Zhurkov’s Stress-Driven Fracture as a Driving Force of the Microcrystalline Cellulose Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cellulose Processing
2.2. Analytical Approaches
3. Results
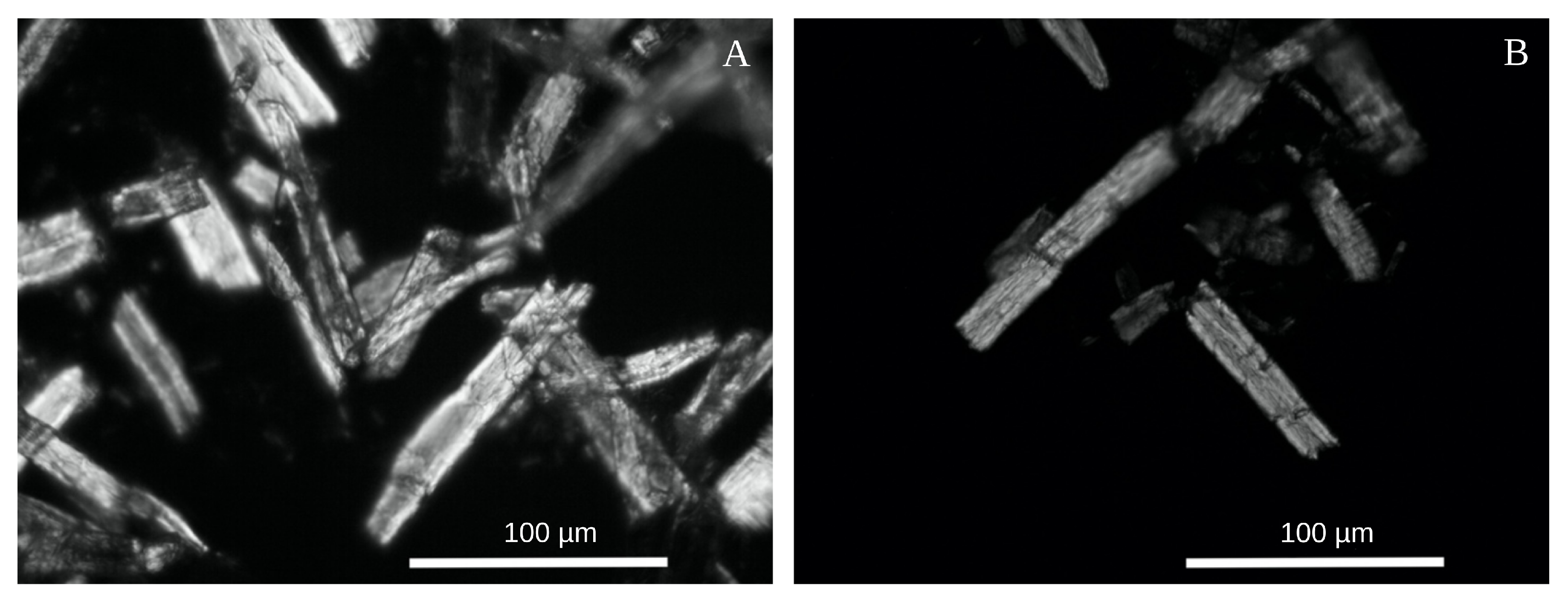
3.1. Characterization of MCC
3.2. Nanocellulose
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Gharehkhani, S.; Sadeghinezhad, E.; Kazi, S.N.; Yarmand, H.; Badarudin, A.; Safaei, M.R.; Zubir, M.N.M. Basic Effects of Pulp Refining on Fiber Properties—A Review. Carbohydr. Polym. 2015, 115, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Braconnot, H. De la Transformation de Plusieurs Substances Végétales en un Principe Nouveau. Ann. Chim. Phys. 1833, 52, 290–294. [Google Scholar]
- Saunders, C.; Taylor, L. A Review of the Synthesis, Chemistry and Analysis of Nitrocellulose. J. Energ. Mat. 1990, 8, 149–203. [Google Scholar] [CrossRef]
- Martina, B.; Kateřina, K.; Miloslava, R.; Jan, G.; Ruta, M. Oxycellulose: Significant Characteristics in Relation to its Pharmaceutical and Medical Applications. Adv. Polym. Technol. 2009, 28, 199–208. [Google Scholar] [CrossRef]
- Cross, C.; Bevan, E. Improvements in Dissolving Cellulose and Allied Compounds. Br. Pat. Appl. 1892, 8, 700. [Google Scholar]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A Critical Review of Manufacturing Processes Used in Regenerated Cellulosic Fibres: Viscose, Cellulose Acetate, Cuprammonium, LiCl/DMAc, Ionic Liquids, and NMMO Based Lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Kuga, S.; Wu, M. Mechanochemistry of Cellulose. Cellulose 2019, 26, 215–225. [Google Scholar] [CrossRef]
- Trache, D.; Khimeche, K.; Mezroua, A.; Benziane, M. Physicochemical Properties of Microcrystalline Nitrocellulose from Alfa Grass Fibres and its Thermal Stability. J. Therm. Anal. Calorim. 2016, 124, 1485–1496. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and Nutritional Aspects of Microcrystalline Cellulose in Food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef]
- Kamel, S.; Ali, N.; Jahangir, K.; Shah, S.; El-Gendy, A. Pharmaceutical Significance of Cellulose: A review. EXPRESS Polym. Lett. 2008, 2, 758–778. [Google Scholar] [CrossRef]
- Nogi, M.; Iwamoto, S.; Nakagaito, A.N.; Yano, H. Optically Transparent Nanofiber Paper. Adv. Mater. 2009, 21, 1595–1598. [Google Scholar] [CrossRef]
- Zlenko, D.V.; Nikolsky, S.N.; Vedenkin, A.S.; Politenkova, G.G.; Skoblin, A.A.; Melnikov, V.P.; Mikhaleva, M.G.; Stovbun, S.V. Twisting of Fibers Balancing the Gel–Sol Transition in Cellulose Aqueous Suspensions. Polymers 2019, 11, 873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-Based Conductive Materials and Their Emerging Applications in Energy Devices—A Review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent Developments on Nanocellulose Reinforced Polymer Nanocomposites: A Review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Trache, D.; Donnot, A.; Khimeche, K.; Benelmir, R.; Brosse, N. Physico-Chemical Properties and Thermal Stability of Microcrystalline Cellulose Isolated from Alfa Fibres. Carbohydr. Polym. 2014, 104, 223–230. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Hassan, M.L. Physical and Mechanical Properties of Microcrystalline Cellulose Prepared from Agricultural Residues. Carbohydr. Polym. 2007, 67, 1–10. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Derradji, M.; Bessa, W. Ecofriendly Isolation and Characterization of Microcrystalline Cellulose from Giant Reed Using Various Acidic Media. Cellulose 2019, 26, 7635–7651. [Google Scholar] [CrossRef]
