Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation
Abstract
1. Introduction
2. Requirements of Ideal Hydrogel Scaffold for Dentin–Pulp Complex Regeneration
3. Mechanism of Action of Hydrogels in Dentin–Pulp Regeneration
4. Hydrogels Used in Dentin–Pulp Complex Regeneration
4.1. Natural Hydrogels
4.1.1. Collagen
4.1.2. Gelatin Hydrogel
4.1.3. Fibrin
4.1.4. Matrigel
4.1.5. Keratin Hydrogel
4.1.6. Alginate
4.1.7. Chitosan Hydrogel
4.1.8. Hyaluronic Acid Hydrogel
4.1.9. Agarose Hydrogel
4.1.10. Cellulose Hydrogel
4.1.11. Extracellular Matrix Hydrogels from Decellularized Tissues
4.2. Synthetic Hydrogels
4.2.1. Poly Lactic Acid-Based Hydrogels
4.2.2. Polydimethylsiloxane Hydrogels
4.2.3. Poly-N-Isopropylacrylamide Gel
4.2.4. Polyethylene Glycol
4.2.5. Synthetic Self-Assembling Peptide Hydrogel
Peptide Amphiphiles
RADA16-I Peptide Hydrogels
Multidomain Peptides Hydrogels
4.2.6. VitroGel 3D
4.3. Hybrid Hydrogels
4.3.1. Alginate/Laponite Hydrogel
4.3.2. PEG-Modified Natural Polymers
5. Comparing Natural and Synthetic Classes of Hydrogels
6. Clinical Application of Different Hydrogels for Dentin–Pulp Complex Regeneration
7. Physical and Chemical Properties of the Hydrogel that Affect Cellular Behavior in Dentin–Pulp Regeneration
7.1. Bioactivity of the Polymer
7.2. Hydrogel Forming Ability
7.3. Concentration of the Monomer
7.4. Concentration of the Crosslinker
7.5. Polymer Degradability
7.6. Biofunctionalization
7.7. Rheological Properties
7.8. Network Structure
7.9. pH of the Polymer Solution
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galler, K.M.; Hartgerink, J.D.; Cavender, A.C.; Schmalz, G.; D’Souza, R.N. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng. Part A 2012, 18, 176–184. [Google Scholar] [CrossRef]
- Galler, K.M.; Aulisa, L.; Regan, K.R.; D’Souza, R.N.; Hartgerink, J.D. Self-Assembling Multidomain Peptide Hydrogels: Designed Susceptibility to Enzymatic Cleavage Allows Enhanced Cell Migration and Spreading. J. Am. Chem. Soc. 2010, 132, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Okiji, T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit. Anom. 2016, 56, 144–153. [Google Scholar] [CrossRef]
- Fahmy, S.H.; Hassanien, E.E.S.; Nagy, M.M.; El Batouty, K.M.; Mekhemar, M.; Fawzy El Sayed, K.; Hassanein, E.H.; Wiltfang, J.; Dorfer, C. Investigation of the regenerative potential of necrotic mature teeth following different revascularisation protocols. Aust. Endod. J. 2017, 43, 73–82. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Elsalawy, R.; Ibrahim, N.; Gadalla, M.; Albargasy, H.; Zahra, N.; Mokhtar, S.; El Nahhas, N.; El Kaliouby, Y.; Dorfer, C.E. The Dental Pulp Stem/Progenitor Cells-Mediated Inflammatory-Regenerative Axis. Tissue Eng. Part B Rev. 2019, 25, 445–460. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Klingebiel, P.; Dorfer, C.E. Toll-like Receptor Expression Profile of Human Dental Pulp Stem/Progenitor Cells. J. Endod. 2016, 42, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The role of matrix metalloproteinases (MMPs) in human caries. J. Dent. Res. 2006, 85, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Smith, A. Cells and extracellular matrices of dentin and pulp: A biological basis for repair and tissue engineering. Crit. Rev. Oral. Biol. Med. 2004, 15, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. JBMR 2003, 18, 696–704. [Google Scholar] [CrossRef]
- Sharma, L.A.; Love, R.M. Scaffolds for regeneration of the pulp–dentine complex. In Handbook of Tissue Engineering Scaffolds: Volume One; Elsevier: Amsterdam, The Netherlands, 2019; pp. 459–478. [Google Scholar]
- Jernvall, J.; Thesleff, I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech. Dev. 2000, 92, 19–29. [Google Scholar] [CrossRef]
- Smith, A.; Lumley, P.; Tomson, P.; Cooper, P. Dental regeneration and materials—A partnership. Clin. Oral Investig. 2008, 12, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Inflammation–regeneration interplay in the dentine–pulp complex. J. Dent. 2010, 38, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Ahmed, G.M.; Abouauf, E.A.; Schwendicke, F. Stem/progenitor cell-mediated pulpal tissue regeneration: A systematic review and meta-analysis. Int. Endod. J. 2019, 52, 1573–1585. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Jakusz, K.; Jochens, A.; Dorfer, C.; Schwendicke, F. Stem Cell Transplantation for Pulpal Regeneration: A Systematic Review. Tissue Eng. Part B Rev. 2015, 21, 451–460. [Google Scholar] [CrossRef]
- Ahmed, G.M.; Abouauf, E.A.; AbuBakr, N.; Dorfer, C.E.; El-Sayed, K.F. Tissue Engineering Approaches for Enamel, Dentin, and Pulp Regeneration: An Update. Stem Cells Int. 2020, 2020, 5734539. [Google Scholar] [CrossRef]
- Lovelace, T.W.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J. Endod. 2011, 37, 133–138. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, G.; Chen, Z. Pulp regeneration: Current approaches and future challenges. Front. Physiol. 2016, 7, 58. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Federlin, M.; Cavender, A.C.; Hartgerink, J.D.; Hecker, S.; Schmalz, G. Dentin conditioning codetermines cell fate in regenerative endodontics. J. Endod. 2011, 37, 1536–1541. [Google Scholar] [CrossRef]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef]
- Galler, K.M.; Eidt, A.; Schmalz, G. Cell-free approaches for dental pulp tissue engineering. J. Endod. 2014, 40, S41–S45. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, H.; Mazidi, A.; Mohammadi Asl, S.; Ellini, M.R.; Moshiri, A.; Nekoofar, M.H.; Dummer, P.M.H. The role of stem cell therapy in regeneration of dentine-pulp complex: A systematic review. Prog. Biomater. 2018, 7, 249–268. [Google Scholar] [CrossRef]
- Mullane, E.M.; Dong, Z.; Sedgley, C.M.; Hu, J.C.C.; Botero, T.M.; Holland, G.R.; Nör, J.E. Effects of VEGF and FGF2 on the revascularization of severved human dental pulps. J. Dent. Res. 2008, 87, 1144–1148. [Google Scholar] [CrossRef]
- Nakashima, M.; Reddi, A.H. The application of bone morphogenetic proteins to dental tissue engineering. Nat. Biotechnol. 2003, 21, 1025–1032. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Hartgerink, J.D. Biomaterials and their potential applications for dental tissue engineering. J. Mater. Chem. 2010, 20, 8730–8746. [Google Scholar] [CrossRef]
- Fan, C.; Wang, D.-A. Macroporous hydrogel scaffolds for three-dimensional cell culture and tissue engineering. Tissue Eng. Part B Rev. 2017, 23, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Olanrele, O.S.; Zhang, X.; Hsu, C.C. Fabrication of Hydrogel Materials for Biomedical Applications. In Novel Biomaterials for Regenerative Medicine; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Springer: Singapore, 2018; pp. 197–224. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C 2017, 79, 958–971. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lee, D.S. Injectable Biodegradable Hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef] [PubMed]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Wong Po Foo, C.T.S.; Lee, J.S.; Mulyasasmita, W.; Parisi-Amon, A.; Heilshorn, S.C. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc. Natl. Acad. Sci. USA 2009, 106, 22067–22072. [Google Scholar] [CrossRef] [PubMed]
- Raddall, G.; Mello, I.; Leung, B.M. Biomaterials and scaffold design strategies for regenerative endodontic therapy. Front. Bioeng. Biotechnol. 2019, 7, 317. [Google Scholar] [CrossRef]
- Yuan, Z.; Nie, H.; Wang, S.; Lee, C.H.; Li, A.; Fu, S.Y.; Zhou, H.; Chen, L.; Mao, J.J. Biomaterial selection for tooth regeneration. Tissue Eng. Part B Rev. 2011, 17, 373–388. [Google Scholar] [CrossRef]
- Partap, S. Scaffolds & Surfaces. In Basic Engineering for Medics and Biologists: An ESEM Primer; Lee, T.C., Niederer, P., Eds.; IOS Press: Amsterdam, The Netherlands, 2010; Volume 152, pp. 187–201. [Google Scholar]
- Temenoff, J.S.; Mikos, A.G. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials 2000, 21, 2405–2412. [Google Scholar] [CrossRef]
- Vermonden, T.; Censi, R.; Hennink, W.E. Hydrogels for protein delivery. Chem. Rev. 2012, 112, 2853–2888. [Google Scholar] [CrossRef]
- Lai, J.-Y. Biocompatibility of chemically cross-linked gelatin hydrogels for ophthalmic use. J. Mater. Sci. Mater. Med. 2010, 21, 1899–1911. [Google Scholar] [CrossRef]
- Babensee, J.E.; Anderson, J.M.; McIntire, L.V.; Mikos, A.G. Host response to tissue engineered devices. Adv. Drug Del. Rev. 1998, 33, 111–139. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Del. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Lutolf, M.; Hubbell, J. Synthesis and physicochemical characterization of end-linked poly (ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 2003, 4, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Zisch, A.H.; Lutolf, M.P.; Ehrbar, M.; Raeber, G.P.; Rizzi, S.C.; Davies, N.; Schmökel, H.; Bezuidenhout, D.; Djonov, V.; Zilla, P. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 2003, 17, 2260–2262. [Google Scholar] [CrossRef] [PubMed]
- O’brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today. 2011, 14, 88–95. [Google Scholar]
- Lai, J.-Y.; Li, Y.-T. Functional Assessment of Cross-Linked Porous Gelatin Hydrogels for Bioengineered Cell Sheet Carriers. Biomacromolecules 2010, 11, 1387–1397. [Google Scholar] [CrossRef]
- Hou, Q.; Paul, A.; Shakesheff, K.M. Injectable scaffolds for tissue regeneration. J. Mater. Chem. 2004, 14, 1915–1923. [Google Scholar] [CrossRef]
- Shung, A.K.; Behravesh, E.; Jo, S.; Mikos, A.G. Crosslinking characteristics of and cell adhesion to an injectable poly (propylene fumarate-co-ethylene glycol) hydrogel using a water-soluble crosslinking system. Tissue Eng. 2003, 9, 243–254. [Google Scholar] [CrossRef]
- Kempen, D.H.; Lu, L.; Kim, C.; Zhu, X.; Dhert, W.J.; Currier, B.L.; Yaszemski, M.J. Controlled drug release from a novel injectable biodegradable microsphere/scaffold composite based on poly (propylene fumarate). J. Biomed. Mater. Res. A 2006, 77, 103–111. [Google Scholar] [CrossRef]
- He, S.; Yaszemski, M.J.; Yasko, A.W.; Engel, P.S.; Mikos, A.G. Injectable biodegradable polymer composites based on poly (propylene fumarate) crosslinked with poly (ethylene glycol)-dimethacrylate. Biomaterials 2000, 21, 2389–2394. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhang, C. Dental Pulp Stem Cells and Hydrogel in Pulp Regeneration. In Dental Stem Cells: Regenerative Potential; Springer: Berlin/Heidelberg, Germany, 2016; pp. 133–145. [Google Scholar]
- Ajay Sharma, L.; Sharma, A.; Dias, G.J. Advances in regeneration of dental pulp—A literature review. J. Investig. Clin. Dent. 2015, 6, 85–98. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Del. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xie, L.; Wu, H.; Yang, T.; Zhang, Q.; Tian, Y.; Liu, Y.; Han, X.; Guo, W.; He, M. Alginate/laponite hydrogel microspheres co-encapsulating dental pulp stem cells and VEGF for endodontic regeneration. Acta Biomater. 2020, 113, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Zein, N.; Harmouch, E.; Lutz, J.-C.; Fernandez De Grado, G.; Kuchler-Bopp, S.; Clauss, F.; Offner, D.; Hua, G.; Benkirane-Jessel, N.; Fioretti, F. Polymer-Based Instructive Scaffolds for Endodontic Regeneration. Materials 2019, 12, 2347. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-F.; Luo, L.-J.; Lai, J.-Y. Gallic acid grafting effect on delivery performance and antiglaucoma efficacy of antioxidant-functionalized intracameral pilocarpine carriers. Acta Biomater. 2016, 38, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lee, S.H.; Hwang, Y.C.; Rosa, V.; Lee, K.W.; Min, K.S. Behaviour of human dental pulp cells cultured in a collagen hydrogel scaffold cross-linked with cinnamaldehyde. Int. Endod. J. 2017, 50, 58–66. [Google Scholar] [CrossRef]
