxyl end-groups. J. Therm. Anal. Calorim. 2015, 122, 1221–1229. [Google Scholar] [CrossRef]
- Weiss, V.M.; Naolou, T.; Groth, T.; Kressler, J.; Mäder, K. In vitro toxicity of stearoyl-poly (glycerol adipate) nanoparticles. J. Appl. Biomater. Funct. Mater. 2012, 10, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Kallinteri, P.; Higgins, S.; Hutcheon, G.A.; Garnett, M.C. Drug incorporation and release of water soluble drugs from novel functionalised poly (glycerol adipate) nanoparticles. J. Control. Release 2008, 125, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Abo-zeid, Y.; Mantovani, G.; Irving, W.L.; Garnett, M.C. Synthesis of nucleoside-boronic esters hydrophobic pro-drugs: A possible route to improve hydrophilic nucleoside drug loading into polymer nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 46, 354–364. [Google Scholar] [CrossRef]
- Meng, W.; Parker, T.; Kallinteri, P.; Walker, D.; Higgins, S.; Hutcheon, G.A.; Garnett, M.C. Uptake and metabolism of novel biodegradable poly (glycerol-adipate) nanoparticles in DAOY monolayer. J. Control. Release 2006, 116, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Abo-zeid, Y.; Urbanowicz, R.A.; Thomson, B.J.; Irving, W.L.; Tarr, A.W.; Garnett, M.C. Enhanced nanoparticle uptake into virus infected cells: Could nanoparticles be useful in antiviral therapy? Int. J. Pharm. 2018, 547, 572–581. [Google Scholar] [CrossRef]
- Weiss, V.M.; Naolou, T.; Hause, G.; Kuntsche, J.; Kressler, J.; Mäder, K. Poly (glycerol adipate)-fatty acid esters as versatile nanocarriers: From nanocubes over ellipsoids to nanospheres. J. Control. Release 2012, 158, 156–164. [Google Scholar] [CrossRef]
- Orafaei, H.; Kallinteri, P.; Huggins, S.; Hutcheon, G.; Pourcain, C. Novel poly (glycerol-adipate) polymers used for nanoparticle making: A study of surface free energy. Iran. J. Pharm. Res. 2008, 7, 11–19. [Google Scholar]