- Vanhatalo, K.M.; Dahl, O.P. Effect of Mild Acid Hydrolysis Parameters on Properties of Microcrystalline Cellulose. BioResources 2014, 9, 4729–4740. [Google Scholar] [CrossRef]
- Leppänen, K.; Andersson, S.; Torkkeli, M.; Knaapila, M.; Kotelnikova, N.; Serimaa, R. Structure of Cellulose and Microcrystalline Cellulose from Various Wood Species, Cotton and Flax Studied by X-ray Scattering. Cellulose 2009, 16, 999–1015. [Google Scholar] [CrossRef]
- Beroual, M.; Boumaza, L.; Mehelli, O.; Trache, D.; Tarchoun, A.F.; Khimeche, K. Physicochemical Properties and Thermal Stability of Microcrystalline Cellulose Isolated from Esparto Grass Using Different Delignification Approaches. J. Polym. Environ. 2020. [Google Scholar] [CrossRef]
- Beroual, M.; Trache, D.; Mehelli, O.; Boumaza, L.; Tarchoun, A.F.; Derradji, M.; Khimeche, K. Effect of the Delignification Process on the Physicochemical Properties and Thermal Stability of Microcrystalline Cellulose Extracted from Date Palm Fronds. Waste Biomass Valoriz. 2020. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose Nanomaterials Review: Structure, Properties and Nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Qing, Y.; Sabo, R.; Zhu, J.; Agarwal, U.; Cai, Z.; Wu, Y. A Comparative Study of Cellulose Nanofibrils Disintegrated via Multiple Processing Approaches. Carbohydr. Polym. 2013, 97, 226–234. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and Modification of Nanofibrillated Cellulose Using Various Mechanical Processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the Isolation of Nanocrystals from Microcrystalline Cellulose by Acid Hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar] [CrossRef]
- Zhurkov, S.; Korsukov, V. Atomic Mechanism of Fracture of Solid Polymers. J. Polym. Sci. Polym. Phys. Ed. 1974, 12, 385–398. [Google Scholar] [CrossRef] [Green Version]
- Zhurkov, S. Kinetic Concept of the Strength of Solids. Int. J. Fract. 1984, 26, 295–307. [Google Scholar] [CrossRef]
- Zhurkov, S.; Kuksenko, V. The Micromechanics of Polymer Fracture. Int. J. Fract. 1975, 11, 629–639. [Google Scholar] [CrossRef]
- Suhir, E. Three-Step Concept (TSC) in Modeling Microelectronics Reliability (MR): Boltzmann-Arrhenius-Zhurkov (BAZ) Probabilistic Physics-of-Failure Equation Sandwiched between Two Statistical Models. Microelectron. Reliab. 2014, 54, 2594–2603. [Google Scholar] [CrossRef]
- Stovbun, S.V.; Lomakin, S.; Shchegolikhin, A.; Skoblin, A.; Melnikov, V. Role of Structural Stresses in the Thermodestruction of Supercoiled Cellulose Macromolecules after Nitration. Russ. J. Phys. Chem. B 2018, 12, 36–45. [Google Scholar] [CrossRef]