- Pankajakshan, D.; Voytik-Harbin, S.L.; Nor, J.E.; Bottino, M.C. Injectable Highly Tunable Oligomeric Collagen Matrices for Dental Tissue Regeneration. ACS Appl. Bio Mater. 2020, 3, 859–868. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Bherwani, A.K.; Simon, M.; Rafailovich, M.H. Biomineralization on enzymatically cross-linked gelatin hydrogels in the absence of dexamethasone. Dent. Mater. 2015, 3, 5210–5219. [Google Scholar] [CrossRef]
- Miyazawa, A.; Matsuno, T.; Asano, K.; Tabata, Y.; Satoh, T. Controlled release of simvastatin from biodegradable hydrogels promotes odontoblastic differentiation. Dent. Mater. J. 2015, 34, 466–474. [Google Scholar] [CrossRef]
- Khayat, A.; Monteiro, N.; Smith, E.; Pagni, S.; Zhang, W.; Khademhosseini, A.; Yelick, P.C. GelMA-encapsulated hDPSCs and HUVECs for dental pulp regeneration. J. Dent. Res. 2017, 96, 192–199. [Google Scholar] [CrossRef]
- Park, J.H.; Gillispie, G.J.; Copus, J.S.; Zhang, W.; Atala, A.; Yoo, J.J.; Yelick, P.C.; Lee, S.J. The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs. Biofabrication 2020, 12, 35029. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Cavender, A.C.; Koeklue, U.; Suggs, L.J.; Schmalz, G.; D’Souza, R.N. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen. Med. 2011, 6, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Moon, J.-H.; Kim, S.G.; Kim, S.-Y. Pulp regeneration with hemostatic matrices as a scaffold in an immature tooth minipig model. Sci. Rep. 2020, 10, 12536. [Google Scholar] [CrossRef] [PubMed]
- Athirasala, A.; Lins, F.; Tahayeri, A.; Hinds, M.; Smith, A.J.; Sedgley, C.; Ferracane, J.; Bertassoni, L.E. A Novel Strategy to Engineer Pre-Vascularized Full-Length Dental Pulp-like Tissue Constructs. Sci. Rep. 2017, 7, 3323. [Google Scholar] [CrossRef] [PubMed]
- Ajay Sharma, L.; Ali, M.A.; Love, R.M.; Wilson, M.J.; Dias, G.J. Novel keratin preparation supports growth and differentiation of odontoblast-like cells. Int. Endod. J. 2016, 49, 471–482. [Google Scholar] [CrossRef]
- Ajay Sharma, L. Wool-derived keratin hydrogel as a potential scaffold for pulp-dentine regeneration: An in vitro and in vivo study. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2016. [Google Scholar]
- Zhang, S.; Thiebes, A.L.; Kreimendahl, F.; Ruetten, S.; Buhl, E.M.; Wolf, M.; Jockenhoevel, S.; Apel, C. Extracellular Vesicles-Loaded Fibrin Gel Supports Rapid Neovascularization for Dental Pulp Regeneration. Int. J. Mol. Sci. 2020, 21, 4226. [Google Scholar] [CrossRef]
- Ha, M.; Athirasala, A.; Tahayeri, A.; Menezes, P.P.; Bertassoni, L.E. Micropatterned hydrogels and cell alignment enhance the odontogenic potential of stem cells from apical papilla in-vitro. Dent. Mater. 2020, 36, 88–96. [Google Scholar] [CrossRef]
- Xiao, M.; Qiu, J.; Kuang, R.; Zhang, B.; Wang, W.; Yu, Q. Synergistic effects of stromal cell-derived factor-1α and bone morphogenetic protein-2 treatment on odontogenic differentiation of human stem cells from apical papilla cultured in the VitroGel 3D system. Cell Tissue Res. 2019, 378, 207–220. [Google Scholar] [CrossRef]
- Galler, K.M.; Cavender, A.; Yuwono, V.; Dong, H.; Shi, S.; Schmalz, G.; Hartgerink, J.D.; D’Souza, R.N. Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng. Part A. 2008, 14, 2051–2058. [Google Scholar] [CrossRef]
- Colombo, J.S.; Jia, S.; D’Souza, R.N. Modeling Hypoxia Induced Factors to Treat Pulpal Inflammation and Drive Regeneration. J. Endod. 2020, 46, S19–S25. [Google Scholar] [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.; Nör, J. Dental pulp tissue engineering in full-length human root canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kaneko, T.; Sueyama, Y.; Kaneko, R.; Okiji, T. Dental pulp tissue engineering of pulpotomized rat molars with bone marrow mesenchymal stem cells. Odontology 2017, 105, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Sueyama, Y.; Kaneko, T.; Ito, T.; Kaneko, R.; Okiji, T. Implantation of Endothelial Cells with Mesenchymal Stem Cells Accelerates Dental Pulp Tissue Regeneration/Healing in Pulpotomized Rat Molars. J. Endod. 2017, 43, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Kaneko, T.; Zaw, S.Y.M.; Sone, P.P.; Murano, H.; Sueyama, Y.; Zaw, Z.C.T.; Okiji, T. Macrophage populations show an M1-to-M2 transition in an experimental model of coronal pulp tissue engineering with mesenchymal stem cells. Int. Endod. J. 2018, 52, 504–514. [Google Scholar] [CrossRef]
- Kaneko, T.; Sone, P.P.; Zaw, S.Y.M.; Sueyama, Y.; Zaw, Z.C.T.; Okada, Y.; Murano, H.; Gu, B.; Okiji, T. In vivo fate of bone marrow mesenchymal stem cells implanted into rat pulpotomized molars. Stem. Cell Res. 2019, 38, 101457. [Google Scholar] [CrossRef]
- Smith, J.G.; Smith, A.J.; Shelton, R.M.; Cooper, P.R. Dental Pulp Cell Behavior in Biomimetic Environments. J. Dent. Res. 2015, 94, 1552–1559. [Google Scholar] [CrossRef]
- Bekhouche, M.; Bolon, M.; Charriaud, F.; Lamrayah, M.; Da Costa, D.; Primard, C.; Costantini, A.; Pasdeloup, M.; Gobert, S.; Mallein-Gerin, F. Development of an antibacterial nanocomposite hydrogel for human dental pulp engineering. J. Mater. Chem. B 2020, 8, 8422–8432. [Google Scholar] [CrossRef]
- Bhoj, M.; Zhang, C.; Green, D.W. A First Step in De Novo Synthesis of a Living Pulp Tissue Replacement Using Dental Pulp MSCs and Tissue Growth Factors, Encapsulated within a Bioinspired Alginate Hydrogel. J. Endod. 2015, 41, 1100–1107. [Google Scholar] [CrossRef]
- Kikuchi, N.; Kitamura, C.; Morotomi, T.; Inuyama, Y.; Ishimatsu, H.; Tabata, Y.; Nishihara, T.; Terashita, M. Formation of dentin-like particles in dentin defects above exposed pulp by controlled release of fibroblast growth factor 2 from gelatin hydrogels. J. Endod. 2007, 33, 1198–1202. [Google Scholar] [CrossRef]
- Nagy, M.M.; Tawfik, H.E.; Hashem, A.A.R.; Abu-Seida, A.M. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J. Endod. 2014, 40, 192–198. [Google Scholar] [CrossRef]
- Dobie, K.; Smith, G.; Sloan, A.J.; Smith, A.J. Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connect. Tissue Res. 2002, 43, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Shi, L.; Pan, S.; He, L.; Niu, Y.; Wang, X. A Customized Self-Assembling Peptide Hydrogel-Wrapped Stem Cell Factor Targeting Pulp Regeneration Rich in Vascular-Like Structures. ACS Omega 2020, 5, 16568–16574. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.K.; Gao, W.; Patel, S.D.; Siddiqui, Z.; Weiner, S.; Shimizu, E.; Sarkar, B.; Kumar, V.A. Self-assembly of a dentinogenic peptide hydrogel. ACS Omega 2018, 3, 5980–5987. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Chen, Z.; Chen, J.; Xu, H.; Xu, Y.; Yang, T.; Zhang, Q. RGD-and VEGF-Mimetic Peptide Epitope-Functionalized Self-Assembling Peptide Hydrogels Promote Dentin-Pulp Complex Regeneration. Int. J. Nanomed. 2020, 15, 6631. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Nagano, A.; Ikada, Y. Biodegradation of Hydrogel Carrier Incorporating Fibroblast Growth Factor. Tissue Eng. 1999, 5, 127–138. [Google Scholar] [CrossRef]
- Ishihara, M.; Obara, K.; Nakamura, S.; Fujita, M.; Masuoka, K.; Kanatani, Y.; Takase, B.; Hattori, H.; Morimoto, Y.; Ishihara, M. Chitosan hydrogel as a drug delivery carrier to control angiogenesis. J. Artif. Organs 2006, 9, 8–16. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- DeVolder, R.; Kong, H.-J. Hydrogels for in vivo-like three-dimensional cellular studies. WIREs Syst. Biol. Med. 2012, 4, 351–365. [Google Scholar] [CrossRef]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K. Molecular Biology of The Cell; Taylor and Francis Group: Abingdon, UK, 2002. [Google Scholar]
- Sharma, S.; Srivastava, D.; Grover, S.; Sharma, V. Biomaterials in tooth tissue engineering: A review. J. Clin. Diagn. Res 2014, 8, 309–315. [Google Scholar] [CrossRef]
- Reilly, G.C.; Engler, A.J. Intrinsic extracellular matrix properties regulate stem cell differentiation. J. Biomech. 2010, 43, 55–62. [Google Scholar] [CrossRef]
- Zoldan, J.; Karagiannis, E.D.; Lee, C.Y.; Anderson, D.G.; Langer, R.; Levenberg, S. The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials 2011, 32, 9612–9621. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Sasirekha, K.; Krishnakumar, S.; Sastry, T.P. A novel cross-linked human amniotic membrane for corneal implantations. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 227, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.H.-K.; Chen, H.-C.; Ma, K.S.-K.; Lai, J.-Y.; Yang, U.; Yeh, L.-K.; Hsueh, Y.-J.; Chu, W.-K.; Lai, C.-H.; Chen, J.-K. Preservation of human limbal epithelial progenitor cells on carbodiimide cross-linked amniotic membrane via integrin-linked kinase-mediated Wnt activation. Acta Biomater. 2016, 31, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Whittington, C.F.; Yoder, M.C.; Voytik-Harbin, S.L. Collagen-polymer guidance of vessel network formation and stabilization by endothelial colony forming cells in vitro. Macromol. Biosci. 2013, 13, 1135–1149. [Google Scholar] [CrossRef]
- Veis, A.; Goldberg, M. Pulp Extracellular Matrix; Springer: Berlin/Heidelberg, Germany, 2014; pp. 35–46. [Google Scholar]
- Smith, A.J.; Scheven, B.A.; Takahashi, Y.; Ferracane, J.L.; Shelton, R.M.; Cooper, P.R. Dentine as a bioactive extracellular matrix. Arch. Oral Biol. 2012, 57, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Souron, J.B.; Petiet, A.; Decup, F.; Tran, X.V.; Lesieur, J.; Poliard, A.; Le Guludec, D.; Letourneur, D.; Chaussain, C.; Rouzet, F.; et al. Pulp cell tracking by radionuclide imaging for dental tissue engineering. Tissue Eng. Part C Methods 2014, 20, 188–197. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Lin, P.-K.; Hsiue, G.-H.; Cheng, H.-Y.; Huang, S.-J.; Li, Y.-T. Low Bloom Strength Gelatin as a Carrier for Potential Use in Retinal Sheet Encapsulation and Transplantation. Biomacromolecules 2009, 10, 310–319. [Google Scholar] [CrossRef]
- Pierce, B.F.; Pittermann, E.; Ma, N.; Gebauer, T.; Neffe, A.T.; Hölscher, M.; Jung, F.; Lendlein, A. Viability of human mesenchymal stem cells seeded on crosslinked entropy-elastic gelatin-based hydrogels. Macromol. Biosci. 2012, 12, 312–321. [Google Scholar] [CrossRef]
- Qu, T.; Liu, X. Nano-structured gelatin/bioactive glass hybrid scaffolds for the enhancement of odontogenic differentiation of human dental pulp stem cells. J. Mater. Chem. B 2013, 1, 4764–4772. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.J.; Ren, J.R.; Huang, N.; Wang, J.; Chen, J.Y.; Leng, Y.X.; Liu, H.Q. Surface engineering of Ti–O films by photochemical immobilization of gelatin. Mater. Sci. Eng. C 2008, 28, 1495–1500. [Google Scholar] [CrossRef]
- Van den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; He, T.; Shen, J. Endothelium regeneration on luminal surface of polyurethane vascular scaffold modified with diamine and covalently grafted with gelatin. Biomaterials 2004, 25, 423–430. [Google Scholar] [CrossRef]