- Taresco, V.; Suksiriworapong, J.; Creasey, R.; Burley, J.C.; Mantovani, G.; Alexander, C.; Treacher, K.; Booth, J.; Garnett, M.C. Properties of acyl modified poly (glycerol-adipate) comb-like polymers and their self-assembly into nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 3267–3278. [Google Scholar] [CrossRef]
- Carnahan, M.A.; Grinstaff, M.W. Synthesis and Characterization of Poly(glycerol−succinic acid) Dendrimers. Macromolecules 2001, 34, 7648–7655. [Google Scholar] [CrossRef]
- Carnahan, M.A.; Grinstaff, M.W. Synthesis of generational polyester dendrimers derived from glycerol and succinic or adipic acid. Macromolecules 2006, 39, 609–616. [Google Scholar] [CrossRef]
- Agach, M.; Delbaere, S.; Marinkovic, S.; Estrine, B.; Nardello-Rataj, V. Characterization, stability and ecotoxic properties of readily biodegradable branched oligoesters based on bio-sourced succinic acid and glycerol. Polym. Degrad. Stab. 2012, 97, 1956–1963. [Google Scholar] [CrossRef]
- Wyatt, V.T.; Nuñez, A.; Foglia, T.A.; Marmer, W.N. Synthesis of hyperbranched P poly (glycerol-diacid) oligomers. J. Am. Oil Chem. Soc. 2006, 83, 1033–1039. [Google Scholar] [CrossRef]
- Baharu, M.N.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B. Synthesis and characterization of polyesters derived from glycerol, azelaic acid, and succinic acid. Green Chem. Lett. Rev. 2015, 8, 31–38. [Google Scholar] [CrossRef]
- Wyatt, V.T.; Strahan, G.D.; Nuñez, A. The Lewis Acid-Catalyzed Synthesis of Hyperbranched Oligo (glycerol-diacid) s in Aprotic Polar Media. J. Am. Oil Chem. Soc. 2010, 87, 1359–1369. [Google Scholar] [CrossRef]
- Wyatt, V.T.; Yadav, M.P.; Latona, N.; Liu, C.-K. Thermal and mechanical properties of glycerol-based polymer films infused with plant cell wall polysaccharides. J. Biobased Mater. Bioenergy 2013, 7, 348–356. [Google Scholar] [CrossRef]
- Unnisa, C.N.; Chitra, S.; Selvasekarapandian, S.; Monisha, S.; Devi, G.N.; Moniha, V.; Hema, M. Development of poly (glycerol suberate) polyester (PGS)–PVA blend polymer electrolytes with NH 4 SCN and its application. Ionics 2018, 24, 1979–1993. [Google Scholar] [CrossRef]