- Satyamurthy, P.; Vigneshwaran, N. A Novel Process for Synthesis of Spherical Nanocellulose by Controlled Hydrolysis of Microcrystalline Cellulose Using Anaerobic Microbial Consortium. Enzym. Microb. Technol. 2013, 52, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L.C. Morphological, Physiochemical and Thermal Properties of Microcrystalline Cellulose (MCC) Extracted from Bamboo Fiber. Molecules 2020, 25, 2824. [Google Scholar] [CrossRef] [PubMed]
- Kuthi, F.A.A.; Norzali, N.R.A.; Badri, K.H. Thermal Characteristics of Microcrystalline Cellulose from Oil Palm Biomass. Malays. J. Anal. Sci. 2016, 20, 1112–1122. [Google Scholar]
- Hult, E.; Larsson, P.; Iversen, T. Cellulose Fibril Aggregation – an Inherent Property of Kraft Pulps. Polymer 2001, 42, 3309–3314. [Google Scholar] [CrossRef]
- Jarvis, M. Cellulose Stacks Up. Nature 2003, 426, 611–612. [Google Scholar] [CrossRef]
- Newman, R. Estimation of the Lateral Dimensions of Cellulose Crystallites Using 13C NMR Signal Strengths. Sol. St. Nucl. Magn. Reson. 1999, 15, 21–29. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J. Excellent Chemical and Material Cellulose from Tunicates: Diversity in Cellulose Production Yield and Chemical and Morphological Structures from Different Tunicate Species. Cellulose 2014, 21, 3427–3441. [Google Scholar] [CrossRef]
- Fernandes, A.; Thomas, L.; Altaner, C.; Callow, P.; Forsyth, V.; Apperley, D.; Kennedy, C.; Jarvis, M. Nanostructure of Cellulose Microfibrils in Spruce Wood. Proc. Natl. Acad. Sci. USA 2011, 108, E1195–E1203. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Shklyaev, O.; Nili, A.; Mohamed, M.; Kubicki, J.; Crespi, V.; Zhong, L. Cellulose Microfibril Twist, Mechanics, and Implication for Cellulose Biosynthesis. J. Phys. Chem. A 2013, 117, 2580–2589. [Google Scholar] [CrossRef]
- O’Sullivan, A.C. Cellulose: The Structure Slowly Unravels. Cellulose 1997, 4, 173–207. [Google Scholar]
- Hanley, S.J.; Revol, J.F.; Godbout, L.; Gray, D.G. Atomic Force Microscopy and Transmission Electron Microscopy of Cellulose from Micrasterias Denticulata; Evid. A Chiral Helical Microfibril Twist. Cellulose 1997, 4, 209–220. [Google Scholar] [CrossRef]
- Hanley, S.J.; Giasson, J.; Revol, J.F.; Gray, D.G. Atomic Force Microscopy of Cellulose Microfibrils: Comparison with Transmission Electron Microscopy. Polymer 1992, 33, 4639–4642. [Google Scholar] [CrossRef]
- Usachev, S.V.; Zlenko, D.V.; Nagornova, I.V.; Koverzanova, E.V.; Mikhaleva, M.G.; Vedenkin, A.S.; Vtyurina, D.N.; Skoblin, A.A.; Nikolsky, S.N.; Politenkova, G.G.; et al. Structure and Properties of Helical Fibers Spun from Cellulose Solutions in [Bmim]Cl. Carbohydr. Polym. 2020, 235, 115866. [Google Scholar] [CrossRef]
- Nikolsky, S.N.; Zlenko, D.V.; Melnikov, V.P.; Stovbun, S.V. The Fibrils Untwisting Limits the Rate of Cellulose Nitration Process. Carbohydr. Polym. 2019, 204, 232–237. [Google Scholar] [CrossRef]
- Tverdislov, V.; Malyshko, E.; Il’chenko, S.; Zhulyabina, O.; Yakovenko, L. A Periodic System of Chiral Structures in Molecular Biology. Biophysics 2017, 62, 331–341. [Google Scholar] [CrossRef]
- Malyshko, E.; Murtazina, A.; Tverdislov, V. Chirality as a Physical Basis of Hierarchical Periodization of Biomacromolecular Structures. Biophysics 2020, 65, 181–185. [Google Scholar] [CrossRef]