- Salamon, A.; van Vlierberghe, S.; van Nieuwenhove, I.; Baudisch, F.; Graulus, G.-J.; Benecke, V.; Alberti, K.; Neumann, H.-G.; Rychly, J.; Martins, J.C.; et al. Gelatin-Based Hydrogels Promote Chondrogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells In Vitro. Materials 2014, 7, 1342–1359. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Radhakrishnan, J.; Krishnan, U.M.; Sethuraman, S. Hydrogel based injectable scaffolds for cardiac tissue regeneration. Biotechnol. Adv. 2014, 32, 449–461. [Google Scholar] [CrossRef]
- Zhou, D.; Ito, Y. Inorganic material surfaces made bioactive by immobilizing growth factors for hard tissue engineering. RSC Adv. 2013, 3, 11095–11106. [Google Scholar] [CrossRef]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef]
- Ishimatsu, H.; Kitamura, C.; Morotomi, T.; Tabata, Y.; Nishihara, T.; Chen, K.-K.; Terashita, M. Formation of dentinal bridge on surface of regenerated dental pulp in dentin defects by controlled release of fibroblast growth factor-2 from gelatin hydrogels. J. Endod. 2009, 35, 858–865. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem. Cell Rev. 2019, 15, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Thrivikraman, G.; Athirasala, A.; Tahayeri, A.; França, C.M.; Ferracane, J.L.; Bertassoni, L.E. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent. Mater. 2018, 34, 389–399. [Google Scholar] [CrossRef]
- Ducret, M.; Montembault, A.; Josse, J.; Pasdeloup, M.; Celle, A.; Benchrih, R.; Mallein-Gerin, F.; Alliot-Licht, B.; David, L.; Farges, J.C. Design and characterization of a chitosan-enriched fibrin hydrogel for human dental pulp regeneration. Dent. Mater. 2019, 35, 523–533. [Google Scholar] [CrossRef]
- Roura, S.; Gálvez-Montón, C.; Bayes-Genis, A. Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. J. Tissue Eng. Regen. Med. 2017, 11, 2304–2313. [Google Scholar] [CrossRef]
- Li, Y.; Meng, H.; Liu, Y.; Lee, B.P. Fibrin Gel as an Injectable Biodegradable Scaffold and Cell Carrier for Tissue Engineering. Sci. World J. 2015, 2015, 685690. [Google Scholar] [CrossRef]
- Brown, A.C.; Barker, T.H. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef]
- Verma, P.; Nosrat, A.; Kim, J.R.; Price, J.B.; Wang, P.; Bair, E.; Xu, H.H.; Fouad, A.F. Effect of Residual Bacteria on the Outcome of Pulp Regeneration In Vivo. J. Dent. Res. 2017, 96, 100–106. [Google Scholar] [CrossRef]
- Shenoi, P.R.; Morey, E.S.; Makade, C.S.; Gunwal, M.K.; Khode, R.T.; Wanmali, S.S. In vitro evaluation of the antimicrobial efficacy of chitosan and other endodontic irrigants against Enterococcus faecalis. Gen. Dent. 2016, 64, 60–63. [Google Scholar] [PubMed]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, F.; Shakir, M. The Influence of Enterococcus faecalis as a Dental Root Canal Pathogen on Endodontic Treatment: A Systematic Review. Cureus 2020, 12, e7257. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Amiaud, J.; Delbos, L.; Charrier, C.; Montembault, A.; Ducret, M.; Farges, F.; David, L.; Alliot-Licht, B.; Gaudin, A. Dental pulp inflammatory/immune response to a chitosan-enriched fibrin hydrogel in the pulpotomised rat incisor. Eur. Cells Mater. 2020, 40, 74–87. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Dorfer, C.E.; Fawzy El-Sayed, K.M. Dental Stem Cell-Derived Secretome/Conditioned Medium: The Future for Regenerative Therapeutic Applications. Stem Cells Int. 2020, 2020, 7593402. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Shaw, L.M. Tumor cell invasion assays In Cell Migration: Developmental Methods and Protocols; Guan, J.-L., Ed.; Springer Science & Business Media: Berlin, Germany, 2005; Volume 294, pp. 97–105. [Google Scholar]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Vukicevic, S.; Kleinman, H.K.; Luyten, F.P.; Roberts, A.B.; Roche, N.S.; Reddi, A.H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 1992, 202, 1–8. [Google Scholar] [CrossRef]
- Taub, M.; Wang, Y.; Szczesny, T.M.; Kleinman, H.K. Epidermal growth factor or transforming growth factor alpha is required for kidney tubulogenesis in matrigel cultures in serum-free medium. Proc. Natl. Acad. Sci. USA 1990, 87, 4002–4006. [Google Scholar] [CrossRef] [PubMed]
- Lambricht, L.; De Berdt, P.; Vanacker, J.; Leprince, J.; Diogenes, A.; Goldansaz, H.; Bouzin, C.; Préat, V.; Dupont-Gillain, C.; Des Rieux, A. The type and composition of alginate and hyaluronic-based hydrogels influence the viability of stem cells of the apical papilla. Dent. Mater. 2014, 30, e349–e361. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dissanayaka, W.L.; Zhang, C. Dental pulp stem cells overexpressing stromal-derived factor-1α and vascular endothelial growth factor in dental pulp regeneration. Clin. Oral Investig. 2019, 23, 2497–2509. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, S.; Jeanneau, C.; Sheibat-Othman, N.; Kalaji, N.; Fessi, H.; About, I. Usefulness of controlled release of growth factors in investigating the early events of dentin-pulp regeneration. J. Endod. 2013, 39, 228–235. [Google Scholar] [CrossRef]
- Coulombe, P.A.; Bousquet, O.; Ma, L.; Yamada, S.; Wirtz, D. The ‘ins’ and ‘outs’ of intermediate filament organization. Trends Cell Biol. 2000, 10, 420–428. [Google Scholar] [CrossRef]
- Rouse, J.G.; Van Dyke, M.E. A Review of Keratin-Based Biomaterials for Biomedical Applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef]
- Hearle, J.W. A critical review of the structural mechanics of wool and hair fibres. Int. J. Biol. Macromol. 2000, 27, 123–138. [Google Scholar] [CrossRef]
- Donato, R.K.; Mija, A. Keratin Associations with Synthetic, Biosynthetic and Natural Polymers: An Extensive Review. Polymers 2019, 12, 32. [Google Scholar] [CrossRef]
- Hill, P.; Brantley, H.; Van Dyke, M. Some properties of keratin biomaterials: Kerateines. Biomaterials 2010, 31, 585–593. [Google Scholar] [CrossRef]
- Ikkai, F.; Naito, S. Dynamic Light Scattering and Circular Dichroism Studies on Heat-Induced Gelation of Hard-Keratin Protein Aqueous Solutions. Biomacromolecules 2002, 3, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ajay Sharma, L.; Love, R.M.; Ali, M.A.; Sharma, A.; Macari, S.; Avadhani, A.; Dias, G.J. Healing response of rat pulp treated with an injectable keratin hydrogel. J. Appl. Biomater. Funct 2017, 15, e244–e250. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Verma, P.; Ray, P.; Ray, A.R. Preparation of scaffolds from human hair proteins for tissue-engineering applications. Biomed. Mater. 2008, 3, 25007. [Google Scholar] [CrossRef] [PubMed]
- Magin, T.M.; Vijayaraj, P.; Leube, R.E. Structural and regulatory functions of keratins. Exp. Cell Res. 2007, 313, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Izawa, I.; Inagaki, M. Regulatory mechanisms and functions of intermediate filaments: A study using site- and phosphorylation state-specific antibodies. Cancer Sci. 2006, 97, 167–174. [Google Scholar] [CrossRef]
- Salas, P.J.; Forteza, R.; Mashukova, A. Multiple roles for keratin intermediate filaments in the regulation of epithelial barrier function and apico-basal polarity. Tissue Barriers 2016, 4, e1178368. [Google Scholar] [CrossRef]
- Kumar, V.; Bouameur, J.-E.; Bär, J.; Rice, R.H.; Hornig-Do, H.-T.; Roop, D.R.; Schwarz, N.; Brodesser, S.; Thiering, S.; Leube, R.E.; et al. A keratin scaffold regulates epidermal barrier formation, mitochondrial lipid composition, and activity. J. Cell Biol. 2015, 211, 1057–1075. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, Y.-K.; Tang, K.-C.; Yang, K.-C.; Cheng, N.-C.; Yu, J. Keratin scaffolds with human adipose stem cells: Physical and biological effects toward wound healing. J. Tissue Eng. Regen. Med. 2019, 13, 1044–1058. [Google Scholar] [CrossRef]
- Hesse, M.; Zimek, A.; Weber, K.; Magin, T.M. Comprehensive analysis of keratin gene clusters in humans and rodents. Eur. J. Cell Biol. 2004, 83, 19–26. [Google Scholar] [CrossRef]
- Lee, H.; Noh, K.; Lee, S.C.; Kwon, I.-K.; Han, D.-W.; Lee, I.-S.; Hwang, Y.-S. Human hair keratin and its-based biomaterials for biomedical applications. J. Tissue Eng. Regen. Med. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate Hydrogels as Biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, K.H.; Lee, K.Y.; Alsberg, E.; Damm, K.L.; Anderson, K.W.; Mooney, D.J. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Melin, M.; Joffre-Romeas, A.; Farges, J.C.; Couble, M.L.; Magloire, H.; Bleicher, F. Effects of TGFbeta1 on dental pulp cells in cultured human tooth slices. J. Dent. Res. 2000, 79, 1689–1696. [Google Scholar] [CrossRef]
- He, H.; Yu, J.; Liu, Y.; Lu, S.; Liu, H.; Shi, J.; Jin, Y. Effects of FGF2 and TGFbeta1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol. Int. 2008, 32, 827–834. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhan, X.; Zhang, C.; Hargreaves, K.M.; Jin, L.; Tong, E.H.Y. Coculture of dental pulp stem cells with endothelial cells enhances osteo-/odontogenic and angiogenic potential in vitro. J. Endod. 2012, 38, 454–463. [Google Scholar] [CrossRef]
- Fouad, A.F. The Microbial Challenge to Pulp Regeneration. Adv. Dent. Res. 2011, 23, 285–289. [Google Scholar] [CrossRef]
- Athirasala, A.; Tahayeri, A.; Thrivikraman, G.; Franca, C.M.; Monteiro, N.; Tran, V.; Ferracane, J.; Bertassoni, L.E. A dentin-derived hydrogel bioink for 3D bioprinting of cell laden scaffolds for regenerative dentistry. Biofabrication 2018, 10, 024101. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Song, W.; Pan, T.; Wang, H.; Ning, T.; Wei, Q.; Xu, H.H.K.; Wu, B.; Ma, D. Effects of 3-dimensional Bioprinting Alginate/Gelatin Hydrogel Scaffold Extract on Proliferation and Differentiation of Human Dental Pulp Stem Cells. J. Endod. 2019, 45, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. J. Carbohydr. Polym. 2006, 64, 98–103. [Google Scholar] [CrossRef]
- Simionato, J.I.; Paulino, A.T.; Garcia, J.C.; Nozaki, J. Adsorption of aluminium from wastewater by chitin and chitosan produced from silkworm chrysalides. Polym. Int. 2006, 55, 1243–1248. [Google Scholar] [CrossRef]
- Miranda, D.G.; Malmonge, S.M.; Campos, D.M.; Attik, N.G.; Grosgogeat, B.; Gritsch, K. A chitosan-hyaluronic acid hydrogel scaffold for periodontal tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, D.; Nie, J. Chitosan/polyethylene glycol diacrylate films as potential wound dressing material. Int. J. Biol. Macromol. 2008, 43, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Neamnark, A.; Sanchavanakit, N.; Pavasant, P.; Rujiravanit, R.; Supaphol, P. In vitro biocompatibility of electrospun hexanoyl chitosan fibrous scaffolds towards human keratinocytes and fibroblasts. Eur. Polym. J. 2008, 44, 2060–2067. [Google Scholar] [CrossRef]
- Zhao, Q.S.; Ji, Q.X.; Xing, K.; Li, X.Y.; Liu, C.S.; Chen, X.G. Preparation and characteristics of novel porous hydrogel films based on chitosan and glycerophosphate. Carbohydr. Polym. 2009, 76, 410–416. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Li, Y.-T.; Wang, T.-P. In vitro response of retinal pigment epithelial cells exposed to chitosan materials prepared with different cross-linkers. Int. J. Mol. Sci. 2010, 11, 5256–5272. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1. [Google Scholar]