- Kadhum, A.A.H.; Baharu, M.N.; Mahmood, M.H. Elastic polyesters from glycerol and azelaic acid. Adv. Mater. Res. 2011, 233–235, 2571–2575. [Google Scholar] [CrossRef]
- Barrett, D.G.; Luo, W.; Yousaf, M.N. Aliphatic polyester elastomers derived from erythritol and α,ω-diacids. Polym. Chem. 2010, 1, 296. [Google Scholar] [CrossRef]
- Dasgupta, Q.; Chatterjee, K.; Madras, G. Combinatorial Approach to Develop Tailored Biodegradable Poly(xylitol dicarboxylate) Polyesters. Biomacromolecules 2014, 15, 4302–4313. [Google Scholar] [CrossRef]
- Li, Y.; Huang, W.; Cook, W.D.; Chen, Q. A comparative study on poly (xylitol sebacate) and poly (glycerol sebacate): Mechanical properties, biodegradation and cytocompatibility. Biomed. Mater. 2013, 8, 035006. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Kumar, A.; Gao, W.; Gross, R.; Kennedy, S.B.; Washburn, N.R.; Amis, E.J.; Elliott, J.T. Biocompatibility of sorbitol-containing polyesters. Part I: Synthesis, surface analysis and cell response in vitro. Biomaterials 2004, 25, 4195–4201. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Kulshrestha, A.S.; Gao, W.; Gross, R.A.; Baiardo, M.; Scandola, M. Physical characterization of sorbitol or glycerol containing aliphatic copolyesters synthesized by lipase-catalyzed polymerization. Macromolecules 2003, 36, 9804–9808. [Google Scholar] [CrossRef]
- Bruggeman, J.P.; De Bruin, B.-J.; Bettinger, C.J.; Langer, R. Biodegradable poly(polyol sebacate) polymers. Biomaterials 2008, 29, 4726–4735. [Google Scholar] [CrossRef]
- Pasupuleti, S.; Madras, G. Synthesis and degradation of sorbitol-based polymers. J. Appl. Polym. Sci. 2011, 121, 2861–2869. [Google Scholar] [CrossRef]
- Kamaruzaman, M.R.; Chin, S.Y.; Pui, E.C.L.; Prasetiawan, H.; Azizan, N. Synthesis of Biobased Polyester Polyol through Esterification of Sorbitol with Azelaic Acid Catalyzed by Tin(II) Oxide: A Kinetic Modeling Study. Ind. Eng. Chem. Res. 2019, 58, 510–516. [Google Scholar] [CrossRef]