- Tverdislov, V.A.; Malyshko, E.V. Chiral Dualism as an Instrument of Hierarchical Structure Formation in Molecular Biology. Symmetry 2020, 12, 587. [Google Scholar] [CrossRef] [Green Version]
- Stovbun, S.V.; Nikolsky, S.N.; Melnikov, V.P.; Mikhaleva, M.G.; Litvin, Y.; Shchegolikhin, A.; Zlenko, D.V.; Tverdislov, V.; Gerasimov, D.; Rogozin, A. Chemical Physics of Cellulose Nitration. Russ. J. Phys. Chem. B 2016, 10, 245–259. [Google Scholar] [CrossRef]
- Zografi, G.; Kontny, M.; Yang, A.; Brenner, G. Surface Area and Water Vapor Sorption of Microcrystalline Cellulose. Int. J. Pharm. 1984, 18, 99–116. [Google Scholar] [CrossRef]
- Terinte, N.; Ibbett, R.; Schuster, K.C. Overview on Native Cellulose and Microcrystalline Cellulose I Structure Studied by X-ray Diffraction (WAXD): Comparison between Measurement Techniques. Lenzing. Berichte 2011, 89, 118–131. [Google Scholar]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; Ouardi, K.E.; Marlair, G.; Len, C. Hydrolysis of Hemicellulose and Derivatives—A Review of Recent Advances in the Production of Furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Duval, A.; Lawoko, M. A Review on Lignin-Based Polymeric, Micro- and Nano-Structured Materials. React. Funct. Polym. 2014, 85, 78–96. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Preparation of Nanocellulose from Micro-Crystalline Cellulose: The Effect on the Performance and Properties of Agar-Based Composite Films. Carbohydr. Polym. 2016, 135, 18–26. [Google Scholar] [CrossRef]
- Stovbun, S.V.; Skoblin, A.A.; Zlenko, D.V. Self Assembly and Gelation in Solutions of Chiral N-trifluoroacetylated α-aminoalcohols. Chem. Phys. 2018, 508, 34–44. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stovbun, S.V.; Mikhaleva, M.G.; Skoblin, A.A.; Usachev, S.V.; Nikolsky, S.N.; Kharitonov, V.A.; Kovaleva, K.I.; Politenkova, G.G.; Vedenkin, A.S.; Zlenko, D.V. Zhurkov’s Stress-Driven Fracture as a Driving Force of the Microcrystalline Cellulose Formation. Polymers 2020, 12, 2952. https://doi.org/10.3390/polym12122952
Stovbun SV, Mikhaleva MG, Skoblin AA, Usachev SV, Nikolsky SN, Kharitonov VA, Kovaleva KI, Politenkova GG, Vedenkin AS, Zlenko DV. Zhurkov’s Stress-Driven Fracture as a Driving Force of the Microcrystalline Cellulose Formation. Polymers. 2020; 12(12):2952. https://doi.org/10.3390/polym12122952
Chicago/Turabian StyleStovbun, Sergey V., Mariya G. Mikhaleva, Aleksey A. Skoblin, Sergey V. Usachev, Sergey N. Nikolsky, Vasily A. Kharitonov, Kseniya I. Kovaleva, Galina G. Politenkova, Alexander S. Vedenkin, and Dmitry V. Zlenko. 2020. "Zhurkov’s Stress-Driven Fracture as a Driving Force of the Microcrystalline Cellulose Formation" Polymers 12, no. 12: 2952. https://doi.org/10.3390/polym12122952
APA StyleStovbun, S. V., Mikhaleva, M. G., Skoblin, A. A., Usachev, S. V., Nikolsky, S. N., Kharitonov, V. A., Kovaleva, K. I., Politenkova, G. G., Vedenkin, A. S., & Zlenko, D. V. (2020). Zhurkov’s Stress-Driven Fracture as a Driving Force of the Microcrystalline Cellulose Formation. Polymers, 12(12), 2952. https://doi.org/10.3390/polym12122952