- Mourya, V.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Park, S.-J.; Li, Z.; Hwang, I.-N.; Huh, K.M.; Min, K.-S. Glycol chitin–based thermoresponsive hydrogel scaffold supplemented with enamel matrix derivative promotes odontogenic differentiation of human dental pulp cells. J. Endod. 2013, 39, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, Y.; Yu, Y.; Zhou, X.; Du, W.; Wan, M.; Fan, Y.; Zhou, X.; Xu, X.; Zheng, L. Evaluation of Chitosan Hydrogel for Sustained Delivery of VEGF for Odontogenic Differentiation of Dental Pulp Stem Cells. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Chatzistavrou, X.; Ge, L.; Qin, M.; Papagerakis, P.; Wang, Y. Biological properties of modified bioactive glass on dental pulp cells. J. Dent. 2019, 83, 18–26. [Google Scholar]
- El Ashiry, E.A.; Alamoudi, N.M.; El Ashiry, M.K.; Bastawy, H.A.; El Derwi, D.A.; Atta, H.M. Tissue engineering of necrotic dental pulp of immature teeth with apical periodontitis in dogs: Radiographic and histological evaluation. J. Clin. Pediatr. Dent. 2018, 42, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.G.; Hales, C.A. Chemistry and Biology of Hyaluronan; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Rice, K.G. The Chemistry, Biology, and Medical Applications of Hyaluronan and Its Derivatives; Laurent, T., Ed.; ACS Publications; Portland Press: London, UK, 1998. [Google Scholar]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef] [PubMed]
- Laurent, T.C.; Fraser, J.R.E. Hyaluronan 1. FASEB J. 1992, 6, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.N.; Birkinshaw, C. Hyaluronic acid based scaffolds for tissue engineering—A review. Carbohydr. Polym. 2013, 92, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef]
- Jiao, Y.; Pang, X.; Zhai, G. Advances in hyaluronic acid-based drug delivery systems. Curr. Drug Targets 2016, 17, 720–730. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Linde, A. A study of the dental pulp glycosaminoglycans from permanent human teeth and rat and rabbit incisors. Arch. Oral Biol. 1973, 18, 49–59. [Google Scholar] [CrossRef]
- Felszeghy, S.; Hyttinen, M.; Tammi, R.; Tammi, M.; Módis, L. Quantitative image analysis of hyaluronan expression in human tooth germs. Eur. J. Oral Sci. 2000, 108, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Tan, L.; Cen, L.; Zhang, Z. An injectable scaffold based on crosslinked hyaluronic acid gel for tissue regeneration. RSC Adv. 2016, 6, 16838–16850. [Google Scholar] [CrossRef]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.; Reis, R.L.; Gomes, M.E. Injectable and tunable hyaluronic acid hydrogels releasing chemotactic and angiogenic growth factors for endodontic regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef]
- Almeida, L.D.; Babo, P.S.; Silva, C.R.; Rodrigues, M.T.; Hebling, J.; Reis, R.L.; Gomes, M.E. Hyaluronic acid hydrogels incorporating platelet lysate enhance human pulp cell proliferation and differentiation. J. Mater. Sci. Mater. Med. 2018, 29, 88. [Google Scholar]
- Niloy, K.K.; Gulfam, M.; Compton, K.B.; Li, D.; Huang, G.T.-J.; Lowe, T.L. Methacrylated Hyaluronic Acid–Based Hydrogels Maintain Stemness in Human Dental Pulp Stem Cells. Regen. Eng. Transl. Med. 2019, 6, 262–272. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Yu, Z.; Chen, C.-A.; Aksel, H.; Azim, A.A.; Huang, G.T.-J. A miniature swine model for stem cell-based de novo regeneration of dental pulp and dentin-like tissue. Tissue Eng. Part C Methods 2018, 24, 108–120. [Google Scholar] [CrossRef]
- Chrepa, V.; Austah, O.; Diogenes, A. Evaluation of a commercially available hyaluronic acid hydrogel (Restylane) as injectable scaffold for dental pulp regeneration: An in vitro evaluation. J. Endod. 2017, 43, 257–262. [Google Scholar] [CrossRef]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and its derivatives as supports for enzyme immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Prakash, O.; Jaiswal, N. Immobilization of a thermostable α-amylase on agarose and agar matrices and its application in starch stain removal. World Appl. Sci. J. 2011, 13, 572–577. [Google Scholar]
- Horinaka, J.-i.; Yasuda, R.; Takigawa, T. Entanglement network of agarose in various solvents. Polym. J. 2011, 43, 1000–1002. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, R.; Yang, Y.; Wu, S.; Cao, Y.; Lu, A.; Zhang, L. Strength enhanced hydrogels constructed from agarose in alkali/urea aqueous solution and their application. Chem. Eng. J. 2018, 331, 177–184. [Google Scholar] [CrossRef]
- Christensen, L. Normal and pathologic tissue reactions to soft tissue gel fillers. Dermatol. Surg. 2007, 33, S168–S175. [Google Scholar] [PubMed]
- Christensen, L.H. Host tissue interaction, fate, and risks of degradable and nondegradable gel fillers. Dermatol. Surg. 2009, 35, 1612–1619. [Google Scholar] [CrossRef]
- Scarano, A.; Carinci, F.; Piattelli, A. Lip augmentation with a new filler (agarose gel): A 3-year follow-up study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e11–e15. [Google Scholar] [CrossRef]
- Cao, Z.; Gilbert, R.J.; He, W. Simple Agarose−Chitosan gel composite system for enhanced neuronal growth in three dimensions. Biomacromolecules 2009, 10, 2954–2959. [Google Scholar] [CrossRef]
- Xiong, J.-Y.; Narayanan, J.; Liu, X.-Y.; Chong, T.K.; Chen, S.B.; Chung, T.-S. Topology evolution and gelation mechanism of agarose gel. J. Phys. Chem. B 2005, 109, 5638–5643. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A novel electroactive agarose-aniline pentamer platform as a potential candidate for neural tissue engineering. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Campos, F.; Bonhome-Espinosa, A.B.; Vizcaino, G.; Rodriguez, I.A.; Durand-Herrera, D.; Lopez-Lopez, M.T.; Sánchez-Montesinos, I.; Alaminos, M.; Sánchez-Quevedo, M.C.; Carriel, V. Generation of genipin cross-linked fibrin-agarose hydrogel tissue-like models for tissue engineering applications. Biomed. Mater. 2018, 13, 025021. [Google Scholar] [CrossRef]
- Sarem, M.; Moztarzadeh, F.; Mozafari, M. How can genipin assist gelatin/carbohydrate chitosan scaffolds to act as replacements of load-bearing soft tissues? Carbohydr. Polym. 2013, 93, 635–643. [Google Scholar] [CrossRef]
- Cecilia, A.; Baecker, A.; Hamann, E.; Rack, A.; van de Kamp, T.; Gruhl, F.; Hofmann, R.; Moosmann, J.; Hahn, S.; Kashef, J. Optimizing structural and mechanical properties of cryogel scaffolds for use in prostate cancer cell culturing. Mater. Sci. Eng. C 2017, 71, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive chitosan–Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009, 5, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- El Moshy, S.; Abbass, M.M.; El-Motayam, A.M. Biomimetic remineralization of acid etched enamel using agarose hydrogel model. F1000Research 2018, 7, 1476. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Y.; Li, Q.-L. Scanning electron microscopic analysis of using agarose hydrogel microenvironment to create enamel prism-like tissue on dentine surface. J. Dent. 2016, 55, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Bourges, X.; Weiss, P.; Daculsi, G.; Legeay, G. Synthesis and general properties of silated-hydroxypropyl methylcellulose in prospect of biomedical use. Adv. Colloid Interface Sci. 2002, 99, 215–228. [Google Scholar] [CrossRef]
- Guo, K.; Chu, C. Synthesis and characterization of novel biodegradable unsaturated poly (ester amide)/poly (ethylene glycol) diacrylate hydrogels. J. Polym. Sci. A Polym. Chem. 2005, 43, 3932–3944. [Google Scholar] [CrossRef]
- Deng, J.; He, Q.; Wu, Z.; Yang, W. Using glycidyl methacrylate as cross-linking agent to prepare thermosensitive hydrogels by a novel one-step method. J. Polym. Sci. A Polym. Chem. 2008, 46, 2193–2201. [Google Scholar] [CrossRef]
- Chen, H.; Fan, M. Novel thermally sensitive pH-dependent chitosan/carboxymethyl cellulose hydrogels. J. Polym. Sci. A Polym. Chem. 2008, 23, 38–48. [Google Scholar]
- Chang, C.; Lue, A.; Zhang, L. Effects of crosslinking methods on structure and properties of cellulose/PVA hydrogels. Macromol. Chem. Phys. 2008, 209, 1266–1273. [Google Scholar] [CrossRef]
- te Nijenhuis, K. On the nature of crosslinks in thermoreversible gels. Polym. Bull. 2007, 58, 27–42. [Google Scholar] [CrossRef]
- Chen, C.-H.; Tsai, C.-C.; Chen, W.; Mi, F.-L.; Liang, H.-F.; Chen, S.-C.; Sung, H.-W. Novel living cell sheet harvest system composed of thermoreversible methylcellulose hydrogels. Biomacromolecules 2006, 7, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Magne, D.; Weiss, P.; Trojani, C.; Rochet, N.; Carle, G.; Vignes-Colombeix, C.; Chadjichristos, C.; Galera, P.; Daculsi, G. A silanized hydroxypropyl methylcellulose hydrogel for the three-dimensional culture of chondrocytes. Biomaterials 2005, 26, 6643–6651. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Magne, D.; Moreau, A.; Gauthier, O.; Malard, O.; Vignes-Colombeix, C.; Daculsi, G.; Weiss, P.; Guicheux, J. Engineering cartilage with human nasal chondrocytes and a silanized hydroxypropyl methylcellulose hydrogel. J. Biomed. Mater. Res. A 2007, 80, 66–74. [Google Scholar] [CrossRef]
- Trojani, C.; Weiss, P.; Michiels, J.-F.; Vinatier, C.; Guicheux, J.; Daculsi, G.; Gaudray, P.; Carle, G.F.; Rochet, N. Three-dimensional culture and differentiation of human osteogenic cells in an injectable hydroxypropylmethylcellulose hydrogel. Biomaterials 2005, 26, 5509–5517. [Google Scholar] [CrossRef]
- Aubeux, D.; Beck, L.; Weiss, P.; Guicheux, J.; Enkel, B.; Pérez, F.; Simon, S. Assessment and quantification of noncollagenic matrix proteins released from human dentin powder incorporated into a silated hydroxypropylmethylcellulose biomedical hydrogel. J. Endod. 2016, 42, 1371–1376. [Google Scholar] [CrossRef]
- Teti, G.; Salvatore, V.; Focaroli, S.; Durante, S.; Mazzotti, A.; Dicarlo, M.; Mattioli-Belmonte, M.; Orsini, G. In vitro osteogenic and odontogenic differentiation of human dental pulp stem cells seeded on carboxymethyl cellulose-hydroxyapatite hybrid hydrogel. Front. Physiol. 2015, 6, 297. [Google Scholar] [CrossRef]
- Iftikhar, S.; Zahid, S.; Safi, S.Z.; Khan, A.F.; Nawshad, M.; Ghafoor, S.; Khan, A.S.; Tufail, A. Smart injectable self-setting bioceramics for dental applications. Mater. Sci. Eng. C 2020, 113, 110956. [Google Scholar]
- Badylak, S.F.; Lantz, G.C.; Coffey, A.; Geddes, L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J. Surg. Res. 1989, 47, 74–80. [Google Scholar] [CrossRef]
- Lantz, G.C.; Badylak, S.F.; Coffey, A.C.; Geddes, L.A.; Blevins, W.E. Small intestinal submucosa as a small-diameter arterial graft in the dog. J. Investig. Surg. 1990, 3, 217–227. [Google Scholar] [CrossRef]
- Lantz, G.C.; Badylak, S.F.; Coffey, A.C.; Geddes, L.A.; Sandusky, G.E. Small intestinal submucosa as a superior vena cava graft in the dog. J. Surg. Res. 1992, 53, 175–181. [Google Scholar] [CrossRef]
- Sandusky Jr, G.; Badylak, S.; Morff, R.; Johnson, W.; Lantz, G. Histologic findings after in vivo placement of small intestine submucosal vascular grafts and saphenous vein grafts in the carotid artery in dogs. AJP 1992, 140, 317. [Google Scholar]
- Lantz, G.C.; Badylak, S.F.; Hiles, M.C.; Coffey, A.C.; Geddes, L.A.; Kokini, K.; Sandusky, G.E.; Morff, R.J. Small intestinal submucosa as a vascular graft: A review. J. Investig. Surg. 1993, 6, 297–310. [Google Scholar] [CrossRef]
- Voytik-Harbin, S.L.; Brightman, A.O.; Waisner, B.Z.; Robinson, J.P.; Lamar, C.H. Small intestinal submucosa: A tissue-derived extracellular matrix that promotes tissue-specific growth and differentiation of cells in vitro. Tissue Eng. 1998, 4, 157–174. [Google Scholar] [CrossRef]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Zantop, T.; Gilbert, T.W.; Yoder, M.C.; Badylak, S.F. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. J. Orthop. Res. 2006, 24, 1299–1309. [Google Scholar] [CrossRef]