- Gustini, L.; Lavilla, C.; Janssen, W.; Martínez de Ilarduya Sáez de Asteasu, D.A.; Muñoz Guerra, S.; Koning, C. Green and selective polycondensation methods toward linear sorbitol-based polyesters: Enzymatic versus organic and metal-based catalysis. ChemSusChem (Weinheim. Print) 2016, 9, 2250–2260. [Google Scholar] [CrossRef]
- Natarajan, J.; Madras, G.; Chatterjee, K. Maltitol-based biodegradable polyesters with tailored degradation and controlled release for bone regeneration. RSC Adv. 2016, 6, 40539–40551. [Google Scholar] [CrossRef]
- Barrett, D.G.; Yousaf, M.N. Design and applications of biodegradable polyester tissue scaffolds based on endogenous monomers found in human metabolism. Molecules 2009, 14, 4022–4050. [Google Scholar] [CrossRef]
- Tillet, G.; Boutevin, B.; Ameduri, B. Chemical reactions of polymer cross-linking and post-cross-linking at room and medium temperature. Prog. Polym. Sci. 2011, 36, 191–217. [Google Scholar] [CrossRef]
- Pellis, A.; Herrero Acero, E.; Ferrario, V.; Ribitsch, D.; Guebitz, G.M.; Gardossi, L. The Closure of the Cycle: Enzymatic Synthesis and Functionalization of Bio-Based Polyesters. Trends Biotechnol. 2016, 34, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, J.P.; Bettinger, C.J.; Nijst, C.L.; Kohane, D.S.; Langer, R. Biodegradable xylitol-based polymers. Adv. Mater. 2008, 20, 1922–1927. [Google Scholar] [CrossRef]
- Valerio, O.; Misra, M.; Mohanty, A.K. Poly (glycerol-co-diacids) polyesters: From glycerol biorefinery to sustainable engineering applications, a review. ACS Sustain. Chem. Eng. 2018, 6, 5681–5693. [Google Scholar] [CrossRef]
- Conejero-García, Á.; Gimeno, H.R.; Sáez, Y.M.; Vilariño-Feltrer, G.; Ortuño-Lizarán, I.; Vallés-Lluch, A. Correlating synthesis parameters with physicochemical properties of poly (glycerol sebacate). Eur. Polym. J. 2017, 87, 406–419. [Google Scholar] [CrossRef]
- Van Bochove, B.; Grijpma, D.W. Photo-crosslinked synthetic biodegradable polymer networks for biomedical applications. J. Biomater. Sci. Polym. Ed. 2019, 30, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.G.; Merkel, T.J.; Luft, J.C.; Yousaf, M.N. One-step syntheses of photocurable polyesters based on a renewable resource. Macromolecules 2010, 43, 9660–9667. [Google Scholar] [CrossRef]
- Nebioglu, A.; Soucek, M.D. Reaction kinetics and network characterization of UV-curing polyester acrylate inorganic/organic hybrids. Eur. Polym. J. 2007, 43, 3325–3336. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Padera, R.F.; Burdick, J.A. Biodegradable and radically polymerized elastomers with enhanced processing capabilities. Biomed. Mater. 2008, 3, 034104. [Google Scholar] [CrossRef]
- Ifkovits, J.L.; Devlin, J.J.; Eng, G.; Martens, T.P.; Vunjak-Novakovic, G.; Burdick, J.A. Biodegradable fibrous scaffolds with tunable properties formed from photo-cross-linkable poly (glycerol sebacate). ACS Appl. Mater. Interfaces 2009, 1, 1878–1886. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Highley, C.B.; Ouyang, L.; Burdick, J.A. 3D printing of photocurable poly (glycerol sebacate) elastomers. Biofabrication 2016, 8, 045004. [Google Scholar] [CrossRef]
- Gerecht, S.; Townsend, S.A.; Pressler, H.; Zhu, H.; Nijst, C.L.; Bruggeman, J.P.; Nichol, J.W.; Langer, R. A porous photocurable elastomer for cell encapsulation and culture. Biomaterials 2007, 28, 4826–4835. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lei, D.; Liu, Z.; Chen, S.; Sun, L.; Lv, Z.; Huang, P.; Jiang, Z.; You, Z. A poly (glycerol sebacate) based photo/thermo dual curable biodegradable and biocompatible polymer for biomedical applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Pashneh-Tala, S.; Owen, R.; Bahmaee, H.; Rekštytė, S.; Malinauskas, M.; Claeyssens, F. Synthesis, characterization and 3D micro-structuring via 2-photon polymerization of poly (glycerol sebacate)-methacrylate—an elastomeric degradable polymer. Front. Phys. 2018, 6, 41. [Google Scholar] [CrossRef]