- Drake, M.; Davison, P.; Bump, S.; Schmitt, F. Action of proteolytic enzymes on tropocollagen and insoluble collagen. Biochemistry 1966, 5, 301–312. [Google Scholar] [CrossRef]
- Miller, E. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry 1972, 11, 4903–4909. [Google Scholar] [CrossRef]
- Uriel, S.; Labay, E.; Francis-Sedlak, M.; Moya, M.L.; Weichselbaum, R.R.; Ervin, N.; Cankova, Z.; Brey, E.M. Extraction and assembly of tissue-derived gels for cell culture and tissue engineering. Tissue Eng. Part C Methods 2009, 15, 309–321. [Google Scholar] [CrossRef]
- Cheng, M.H.; Uriel, S.; Moya, M.L.; Francis-Sedlak, M.; Wang, R.; Huang, J.J.; Chang, S.Y.; Brey, E.M. Dermis-derived hydrogels support adipogenesis in vivo. J. Biomed. Mater. Res. A 2010, 92, 852–858. [Google Scholar]
- Pilipchuk, S.P.; Vaicik, M.K.; Larson, J.C.; Gazyakan, E.; Cheng, M.H.; Brey, E.M. Influence of crosslinking on the stiffness and degradation of dermis-derived hydrogels. J. Biomed. Mater. Res. A 2013, 101, 2883–2895. [Google Scholar] [CrossRef]
- Poon, C.J.; Cotta, M.V.P.E.; Sinha, S.; Palmer, J.A.; Woods, A.A.; Morrison, W.A.; Abberton, K.M. Preparation of an adipogenic hydrogel from subcutaneous adipose tissue. Acta Biomater. 2013, 9, 5609–5620. [Google Scholar] [CrossRef]
- Freytes, D.O.; Martin, J.; Velankar, S.S.; Lee, A.S.; Badylak, S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 2008, 29, 1630–1637. [Google Scholar] [CrossRef]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Chatzistavrou, X.; Faulk, D.; Badylak, S.; Zheng, L.; Papagerakis, S.; Ge, L.; Liu, H.; Papagerakis, P. Biological and bactericidal properties of Ag-doped bioactive glass in a natural extracellular matrix hydrogel with potential application in dentistry. Eur. Cell Mater. 2015, 29, 342–355. [Google Scholar] [CrossRef]
- Chatzistavrou, X.; Fenno, J.C.; Faulk, D.; Badylak, S.; Kasuga, T.; Boccaccini, A.R.; Papagerakis, P. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater. 2014, 10, 3723–3732. [Google Scholar] [CrossRef]
- Li, J.; Rao, Z.; Zhao, Y.; Xu, Y.; Chen, L.; Shen, Z.; Bai, Y.; Lin, Z.; Huang, Q. A Decellularized Matrix Hydrogel Derived from Human Dental Pulp Promotes Dental Pulp Stem Cell Proliferation, Migration, and Induced Multidirectional Differentiation In Vitro. J. Endod. 2020. [Google Scholar] [CrossRef]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M.; Kawana, K.Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin. Oral Investig. 2020, 1–12. [Google Scholar] [CrossRef]
- Meza, G.; Urrejola, D.; Saint Jean, N.; Inostroza, C.; López, V.; Khoury, M.; Brizuela, C. Personalized Cell Therapy for Pulpitis Using Autologous Dental Pulp Stem Cells and Leukocyte Platelet-rich Fibrin: A Case Report. J. Endod. 2019, 45, 144–149. [Google Scholar] [CrossRef]
- Mittal, N.; Parashar, V. Regenerative Evaluation of Immature Roots using PRF and Artificial Scaffolds in Necrotic Permanent Teeth: A Clinical Study. JCDP 2019, 20, 720–726. [Google Scholar] [CrossRef]
- Garnica-Palafox, I.M.; Sánchez-Arévalo, F.M. Influence of natural and synthetic crosslinking reagents on the structural and mechanical properties of chitosan-based hybrid hydrogels. Carbohydr. Polym. 2016, 151, 1073–1081. [Google Scholar] [CrossRef]
- Munim, S.A.; Raza, Z.A. Poly(lactic acid) based hydrogels: Formation, characteristics and biomedical applications. J. Porous Mater. 2019, 26, 881–901. [Google Scholar] [CrossRef]
- Gibas, I.; Janik, H. Review: Synthetic polymer hydrogels for biomedical applications. Chem. Chem. Technol 2010, 4, 297–304. [Google Scholar]
- Böstman, O.M.; Pihlajamäki, H.K. Adverse tissue reactions to bioabsorbable fixation devices. Clin. Orthop. Relat. Res. 2000, 371, 216–227. [Google Scholar] [CrossRef]
- Basu, A.; Kunduru, K.R.; Doppalapudi, S.; Domb, A.J.; Khan, W. Poly(lactic acid) based hydrogels. Adv. Drug Del. Rev. 2016, 107, 192–205. [Google Scholar] [CrossRef]
- Tsuji, H. Poly(Lactic Acid). In Bio-Based Plastics; Kabasci, S., Ed.; Wiley Online Books: Hoboken, NJ, USA, 2013; pp. 171–239. [Google Scholar] [CrossRef]
- Kuang, R.; Zhang, Z.; Jin, X.; Hu, J.; Gupte, M.J.; Ni, L.; Ma, P.X. Nanofibrous Spongy Microspheres Enhance Odontogenic Differentiation of Human Dental Pulp Stem Cells. Adv. Healthc. Mater. 2015, 4, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Wang, G.; Song, F.; Shi, X. Investigation of human dental pulp cells on a potential injectable poly (lactic-co-glycolic acid) microsphere scaffold. J. Endod. 2017, 43, 745–750. [Google Scholar] [CrossRef]
- Jeong, B.; Bae, Y.H.; Lee, D.S.; Kim, S.W. Biodegradable block copolymers as injectable drug-delivery systems. Nature 1997, 388, 860–862. [Google Scholar] [CrossRef]
- Shiehzadeh, V.; Aghmasheh, F.; Shiehzadeh, F.; Joulae, M.; Kosarieh, E.; Shiehzadeh, F. Healing of large periapical lesions following delivery of dental stem cells with an injectable scaffold: New method and three case reports. Indian J. Dent. Res. 2014, 25, 248. [Google Scholar] [CrossRef]
- Stringer, D. Linear polydimethylsiloxanes:(Viscosity 10–10,000 Centistokes): CAS; European Centre for Ecotoxicology and Toxicology of Chemicals: Brussels, Belgium, 1994. [Google Scholar]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, M.; Zhang, Q.; Zhang, T.; Tian, T.; Ma, Q.; Xue, C.; Lin, S.; Cai, X. Stiffness regulates the proliferation and osteogenic/odontogenic differentiation of human dental pulp stem cells via the WNT signalling pathway. Cell Prolif. 2018, 51, e12435. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Xie, J.; Yao, Y.; Cun, X.; Lin, S.; Tian, T.; Zhu, B.; Lin, Y. The role of WNT Signaling in engineering functional vascular networks for tissue regeneration. Bone Res. 2017, 5, 17048. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Xiao, D.; Weng, J.; Xiong, A.; Kang, B.; Zeng, H. Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway. Toxicol. Lett. 2016, 240, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bardet, C.; Mouraret, S.; Liu, B.; Singh, G.; Sadoine, J.; Dhamdhere, G.; Smith, A.; Tran, X.V.; Joy, A. Wnt acts as a prosurvival signal to enhance dentin regeneration. JBMR 2015, 30, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sasaki, J.; Hashimoto, M.; Katata, C.; Hayashi, M.; Imazato, S. Pulp regeneration by 3-dimensional dental pulp stem cell constructs. J. Dent. Res. 2018, 97, 1137–1143. [Google Scholar] [CrossRef]
- Mano, J.F. Stimuli-responsive polymeric systems for biomedical applications. Adv. Eng. Mater 2008, 10, 515–527. [Google Scholar] [CrossRef]
- Yoshida, R.; Okano, T. Stimuli-responsive hydrogels and their application to functional materials. In Biomedical Applications of Hydrogels Handbook; Ottenbrite, R.M., Park, K., Okano, T., Eds.; Springer Science & Business Media: New York, NY, USA, 2010; pp. 19–43. [Google Scholar]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef]
- Buxton, A.N.; Zhu, J.; Marchant, R.; West, J.L.; Yoo, J.U.; Johnstone, B. Design and characterization of poly (ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007, 13, 2549–2560. [Google Scholar] [CrossRef]
- Beamish, J.A.; Zhu, J.; Kottke-Marchant, K.; Marchant, R.E. The effects of monoacrylated poly (ethylene glycol) on the properties of poly (ethylene glycol) diacrylate hydrogels used for tissue engineering. J. Biomed. Mater. Res. A 2010, 92, 441–450. [Google Scholar] [CrossRef]
- Yang, F.; Williams, C.G.; Wang, D.-A.; Lee, H.; Manson, P.N.; Elisseeff, J. The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 26, 5991–5998. [Google Scholar] [CrossRef]
- Gyawali, D.; Nair, P.; Zhang, Y.; Tran, R.T.; Zhang, C.; Samchukov, M.; Makarov, M.; Kim, H.K.; Yang, J. Citric acid-derived in situ crosslinkable biodegradable polymers for cell delivery. Biomaterials 2010, 31, 9092–9105. [Google Scholar] [CrossRef] [PubMed]
- Komabayashi, T.; Wadajkar, A.; Santimano, S.; Ahn, C.; Zhu, Q.; Opperman, L.A.; Bellinger, L.L.; Yang, J.; Nguyen, K.T. Preliminary study of light-cured hydrogel for endodontic drug delivery vehicle. J. Investig. Clin. Dent. 2016, 7, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng. Part A 2015, 21, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Webber, M.J.; Stupp, S.I. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers 2010, 94, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Jonker, A.M.; Löwik, D.W.; van Hest, J.C. Peptide-and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Rivas, M.; Del Valle, L.J.; Alemán, C.; Puiggalí, J. Peptide self-assembly into hydrogels for biomedical applications related to hydroxyapatite. Gels 2019, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Bao, L.; Lin, T.; Lu, Y.; Wu, Y. Proliferation and odontogenic differentiation of human umbilical cord mesenchymal stem cells and human dental pulp cells co-cultured in hydrogel. Arch. Oral Biol. 2020, 109, 104582. [Google Scholar] [CrossRef]
- Yokoi, H.; Kinoshita, T.; Zhang, S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005, 102, 8414–8419. [Google Scholar] [CrossRef]
- Narmoneva, D.A.; Oni, O.; Sieminski, A.L.; Zhang, S.; Gertler, J.P.; Kamm, R.D.; Lee, R.T. Self-assembling short oligopeptides and the promotion of angiogenesis. Biomaterials 2005, 26, 4837–4846. [Google Scholar] [CrossRef]
- Thonhoff, J.R.; Lou, D.I.; Jordan, P.M.; Zhao, X.; Wu, P. Compatibility of human fetal neural stem cells with hydrogel biomaterials in vitro. Brain Res. 2008, 1187, 42–51. [Google Scholar] [CrossRef]
- Davis, M.E.; Hsieh, P.C.; Takahashi, T.; Song, Q.; Zhang, S.; Kamm, R.D.; Grodzinsky, A.J.; Anversa, P.; Lee, R.T. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl. Acad. Sci. USA 2006, 103, 8155–8160. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.M.; Tokunou, T.; Higgins, L.J.; MacGillivray, C.; Gannon, J.; Lee, R.T. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation 2007, 116, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Motion, J.M.; Narmoneva, D.A.; Takahashi, T.; Hakuno, D.; Kamm, R.D.; Zhang, S.; Lee, R.T. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation 2005, 111, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.C.; Davis, M.E.; Gannon, J.; MacGillivray, C.; Lee, R.T. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J. Clin. Investig. 2006, 116, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Semino, C.E.; Kasahara, J.; Hayashi, Y.; Zhang, S. Entrapment of migrating hippocampal neural cells in three-dimensional peptide nanofiber scaffold. Tissue Eng. 2004, 10, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nagrath, D.; Chen, P.C.; Berthiaume, F.; Yarmush, M.L. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng. Part A. 2008, 14, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Misawa, H.; Kobayashi, N.; Soto-Gutierrez, A.; Chen, Y.; Yoshida, A.; Rivas-Carrillo, J.D.; Navarro-Alvarez, N.; Tanaka, K.; Miki, A.; Takei, J. PuraMatrix™ facilitates bone regeneration in bone defects of calvaria in mice. Cell Transplant. 2006, 15, 903–910. [Google Scholar] [CrossRef]
- Cavalcanti, B.N.; Zeitlin, B.D.; Nör, J.E. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dent. Mater. 2013, 29, 97–102. [Google Scholar] [CrossRef]