- Wu, Y.-L.; D’Amato, A.R.; Yan, A.M.; Wang, R.Q.; Ding, X.; Wang, Y. Three-Dimensional Printing of Poly (glycerol sebacate) Acrylate Scaffolds via Digital Light Processing. ACS Appl. Bio Mater. 2020. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Ouyang, L.; Highley, C.B.; Burdick, J.A. Norbornene-modified poly (glycerol sebacate) as a photocurable and biodegradable elastomer. Polym. Chem. 2017, 8, 5091–5099. [Google Scholar] [CrossRef]
- Williams, C.G.; Malik, A.N.; Kim, T.K.; Manson, P.N.; Elisseeff, J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials 2005, 26, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Greim, H.; Ahlers, J.; Bias, R.; Broecker, B.; Hollander, H.; Gelbke, H.-P.; Jacobi, S.; Klimisch, H.-J.; Mangelsdorf, I.; Mayr, W. Assessment of structurally related chemicals: Toxicity and ecotoxicity of acrylic acid and acrylic acid alkyl esters (acrylates), methacrylic acid and methacrylic acid alkyl esters (methacrylates). Chemosphere 1995, 31, 2637–2659. [Google Scholar] [CrossRef]
- Zondlo, F.M. Final report on the safety assessment of Acrylates Copolymer and 33 related cosmetic ingredients. Int. J. Toxicol. 2002, 21, 1. [Google Scholar]
- Freidig, A.P.; Verhaar, H.J.; Hermens, J.L. Comparing the potency of chemicals with multiple modes of action in aquatic toxicology: Acute toxicity due to narcosis versus reactive toxicity of acrylic compounds. Environ. Sci. Technol. 1999, 33, 3038–3043. [Google Scholar] [CrossRef]
- Fertier, L.; Koleilat, H.; Stemmelen, M.; Giani, O.; Joly-Duhamel, C.; Lapinte, V.; Robin, J.-J. The use of renewable feedstock in UV-curable materials–A new age for polymers and green chemistry. Prog. Polym. Sci. 2013, 38, 932–962. [Google Scholar] [CrossRef]
- Bednarek, M.; Kubisa, P. Reversible networks of degradable polyesters containing weak covalent bonds. Polym. Chem. 2019, 10, 1848–1872. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Lima, M.S.; Sousa, A.F.; Silvestre, A.J.; Coelho, J.F.; Serra, A.C. Cinnamic acid derivatives as promising building blocks for advanced polymers: Synthesis, properties and applications. Polym. Chem. 2019, 10, 1696–1723. [Google Scholar] [CrossRef]
- Zhu, C.; Kustra, S.R.; Bettinger, C.J. Photocrosslinkable biodegradable elastomers based on cinnamate-functionalized polyesters. Acta Biomater. 2013, 9, 7362–7370. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.N.; Ouyang, B.; Sundback, C.A.; Lang, N.; Friehs, I.; Mureli, S.; Pomerantseva, I.; McFadden, J.; Mochel, M.C.; Mwizerwa, O. A highly tunable biocompatible and multifunctional biodegradable elastomer. Adv. Mater. 2013, 25, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Frydrych, M.; Chen, B. Fabrication, structure and properties of three-dimensional biodegradable poly (glycerol sebacate urethane) scaffolds. Polymer 2017, 122, 159–168. [Google Scholar] [CrossRef]
- Krook, N.M.; LeBlon, C.; Jedlicka, S.S. In Vitro Examination of Poly (glycerol sebacate) Degradation Kinetics: Effects of Porosity and Cure Temperature. MRS Online Proc. Library Arch. 2014, 1621, 87–92. [Google Scholar] [CrossRef]
- Li, Y. Developing Poly (Polyol Sebacate)-Based Elastomeric Biomaterials for Soft Tissue Engineering. Ph.D. Thesis, Monash University, Melbourne, Australia, February 2017. [Google Scholar]
- Bamford, C.H. Degradation of polymers. Compr. Chem. Kinet. 1975, 14, 37. [Google Scholar]
- Grassie, N. Developments in Polymer Degradation—7; Springer Science & Business Media: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Mueller, R.-J. Biological degradation of synthetic polyesters—Enzymes as potential catalysts for polyester recycling. Process Biochem. 2006, 41, 2124–2128. [Google Scholar] [CrossRef]
- Li, Y.; Thouas, G.A.; Shi, H.; Chen, Q. Enzymatic and oxidative degradation of poly (polyol sebacate). J. Biomater. Appl. 2014, 28, 1138–1150. [Google Scholar] [CrossRef]