- Sakai, V.; Zhang, Z.; Dong, Z.; Neiva, K.; Machado, M.; Shi, S.; Santos, C.; Nör, J. SHED differentiate into functional odontoblasts and endothelium. J. Dent. Res. 2010, 89, 791–796. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. In vitro analysis of scaffold-free prevascularized microtissue spheroids containing human dental pulp cells and endothelial cells. J. Endod. 2015, 41, 663–670. [Google Scholar] [CrossRef]
- Rombouts, C.; Giraud, T.; Jeanneau, C.; About, I. Pulp vascularization during tooth development, regeneration, and therapy. J. Dent. Res. 2017, 96, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Zentilin, L.; Tafuro, S.; Zacchigna, S.; Arsic, N.; Pattarini, L.; Sinigaglia, M.; Giacca, M. Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood 2006, 107, 3546–3554. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF gene therapy: Stimulating angiogenesis or angioma-genesis? Nat. Med. 2000, 6, 1102–1103. [Google Scholar] [CrossRef] [PubMed]
- Marsboom, G.; Pokreisz, P.; Gheysens, O.; Vermeersch, P.; Gillijns, H.; Pellens, M.; Liu, X.; Collen, D.; Janssens, S. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem. Cells 2008, 26, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Jahr, H.; van Osch, G.J.; Farrell, E. The role of hypoxia in bone marrow–derived mesenchymal stem cells: Considerations for regenerative medicine approaches. Tissue Eng. Part B Rev. 2010, 16, 159–168. [Google Scholar] [CrossRef]
- Grunewald, M.; Avraham, I.; Dor, Y.; Bachar-Lustig, E.; Itin, A.; Yung, S.; Chimenti, S.; Landsman, L.; Abramovitch, R.; Keshet, E. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell 2006, 124, 175–189. [Google Scholar] [CrossRef]
- Cordeiro, M.M.; Dong, Z.; Kaneko, T.; Zhang, Z.; Miyazawa, M.; Shi, S.; Smith, A.J.; Nör, J.E. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J. Endod. 2008, 34, 962–969. [Google Scholar] [CrossRef]
- Rouwkema, J.; Rivron, N.C.; van Blitterswijk, C.A. Vascularization in tissue engineering. Trends Biotechnol. 2008, 26, 434–441. [Google Scholar] [CrossRef]
- Rouwkema, J.; Boer, J.D.; Blitterswijk, C.A.V. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006, 12, 2685–2693. [Google Scholar] [CrossRef]
- Kelm, J.M.; Djonov, V.; Ittner, L.M.; Fluri, D.; Born, W.; Hoerstrup, S.P.; Fussenegger, M. Design of custom-shaped vascularized tissues using microtissue spheroids as minimal building units. Tissue Eng. 2006, 12, 2151–2160. [Google Scholar] [CrossRef]
- Dong, H.; Paramonov, S.E.; Aulisa, L.; Bakota, E.L.; Hartgerink, J.D. Self-assembly of multidomain peptides: Balancing molecular frustration controls conformation and nanostructure. J. Am. Chem. Soc. 2007, 129, 12468–12472. [Google Scholar] [CrossRef]
- Ferrara, N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: Therapeutic implications. Semin. Oncol. 2002, 29, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, Y.; Ueda, M.; Ozasa, M.; Anzai, J.; Takedachi, M.; Yanagita, M.; Ito, M.; Hashikawa, T.; Yamada, S.; Murakami, S. Fibroblast growth factor-2 regulates the cell function of human dental pulp cells. J. Endod. 2009, 35, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Kiick, K.L. Hybrid multicomponent hydrogels for tissue engineering. Macromol. Biosci. 2009, 9, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Del. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.b.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef]
- Xavier, J.R.; Thakur, T.; Desai, P.; Jaiswal, M.K.; Sears, N.; Cosgriff-Hernandez, E.; Kaunas, R.; Gaharwar, A.K. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factor-free approach. ACS Nano 2015, 9, 3109–3118. [Google Scholar] [CrossRef]
- Feng, S.; Liu, J.; Ramalingam, M. 3D Printing of Stem Cell Responsive Ionically-Crosslinked Polyethylene Glycol Diacrylate/Alginate Composite Hydrogels Loaded with Basic Fibroblast Growth Factor for Dental Pulp Tissue Engineering: A Preclinical Evaluation in Animal Model. J. Biomater. Tissue Eng. 2020, 9, 1635–1643. [Google Scholar] [CrossRef]
- Lu, Q.; Pandya, M.; Rufaihah, A.J.; Rosa, V.; Tong, H.J.; Seliktar, D.; Toh, W.S. Modulation of dental pulp stem cell odontogenesis in a tunable PEG-fibrinogen hydrogel system. Stem Cells Int. 2015, 2015, 525367. [Google Scholar] [CrossRef]
- Jones, T.; Kefi, A.; Sun, S.; Cho, M.; Alapati, S. An optimized injectable hydrogel scaffold supports human dental pulp stem cell viability and spreading. Adv. Med. 2016, 2016, 7363579. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine. JCR 2011, 155, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sumita, Y.; Honda, M.J.; Ohara, T.; Tsuchiya, S.; Sagara, H.; Kagami, H.; Ueda, M. Performance of collagen sponge as a 3-D scaffold for tooth-tissue engineering. Biomaterials 2006, 27, 3238–3248. [Google Scholar] [CrossRef] [PubMed]
- Radice, M.; Brun, P.; Cortivo, R.; Scapinelli, R.; Battaliard, C.; Abatangelo, G. Hyaluronan-based biopolymers as delivery vehicles for bone-marrow-derived mesenchymal progenitors. J. Biomed. Mater. Res. 2000, 50, 101–109. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Toh, W.S.; Loh, X.J. Advances in hydrogel delivery systems for tissue regeneration. Mater. Sci. Eng. C 2014, 45, 690–697. [Google Scholar] [CrossRef]
- Liu, L.; Kuffová, L.; Griffith, M.; Dang, Z.; Muckersie, E.; Liu, Y.; McLaughlin, C.R.; Forrester, J.V. Immunological responses in mice to full-thickness corneal grafts engineered from porcine collagen. Biomaterials 2007, 28, 3807–3814. [Google Scholar] [CrossRef]
- Chirani, N.; Gritsch, L.; Motta, F.L.; Fare, S. History and applications of hydrogels. J. Biomed. Sci. 2015, 4, 1–23. [Google Scholar]
- Varghese, S.; Elisseeff, J.H. Hydrogels for Musculoskeletal Tissue Engineering. In Polymers for Regenerative Medicine; Werner, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 95–144. [Google Scholar] [CrossRef]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef]
- Guvendiren, M.; Burdick, J.A. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 2013, 24, 841–846. [Google Scholar] [CrossRef]
- Galler, K.M.; Brandl, F.P.; Kirchhof, S.; Widbiller, M.; Eidt, A.; Buchalla, W.; Goepferich, A.; Schmalz, G. Suitability of different natural and synthetic biomaterials for dental pulp tissue engineering. Tissue Eng. Part A. 2018, 24, 234–244. [Google Scholar] [CrossRef]
- Del Amo, C.; Perez-Valle, A.; Perez-Zabala, E.; Perez-del-Pecho, K.; Larrazabal, A.; Basterretxea, A.; Bully, P.; Andia, I. Wound Dressing Selection Is Critical to Enhance Platelet-Rich Fibrin Activities in Wound Care. Int. J. Mol. Sci. 2020, 21, 624. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Winer, J.P.; Weisel, J.W. Fibrin gels and their clinical and bioengineering applications. J. R. Soc. Interface 2009, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gong, T.; Xie, J.; Lin, S.; Liu, Y.; Zhou, T.; Lin, Y. Softening Substrates Promote Chondrocytes Phenotype via RhoA/ROCK Pathway. ACS Appl. Mater. Interfaces 2016, 8, 22884–22891. [Google Scholar] [CrossRef]
- Rony, R. Transcriptional Characterization of Osteogenic and Adipogenic Differentiation of human Bone Marrow Derived Mesenchymal Stem Cells in 2D and 3D Peptide Hydrogel Culture System. Ph.D. Thesis, Wright State University, Dayton, OH, USA, 2018. [Google Scholar]
- Casagrande, L.; Demarco, F.; Zhang, Z.; Araujo, F.; Shi, S.; Nör, J. Dentin-derived BMP-2 and odontoblast differentiation of SHED. J. Dent. Res. 2010, 89, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.C.; Septier, D.; Bonnefoix, M.; Lecolle, S.; Lebreton-Decoster, C.; Coulomb, B.; Pellat, B.; Godeau, G. Human dermal and gingival fibroblasts in a three-dimensional culture: A comparative study on matrix remodeling. Clin. Oral Investig. 2002, 6, 39–50. [Google Scholar] [CrossRef]
- Smith, J.; Smith, A.; Shelton, R.; Cooper, P. Recruitment of dental pulp cells by dentine and pulp extracellular matrix components. Exp. Cell Res. 2012, 318, 2397–2406. [Google Scholar] [CrossRef]
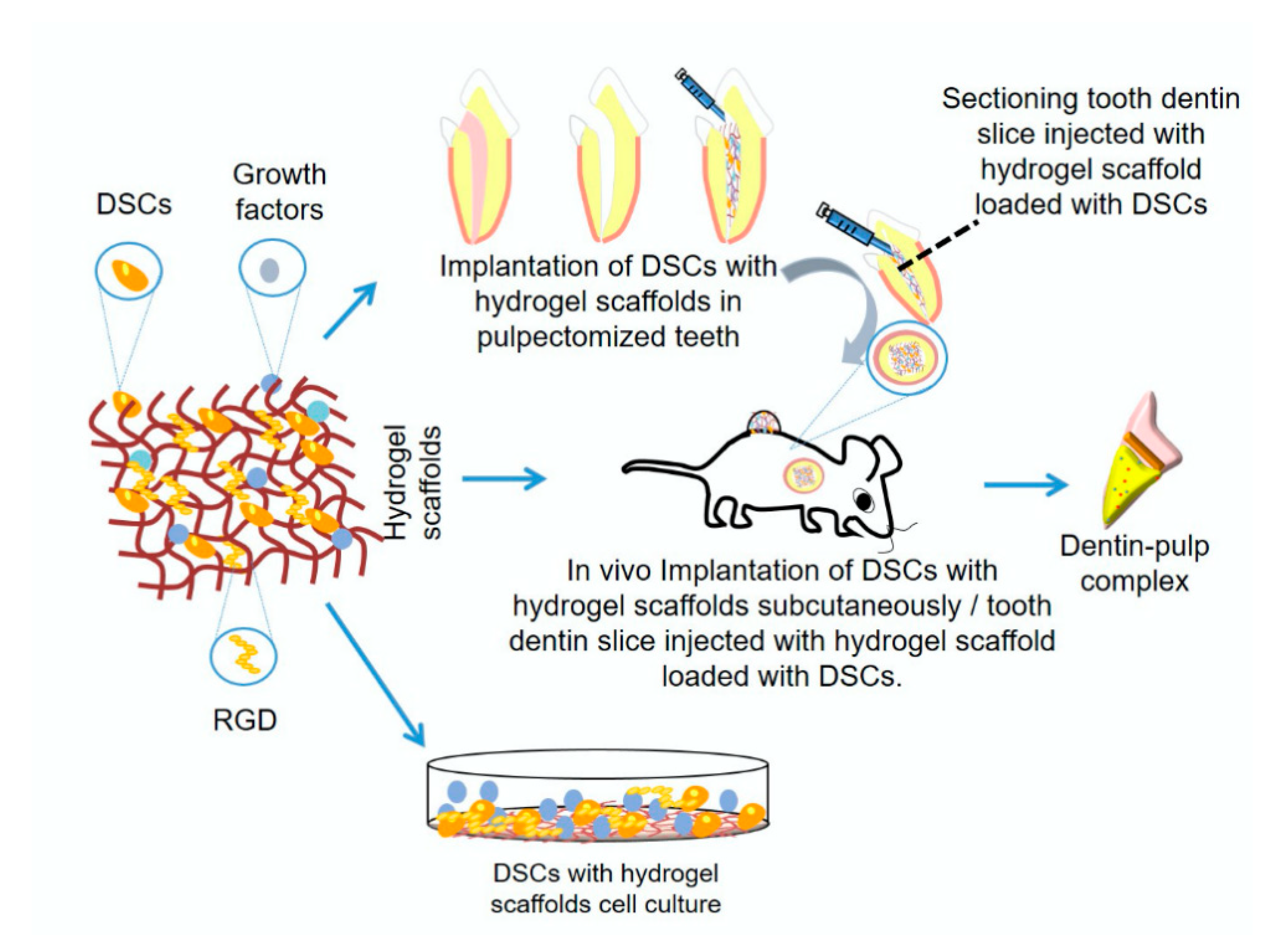
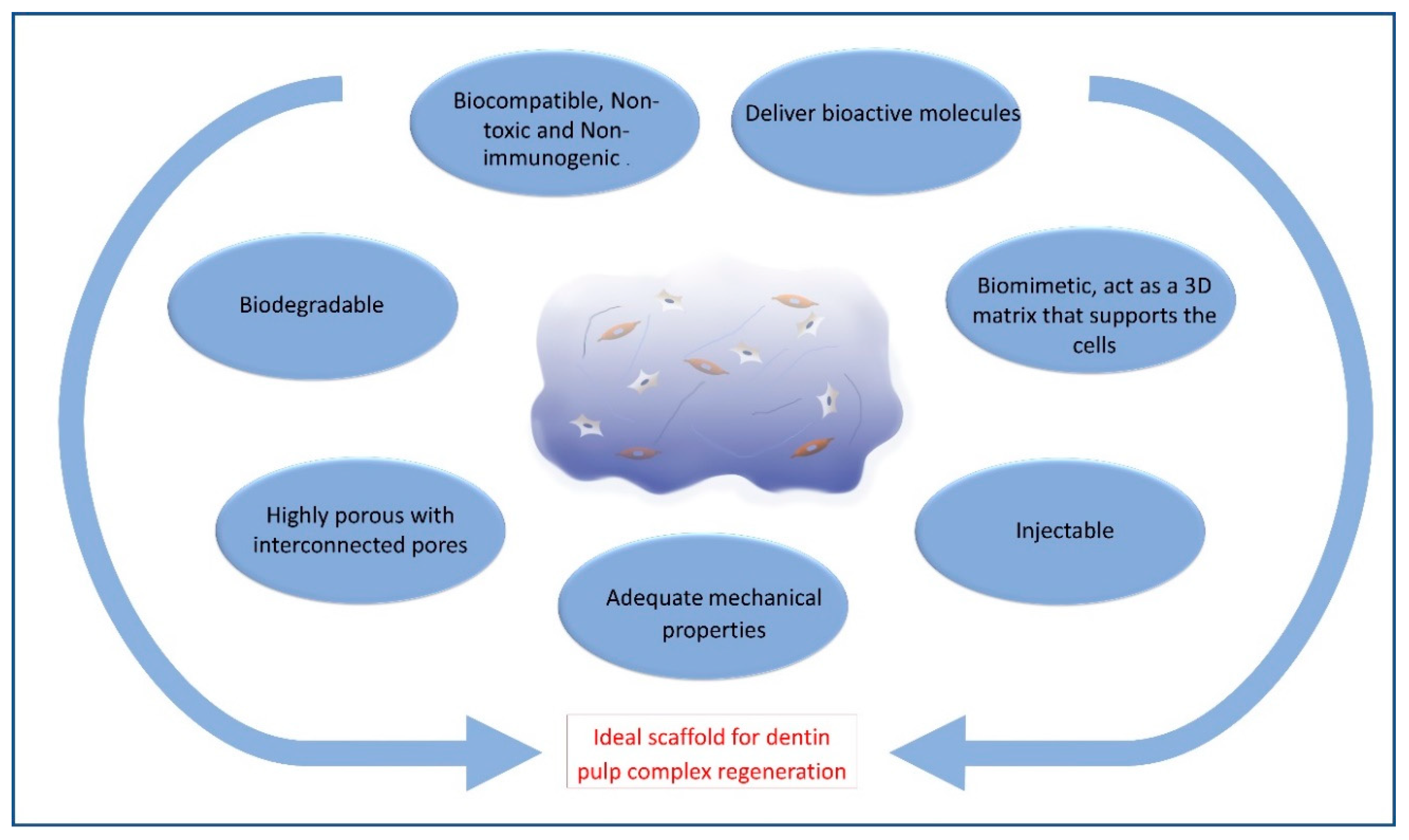
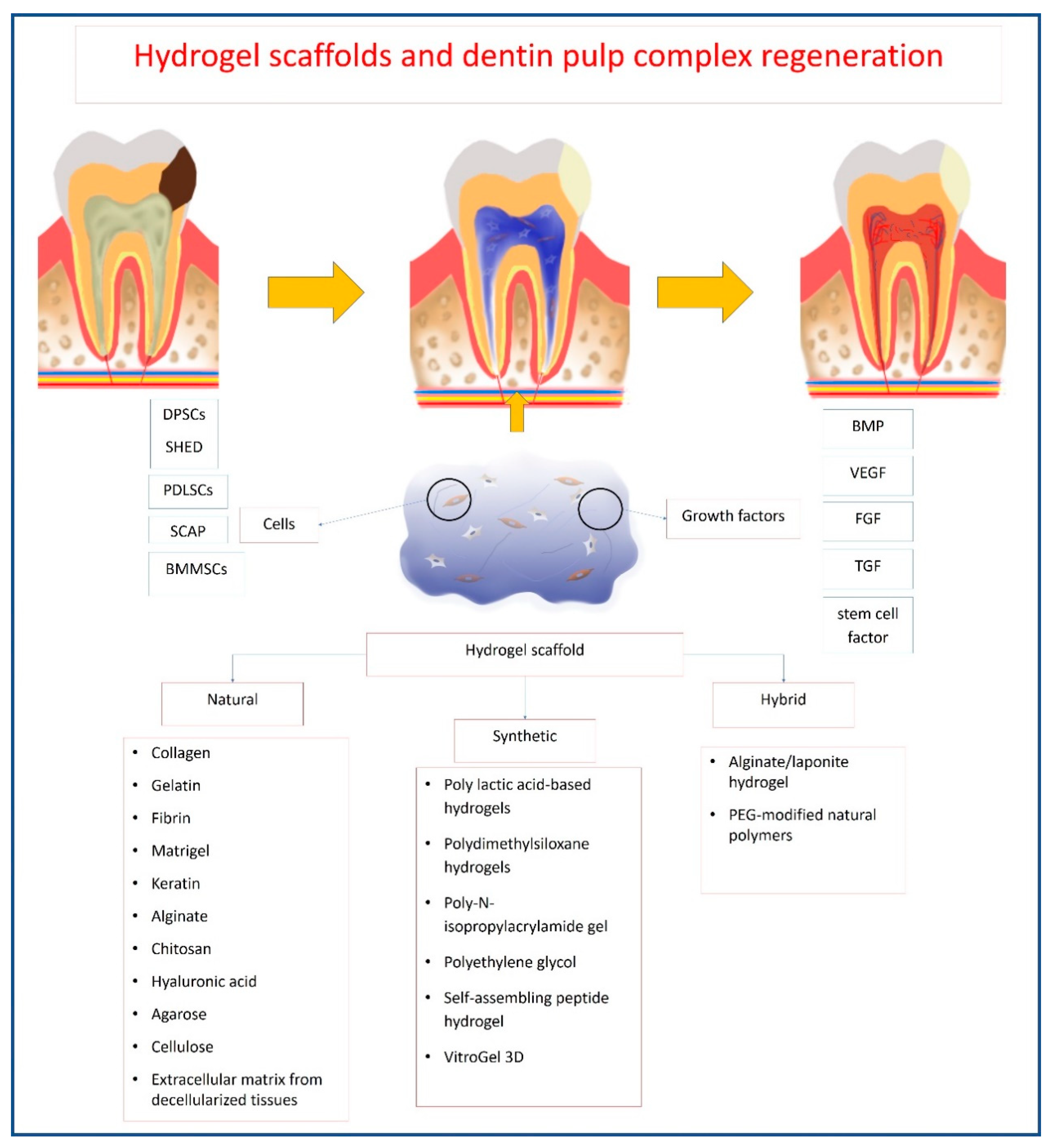
| Natural Hydrogels | |||||||
|---|---|---|---|---|---|---|---|
| Author | Hydrogel Used | Type of Study | Hydrogel Modification | Hydrogel Properties | Cells Used | Upregulated Biological Molecules | Outcomes |
| Collagen Hydrogel | |||||||
| Souron et al., 2014 [104] | Collagen | In vivo | 3D collagen matrices, mimic in vivo cell–cell and cell–matrix interactions, and regulate cell growth | Rat pulp cells labeled with indium-111-oxine | 1 month following implantation, active fibroblasts, new blood vessels and nervous fibers were present in the cellularized 3D collagen hydrogel. | ||
| Kwon et al., 2017 [60] | Collagen | In vitro | Crosslinked with cinnamaldehyde (CA). | Crosslinked collagen with shorter gelation time enhances cellular adhesion. Higher stiffness enhances odontogenic differentiation. | Human dental pulp stem cells (DPSCs) | Dentin sialophosphoprotein (DSPP), Dentin matrix protein 1 (DMP-1), Matrix extracellular phosphoglycoprotein (MEPE), Osteonectin (ON) | CA shortened the setting time, increased compressive strength and surface roughness of collagen hydrogels. CA-crosslinked hydrogels promoted the proliferation and odontogenic differentiation of human DPSCs |
| Pankajakshan et al., 2020 [61] | Collagen | In vitro | Varying hydrogel stiffnesses through varying oligomer concentrations. Incorporation of Vascular endothelial growth factor (VEGF) into 235 Pa collagen or Bone morphogenetic protein 2 (BMP-2) into the 800 Pa ones. | Stiffness affect cytoskeletal organization and cell shape and specify stem cell lineage. | DPSCs | von Willebrand Factor (vWF), platelet endothelial cell adhesion molecule 1 (PECAM-1), vascular endothelial-cadherin | Collagen hydrogels with tunable stiffness supported cell survival, and favored differentiation of cells to a specific lineage. DPSCs cultured in 235 Pa matrices showed an increased expression of endothelial markers, cells cultured in 800 Pa showed increased alkaline phosphatase (ALP) activity and Alizarin S staining. |
| Gelatin Hydrogel | |||||||
| Kikuchi et al., 2007 [84] | Gelatin hydrogel | In vivo | Crosslinked gelatin hydrogel microspheres were impregnated with fibroblast growth factor 2 (FGF-2) and mixed with collagen sponge pieces. | Gelatin hydrogel microspheres (the water content is 95 vol%; diameters ranged from 5–15 µm; the average of diameter was 10 µm) | DSPP | Controlled release of FGF2 from gelatin hydrogels induced the formation of dentin-like particles with dentin defects above exposed pulp. | |
| Ishimatsu et al., 2009 [117] | Gelatin hydrogel | In vivo | Gelatin hydrogel microspheres incorporating FGF-2. | Gelatin hydrogel microspheres (the water content is 95 vol%, the average diameter was 10 µm) | DMP-1 | Dentin regeneration on amputated pulp can be regulated by adjusting the dosage of FGF-2 incorporated in biodegradable gelatin hydrogels. | |
| Nageh et al., 2013 [85] | Gelatin hydrogel | Clinical trial | FGF incorporated in gelatin hydrogel. | Acidic gelatin hydrogel microspheres with a mean diameter of 59 µm and 95.2% water content. | Follow-up X-ray revealed an increase in root length and width with a reduction in apical diameter confirming the root’s development. | ||
| Bhatnagar et al., 2015 [62] | Gelatin hydrogel | In vitro | enzymatically crosslinked with microbial transglutaminase (mTG). | Hard and soft gelatin–mTG gels consist of 1.125 mL and 1.488 mL of a 10% gelatin solution with 0.375 mL (3:1 (v/v) gelatin: mTG), and 0.012 mL (125:1 (v/v) gelatin: mTG) of mTG respectively | DPSCs | Osteocalcin (OCN), ALP, DSPP | Enzymatically crosslinked gelatin hydrogels are a potential effective scaffold for dentin regeneration regardless of matrix stiffness or chemical stimulation using dexamethasone. |