- Chen, D.; Bei, J.; Wang, S. Polycaprolactone microparticles and their biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Suzuki, T. Hydrolysis of polyesters by lipases. Nature 1977, 270, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Herzog, K.; Müller, R.-J.; Deckwer, W.-D. Mechanism and kinetics of the enzymatic hydrolysis of polyester nanoparticles by lipases. Polym. Degrad. Stab. 2006, 91, 2486–2498. [Google Scholar] [CrossRef]
- Marten, E.; Müller, R.-J.; Deckwer, W.-D. Studies on the enzymatic hydrolysis of polyesters I. Low molecular mass model esters and aliphatic polyesters. Polym. Degrad. Stab. 2003, 80, 485–501. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
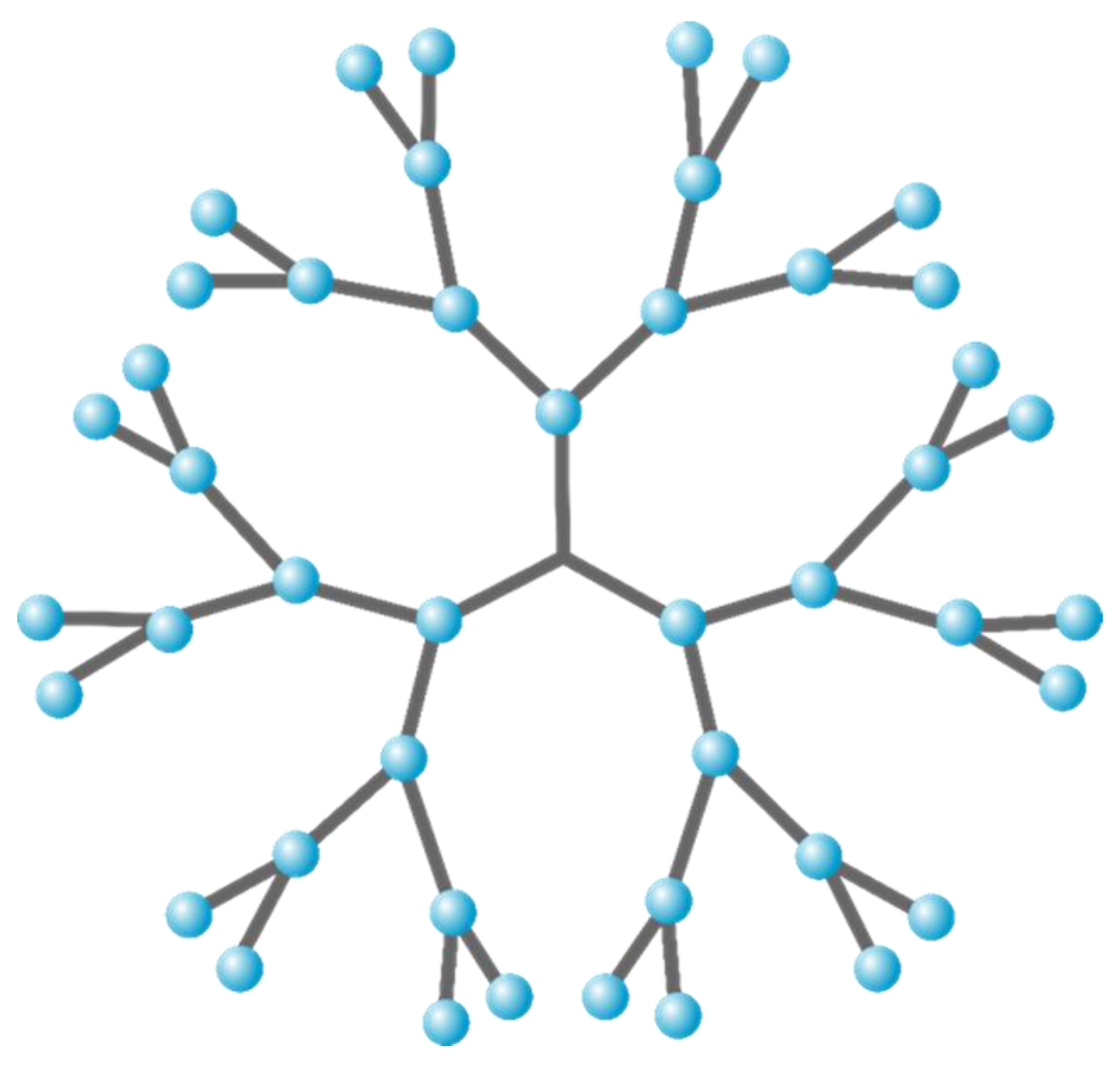

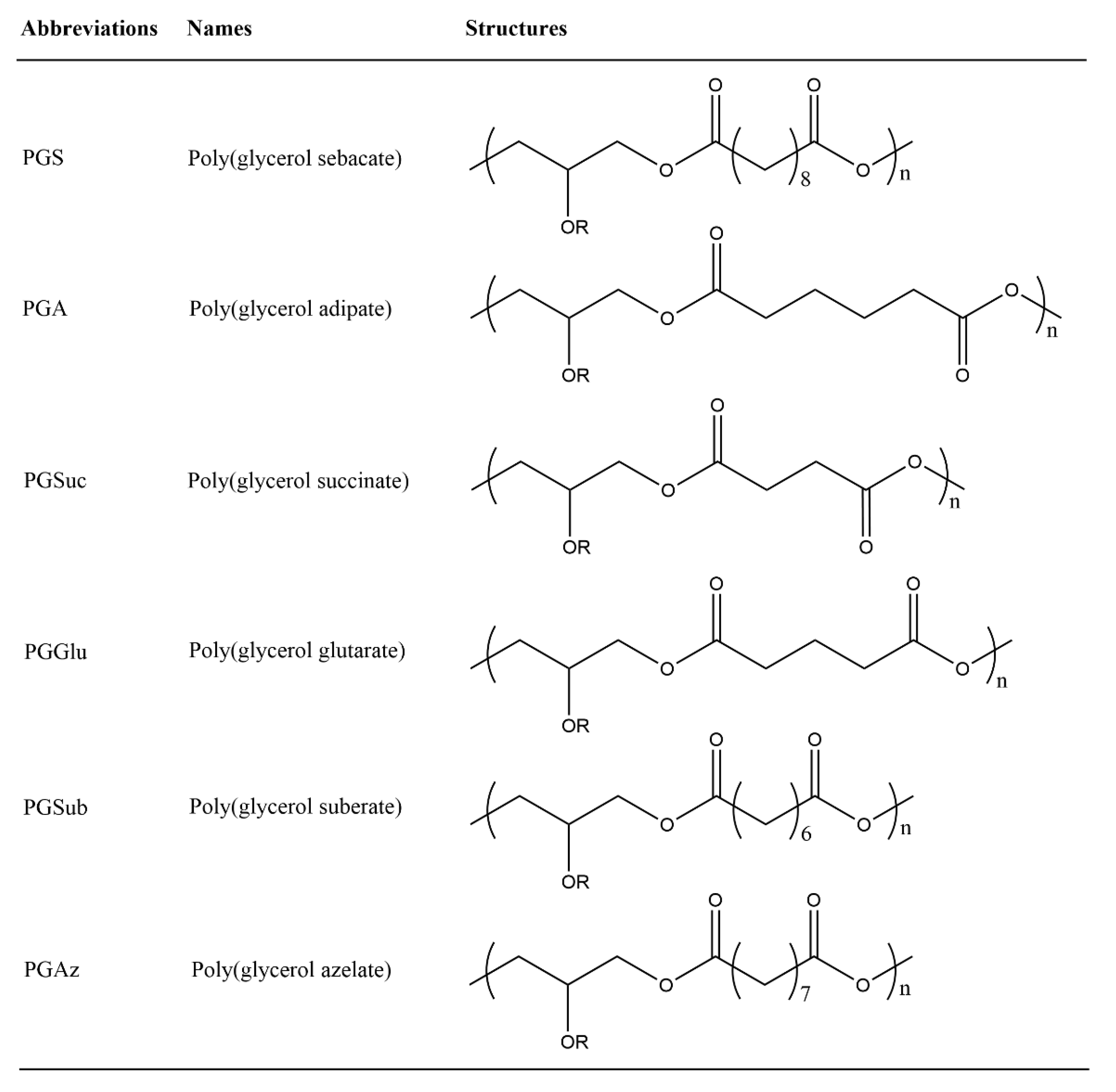
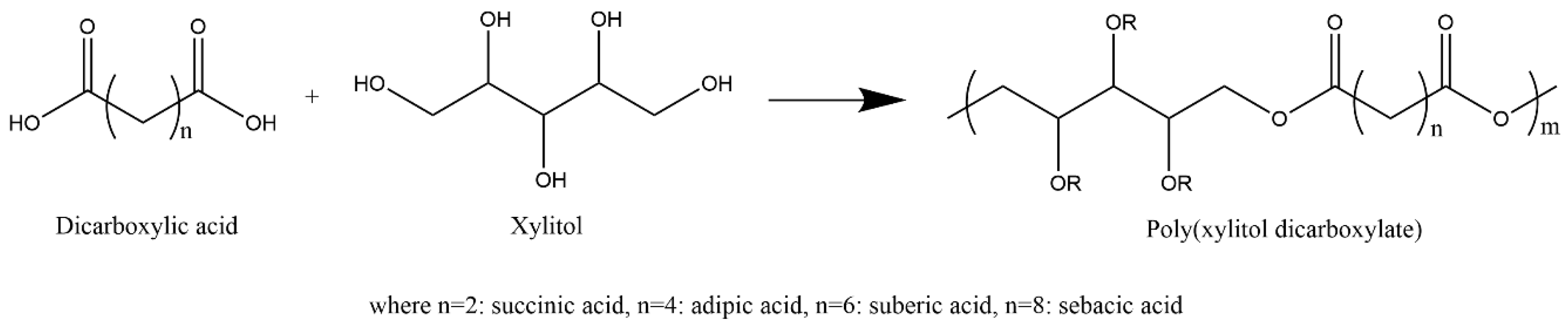
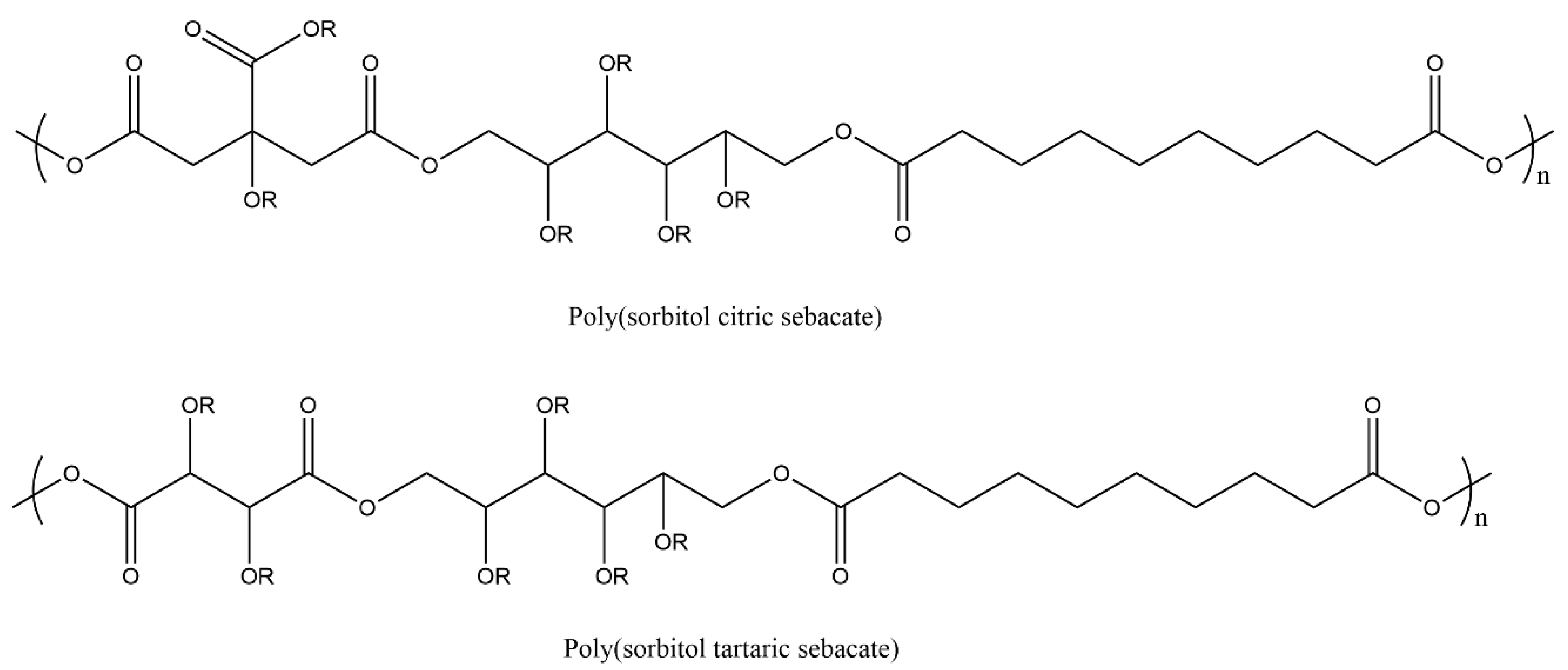
| Prepolymer | Mn a/g mol−1 | Tg(Tm) b/ °C | Đ a |
|---|---|---|---|
| Poly(erythritol glutarate) | 710 | −7.0 | 1.4 |
| Poly(erythritol adipate) | 810 | −15.7 | 1.1 |
| Poly(erythritol pimelate) | 790 | −18.1 | 1.4 |
| Poly(erythritol suberate) | 860 | −21.4 | 1.2 |
| Poly(erythritol azelate) | 820 | −23.6 | 1.5 |
| Poly(erythritol sebacate) | 1020 | −36.9 (60.2) | 1.6 |
| Poly(erythritol dodecanedioate) | 1470 | −41.5 (63.4) | 1.9 |
| Poly(erythritol tetradecanedioate) | 1450 | −46.8 (66.0) | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, K.; Sánchez-Leija, R.J.; Gross, R.A.; Linhardt, R.J. Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers 2020, 12, 2969. https://doi.org/10.3390/polym12122969
Lang K, Sánchez-Leija RJ, Gross RA, Linhardt RJ. Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers. 2020; 12(12):2969. https://doi.org/10.3390/polym12122969
Chicago/Turabian StyleLang, Kening, Regina J. Sánchez-Leija, Richard A. Gross, and Robert J. Linhardt. 2020. "Review on the Impact of Polyols on the Properties of Bio-Based Polyesters" Polymers 12, no. 12: 2969. https://doi.org/10.3390/polym12122969
APA StyleLang, K., Sánchez-Leija, R. J., Gross, R. A., & Linhardt, R. J. (2020). Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers, 12(12), 2969. https://doi.org/10.3390/polym12122969