| Miyazawa et al., 2015 [63] | Gelatin hydrogel | In vitro In vivo | Simvastatin-lactic acid grafted gelatin micelles, mixed with gelatin, followed by chemical crosslinking to form gelatin hydrogels. | Carboxymethylcellulose (CMC) value of micelles was 79 μg/mL. Water solubilization of Simvastatin 43 wt.%. Simvastatin in the gelatin 3.23 wt.%. The sizes of granules 500 μm with rough surfaces and uniformly sized pores | DPSCs | ALP, Dentin sialoprotein (DSP), BMP-2 | It is possible to achieve odontoblastic differentiation of DPSCs through the controlled release of Simvastatin from gelatin hydrogel. |
| Gelatin Methacrylate Hydrogel | |||||||
| Athirasala et al., 2017 [68] | Gelatin Methacrylate hydrogel (GelMA) | In vitro | Gelatin with methacrylic anhydride. | GelMA hydrogels of 5, 10 and 15% (w/v) concentrations showed a honeycomb-like structure. Both 10% and 15% hydrogel groups appeared to have smaller pore sizes than 5% GelMA. | Odontoblasts like cells (OD21) and endothelial colony- forming cells | Pre-vascularized hydrogel scaffolds with microchannels fabricated using GelMA is a simple and effective strategy for dentin–pulp complex regeneration. | |
| Khayat et al., 2017 [64] | GelMA | In vivo | Gelatin with methacrylic anhydride. | DPSCs and human umbilical vein endothelial cells (HUVECs) | GelMA hydrogel combined with human DPSC/human umbilical vein endothelial cells as promising pulpal revascularization treatment to regenerate human dental pulp tissues. | ||
| Ha et al., 2020 [72] | GelMA | In vitro | Gelatin with methacrylic anhydride hydrogels of increasing concentrations. | Increasing polymer concentrations from 5% to 10% and 15% (w/v), resulting in increasing extents of crosslinking. The elastic moduli of hydrogels, increased with increase in polymer concentration from 1.7 kPa for 5% GelMA to 7 kPa and 16.4 kPa for 10% and 15% GelMA hydrogels, respectively. | stem cells of the apical papilla (SCAP) | Substrate mechanics and geometry have a statistically significant influence on SCAP response. | |
| Park et al., 2020 [65] | GelMA | In vitro | GelMA conjugated with synthetic BMP-2 mimetic peptide prepared into bioink. | GelMA exhibited a ~2 kPa storage modulus (G’) before crosslinking and ~4 kPa after crosslinking. | DPSCs | DSPP, OCN | BMP peptide-tethering bioink could accelerate the differentiation of human DPSCs in 3D bioprinted dental constructs. |
| Jang et al., 2020 [67] | GelMA | In vitro In vivo | Thrombin solution added to GelMA hydrogel. | DPSCs | Gelatin hemostatic hydrogels may serve as a viable regenerative scaffold for pulp regeneration. | ||
| Fibrin Hydrogel | |||||||
| Meza 2019 [249] | Platelet Rich Fibrin (PRF) | Case report | DPSCs | Autologous DPSCs isolated from extirpated autologous inflamed dental pulp were loaded on autologous PRF in lower premolar tooth with irreversible pulpitis for successful regeneration. | |||
| Ducret et al., 2019 [123] | Fibrin–chitosan | In vitro | Enriching the fibrin-hydrogel with chitosan. | 10 mg/mL fibrinogen and 0.5% (w/w), 40% DA chitosan, formed a hydrogel at physiological pH (≈7.2), which was sufficiently fluid to preserve its injectability without affecting fibrin biocompatibility | DPSCs | Chitosan imparted antibacterial activity to fibrin hydrogel, reducing E. faecalis growth. The blending of chitosan in fibrin hydrogels did not affect the viability, proliferation and collagen-forming capacity of encapsulated DPSCs as compared to unmodified fibrin. | |
| Mittal et al., 2019 [250] | PRF | Clinical trial | PRF and collagen are better scaffolds than placentrex and chitosan for apexogenesis of immature necrotic permanent teeth. | ||||
| Bekhouche et al., 2020 [82] | Fibrin | In vitro | Incorporation of clindamycin loaded Poly (D, L) Lactic Acid nanoparticles (CLIN-loaded PLA NPs). | Fibrin hydrogel constituted a reservoir of CLIN-loaded PLA NPs inhibiting E. faecalis growth without affecting cell viability and function. | DPSCs | Fibrin hydrogels containing CLIN-loaded PLA NPs showed an antibacterial effect against E. faecalis and inhibited biofilm formation. DPSCs viability and type I collagen synthesis in cellularized hydrogels were similar to the unmodified groups. | |
| Renard et al., 2020 [131] | Fibrin-chitosan | In vivo | Same formulation of fibrin–chitosan hydrogel as used by Ducret et al., 2019 [123] | 40% DA chitosan incorporation in the fibrin hydrogel did not modify modify dental pulpi inflammatory/immune response and triggered polarization of pro-regenerative M2 macrophages. | In in vivo model of rat incisor pulpotomy, fibrin–chitosan hydrogels imparted a similar inflammatory response in the amputated pulp as unmodified fibrin. Both groups enhanced the polarization of pro-regenerative M2 macrophages. | ||
| Zhang et al., 2020 [71] | Fibrin | In vitro | Fibrin hydrogel loaded with DPSCs-derived extracellular vesicles (EVs). | 2 mg/mL fibrinogen formed a hydrogel which was able to retain and preserve the activity of EVs. Forming the most extensive tubular network forming at an EVs concentration of 200 µg/mL | Co-culture of DPSCs and HUVECs | VEGF | Investigated hydrogels enhanced rapid neovascularization under starvation culture, increased deposition of collagen I, III, and IV, and promoted the release of VEGF. |
| Matrigel 3D | |||||||
| Mathieu et al., 2013 [143] | Matrigel | In vitro | DPSCs | Encapsulating transforming growth factor beta1 (TGF-b1) and FGF-2 in a biodegradable Poly glycolide-co-lactide (PGLA) microsphere | |||
| Ito et al., 2017 [77] | Matrigel | In vivo | Bone marrow mesenchymal stem cells (BMMSCs) | Nestin, DSPP | Pulp tissue regeneration was successfully achieved. | ||
| Sueyama et al., 2017 [78] | Matrigel | In vivo | BMMSCs and endothelial cells (ECs). | DSPP, Nestin Bcl-2, Cxcl1, Cxcr2, VEGF | The implantation of ECs with mesenchymal stem cells accelerated pulp tissue regeneration/healing and dentin bridge formation. | ||
| Gu et al., 2018 [79] | Matrigel | In vivo | BMMSCs | M1-to-M2 transition of macrophages plays an important role in creating a favorable microenvironment necessary for pulp tissue regeneration. | |||
| Kaneko et al., 2019 [80] | Matrigel | In vivo | BMMSCs nucleofected with pVectOZ-LacZ plasmid encoding β-galactosidase | DSPP | BMMSCs could differentiate into cells involved in mineralized tissue formation in the functionally relevant region. | ||
| Keratin Hydrogel | |||||||
| Sharma et al., 2016 [69] | Keratin hydrogel | In vitro | Highly branched interconnected porous micro-architecture with a maximum average pore size of 160 µm and minimum pore size of 25 µm. G′ > G″ indicates the elastic solid-like nature of the gel. After 3 months the degradation rate was 68%. | odontoblast-like cells (MDPC-23) | ALP, DMP-1 | Keratin enhanced proliferation and odontoblastic differentiation of odontoblast-like cells. Keratin hydrogels may be a potential scaffold for pulp–dentin regeneration. | |
| Sharma et al., 2016 [70] | Keratin hydrogel | In vitro In vivo | Highly branched interconnected porous micro-architecture with a maximum average pore size of 160 µm and minimum pore size of 25 µm. G′ > G′′ indicates the elastic solid-like nature of the gel. After 3 months the degradation rate was 68%. | odontoblast-like cells (MDPC-23) and DPSCs | Keratin hydrogel enhanced odontogenic differentiation of odontoblast-like cells and enhanced reparative dentin formation. | ||
| Sharma et al., 2017 [150] | Keratin hydrogel | In vivo | Branched interconnected porous micro-architecture with average pore size 163.5 and porosity 82.8%. There was a gradual increase in G’ from 7% to 20% (w/v) gel concentration. The average contact angle was 35.5°. | Keratins hydrogel can be a source for biological treatment options for dentin–pulp complex. | |||
| Alginate Hydrogel | |||||||
| Dobie et al., 2002 [86] | Alginate | In vitro | TGF- β1 or HCL acid-treatment of the hydrogels. | Alginate hydrogels are valuable for delivery of growth factors (GFs) (or agents to release endogenous GFs) to enhance reparative processes of dentin–pulp complex. | Alginate hydrogel acted as an efficient carrier for TGF- β1. Furthermore, acid treatment of the hydrogel aided in the release of TGF- β1 from dentin matrix. Alginate–TGF–β1 blends stimulated reactionary dentinogenic responses with increased predentin width. | ||
| Bhoj et al., 2015 [83] | Alginate | In vitro | Arginine-glycine-aspartic acid (RGD)-modified alginate hydrogels, loaded with VEGF and FGF-2. | RGD–alginate matrix acted as pulp replacement, compatible with the DPSCs and HUVECs, and can deliver VEGF and FGF-2. | Co-culture of DPSCs and HUVECs | Combined addition of FGF and VEGF led to an increased proliferation of both DPSCs and HUVECs in the hydrogels. RGD-modified alginate can efficiently retain VEGF and FGF-2. | |
| Smith et al., 2015 [81] | Alginate | In vitro | Alginate hydrogel doped with bovine dental pulp extracellular matrix (pECM). | 3D Alginate hydrogel doped with pECM formed 3D matrices. pECM provides additional signals for differentiation. | Primary dental pulp cells | Induced differentiation in the mineralizing medium resulted in time-dependent mineral deposition at the periphery of the hydrogel. | |
| Verma et al., 2017 [127] | Alginate–fibrin | In vivo | Oxidized alginate–fibrin hydrogel microbeads. | 7.5% oxidized alginate coupled with fibrinogen concentration of 0.1% enhanced microbead degradation, cell release, and proliferation. | DPSCs | Oxidized alginate–fibrin hydrogel microbeads encapsulating DPSCs showed similar regenerative potential to traditional revascularization protocol in ferret teeth. In both groups, the presence of residual bacteria affected root development. | |
| Athirasala et al., 2018 [168] | Alginate | In vitro | Blending alginate hydrogels with soluble and insoluble fractions of the dentin matrix as a bioink for 3D printing. | Dentin matrix proteins preserve the natural cell-adhesive (RGD) and MMP-binding sites, which are lacking in unmodified alginate, that are important for viability, proliferation, and differentiation. | SCAP | ALP, Runt-related transcription factor -2 (RUNX2) | Alginate and insoluble dentin matrix (in 1:1 ratio) hydrogels bioink significantly enhanced odontogenic differentiation of SCAP under the effect of the soluble dentin molecules in the hydrogel. |
| Yu et al., 2019 [169] | Alginate and gelatin hydrogels | In vitro | 3D bioprinted crosslinked composite alginate and gelatin hydrogels (4% and 20% by weight, respectively). | 3D printing accurately controls the interconnected porosity and pore diameter of the scaffold, and imitate natural cell tissue in vivo. | DPSCs | ALP, OCN, DSPP | 3D-printed alginate and gelatin hydrogels aqueous extracts are more suitable for the growth of DPSCs, and can better promote cell proliferation and differentiation. |
| Chitosan Hydrogel | |||||||
| Park et al., 2013 [179] | Chitosan hydrogel | In vitro | N-acetylation of glycol chitosan | Glycol chitosan (0.2 g) and acetic anhydride (0.87 g) were dissolved in 50 mL of a mixture of distilled water and methanol (50/50, v/v) degree of acetylation 90% pore size ranged from 5 to 40 mm. | Human DPSCs | DSPP, DMP-1, ON, osteopontin | Glycol chitin-based thermo-responsive hydrogel scaffold promoted the proliferation and odontogenic differentiation of human DPSCs. |
| El Ashiry et al., 2018 [182] | Chitosan hydrogel | In vivo | Chitosan 1 g; 77% deacylation, high molecular weight, was dissolved in 2% acetic acid. | DPSCs | DPSCs and GFs incorporated in chitosan hydrogel can regenerate pulp–dentin-like tissue in non-vital immature permanent teeth with apical periodontitis in dogs. | ||
| Wu et al., 2019 [180] | Chitosan hydrogel | In vitro | beta-sodium glycerophosphate added to chitosan (CS/β-GP). | viscosity: 200–400 m Pa·s 2% (w/v) chitosan solution 56% (w/v) beta-sodium glycerophosphate (β-GP) solution CS: β-GP is 5/L | DPSCs | VEGF, ALP, OCN, Osterix, DSPP | CS/β-GP hydrogel could release VEGF continually and promote odontogenic differentiation of DPSCs. |
| Zhu et al., 2019 [181] | Chitosan hydrogel | In vitro | Ag-doped bioactive glass micro-size powder particles added to chitosan (Ag-BG/CS). | Ag-BG/CS pore diameter reaching around 60–120 μm. | DPSCs | OCN, ALP, RUNX-2 | Ag-BG/CS enhanced the odontogenic differentiation potential of lipopolysaccharide-induced inflammatory-reacted dental pulp cells and expressed antibacterial and anti-inflammatory activity. |
| Hyaluronic Acid Hydrogel | |||||||
| Chrepa et al., 2016 [198] | Hyaluronic acid (HA) hydrogel | In vitro | SCAP/Restylane 1:10 concentration SCAP/Matrigel mixture at 1:1 concentration 1,4-butanediol diglycidyl ether; DVS, divinyl sulphone crosslinking agent | SCAP | ALP, DSPP, DMP-1, MEPE gene | HA injectable hydrogel promoted SCAP survival, mineralization and differentiation into an odontoblastic phenotype. | |
| Yang et al., 2016 [193] | Hyaluronic acid hydrogel | In vivo | HA crosslinked with 1,4-butanediol diglycidyl ether | HA (1.5 × 106 Da) 1,4-Butanediol diglycidyl ether crosslinking agent HA concentration of 20 mg mL−1 gel particles of 0– 400 mm. | Dental mesenchymal cells | HA is an injectable scaffold that can regenerate cartilage and dentin–pulp complex. | |
| Almeida et al., 2018 [195] | Hyaluronic acid hydrogel | In vitro | Photo crosslinking of methacrylated HA incorporated with PL. | High molecular weight (1.5–1.8 MDa) HA 1% (w/v) HA solution (in distilled water) reacted with methacrylic anhydride (10 times molar excess) methacrylated disaccharides% was 10.9 ± 1.07% HA hydrogels incorporating 100% (v/v) PL Met-HA was dissolved at a concentration of 1.5% (w/v) in both of the PBS and PL photoinitiator solutions. | Human DPSCs | ALP, collagen type I A 1 strand (COLIA1) | HA hydrogels incorporating PL increased the cellular metabolism and stimulate the mineralized matrix deposition by hDPSCs. |
| Silva et al., 2018 [194] | Hyaluronic acid hydrogel | In vitro Ex vivo | HA hydrogels incorporating cellulose nanocrystals and enriched with Platelet lysate (PL). | 1 wt. % ADH-HA, 1 wt. % a-HA, 0.125 to 0.5 wt. % a-CNCs in 50 v/v% PL solution. | Human DPSCs | HA hydrogel enabled human DPSCs survival and migration. | |
| Zhu et al., 2018 [197] | Hyaluronic acid hydrogel | In vivo | Crosslinked HA hydrogel | Crosslinked HA gel (24 mg/mL) mixed with cells at 1:1–1:1.4 (v/v, i.e., gel/cells) ratio with final cell concentration of *2 · 107/mL 1,4-butanediol diglycidyl ether; DVS, divinyl sulphone crosslinking agent 9% | DPSCs | Nestin, DSPP, DMP-1, Bone sialoprotein | HA hydrogel regenerated pulp-like tissue with a layer of dentin-like tissue or osteodentin along the canal walls. |
| Niloy et al., 2020 [196] | Hyaluronic acid hydrogel | In vitro | Converting sodium salt of HA into Tetrabutylammonium salt and subsequent conjugation of Aminoethyl methacrylate (AEMA) to HA backbone. | AEMA-HA macromers of two different molecular weights (18 kD and 270 kD) 1 g of H/100 mL deionized water, mixed with 12.5 g of ion exchange resin was converted from its hydrogen form to its TBA form AEMA hydrochloride (0.25 equivalent to HA repeat units) | DPSCs | NANOG, SOX2 | HA hydrogels have great potential to mimic the in vivo 3D environment to maintain the native morphological property and stemness of DPSCs. |
| Agarose Hydrogel | |||||||
| Cao et al., 2016 [214] | Agarose hydrogel | In vitro | Calcium chloride (CaCl 2) Agarose hydrogel | 1.0 g of Agarose powder, 1.9 g of CaCl 2 H20 | Agarose hydrogel promoted occlusion of dentinal tubules and formation of enamel prisms-like tissue on human dentin surface. | ||
| Cellulose Hydrogel | |||||||
| Teti et al., 2015 [227] | Cellulose hydrogel | In vitro | Hydroxyapatite was loaded inside CMC hydrogel | Degree of carboxymethylation of 95% (CMC) (average MW 700 KDa) | DPSCs | ALP, RUNX2, COL-IA1, SPARC, DMP-1, DSPP | CMC–hydroxyapatite hydrogel up regulated the osteogenic and odontogenic markers expression and promoted DPSCs adhesion and viability. |
| Aubeux et al., 2016 [226] | Cellulose hydrogel | In vitro | Silanes grafted along the hydroxy-propyl-methyl-cellulose chains. | nanoporous macromolecular structure. Pores have an average diameter of 10 nm | TGF-b1 | Cellulose hydrogel enhanced non-collagenous matrix proteins release from smashed dentin powder. | |
| Iftikhar et al., 2020 [228] | Cellulose hydrogel | In vitro | The surface area, average pore size and particle size of BAG (45S5 Bioglass®) were 65m2/g 5.7 nm, and 92 nm, respectively. | MC3T3-E1 cells differentiated into osteoblasts and osteocytes. | The prepared injectable bioactive glass, hydroxypropylmethyl cellulose (HPMC) and Pluronic F127 was biocompatible in an in vitro system and has the ability to regenerate dentin. | ||
| Extracellular Matrix Hydrogel | |||||||
| Chatzistavrou et al., 2014 [246] | Extracellular matrix (ECM) hydrogel | In vitro | silver-doped bioactive glass (Ag-BG) incorporated into ECM | Ag-BG powder form with particle size < 35μm. ECM concentration of 10 mg/mL ECM60/Ag-BG40, ECM50/Ag-BG50, ECM30/Ag-BG70 weight ratio. | DPSCs | Ag-BG/ECM presented enhanced regenerative properties and anti-bacterial action. | |
| Wang et al., 2015 [245] | ECM hydrogel | In vivo In vitro | silver-doped bioactive glass (Ag-BG) incorporated into ECM | Ag-BG powder form with particle size < 35μm. ECM pepsin digest stock solutions of 10 mg ECM/mL (dry weight) Ag-BG: ECM = 1:1 in wt. %. | DPSCs | Ag-BG/ECM showed antibacterial property, induced dental pulp cells proliferation and differentiation. The in vivo results supported the potential use of Ag-BG/ECM as an injectable material for the restoration of lesions involving pulp injury. | |
| Li et al., 2020 [247] | ECM hydrogel | In vitro | Pre-gel solution was diluted into 0.75% w/v and 0.25% w/v. | Human DPSCs | DSPP, DMP-1 | decellularized matrix hydrogel derived from human dental pulp effectively contributed to promoting human DPSCs proliferation, migration, and induced multi-directional differentiation. | |
| Holiel et al., 2020 [248] | ECM hydrogel | Clinical trial | Human treated dentin matrix hydrogel was dispersed in sodium alginate solution | Particle sized powder (range 350–500 μm) 5% (w/v) of sodium alginate 0.125 g of sterile human treated dentin matrix was dispersed in the sodium alginate solution with a mass ratio of 1:1 | Treated dentin matrix hydrogel attained dentin regeneration and conservation of pulp vitality. | ||
| Synthetic Hydrogels | |||||||
|---|---|---|---|---|---|---|---|
| Author | Hydrogel Used | Type of Study | Hydrogel Modification | Hydrogel Properties | Cells Used | Upregulated Biological Molecules | Outcomes |
| PLA Based Polymers | |||||||
| Shiehzadeh et al., 2014 [260] | Polylactic polyglycolic acid–polyethylene glycol (PLGA-PEG) | Clinical trial | Stem/progenitor cells from the apical dental papilla (SCAP) | Biologic approach can provide a favorable environment for clinical regeneration of dental and paradental tissues. | |||
| Synthetic Self-Assembling Peptide Hydrogel (Peptide Amphiphiles) | |||||||
| Galler et al., 2008 [74] | Synthetic peptide amphiphiles | In vitro | Peptide amphiphiles involves arginine–glycine–aspartic acid (RGD) and an enzyme-cleavable site | Peptide was dissolved at pH 7.0 to attain stock solution of 2% by weight | stem/progenitor cells of exfoliated deciduous teeth (SHED) and dental pulp stem cells (DPSCs) | The hydrogels are easy to handle and can be introduced into small defects, therefore this novel system might be suitable for dental tissue regeneration. | |
| Multi Domain Self-Assembling Peptide (MDP) Hydrogel | |||||||
| Galler et al., 2011 [20] | In vivo | MDP functionalized with transforming growth factor (TGF)-β1, fibroblast growth factor (FGF)-2, and vascular endothelial growth factor (VEGF) via heparin binding | DPSCs | In tooth slices, implanted hydrogel degraded and replaced by a vascularized connective tissue similar to dental pulp. Pretreatment of the tooth cylinders with NoOCl showed resorption lacunae. With NaOCl followed by ethylenediaminetetraacetic acid (EDTA), DPSCs differentiated into odontoblasts-like cells intimately associated with the dentin surface. | |||
| Galler et al., 2012 [1] | MDP | In vivo | MDP functionalized with TGF-β1, FGF-2, and VEGF via heparin binding | DPSCs | Hydrogels implanted into the backs of immunocompromised mice resulted in the formation of vascularized soft connective tissue similar to dental pulp. | ||
| Colombo et al., 2020 [75] | MPD hydrogel | In vitro | SHED | Decellularized and lyophilized MDP produced a biomaterial containing anti-inflammatory bioactive molecules that can provide a tool to reduce pulpal inflammation to promote dentin–pulp complex regeneration. | |||
| RADA16-I Hydrogels Self-Assembling Peptide | |||||||
| Cavalcanti et al., 2013 [291] | A commercial self-assembling peptide | In vitro | 0.2% Puramatrix™ (1% w/v) | DPSCs | Dentin matrix protein (DMP)-1, Dentin sialophosphoprotein (DSPP) | DPSCs expressed DMP-1 and DSPP after 21 days culturing in dentin slices containing PuramatrixTM. The surviving dentin provided signaling molecules to cells suspended in PuramatrixTM. | |
| Rosa et al., 2013 [76] | A commercial self-assembling peptide | In vitro In vivo | 0.2% Puramatrix™ (1% w/v) | SHED | DMP-I, DSPP, matrix extracellular phosphoglycoprotein (MEPE) | Upon mixing SHED with Puramatrix™ hydrogel for 7 days and injecting the construct into roots of human premolars, the cells survived and expressed (DMP-I, DSPP, MEPE) in vitro. Pulp-like tissue with odontoblasts able to form neo-dentinal tubules was observed in vivo. | |
| Dissanayaka et al., 2015 [276] | A commercial self-assembling peptide | In vitro In vivo | Among different Puramatrix™ (1% w/v) concentrations, 0.15% was the optimal. | DPSCs and human umbilical vein endothelial cells (HUVECs) | PuramatrixTM enhanced in vitro cell survival, migration and capillary formation. Co-cultured groups on PuramatrixTM exhibited more extracellular matrix, mineralization and vascularization than DPSC-monocultures in vivo. | ||
| Nguyen et al., 2018 [88] | RADA16-I | In vitro | incorporation of dentonin sequence | Ribbonlike nanofibers with height (∼2 nm) and width (∼14 nm) | DPSCs | The self-assembled peptide platform holds promise for guided dentinogenesis. | |
| Huang, 2020 [280] | RADA16-I | In vitro | Low concentration (0.125%, 0.25%) caused higher cell proliferation rate than high concentration (0.5%, 0.75%, 1%) | DPSCs and umbilical cord mesenchymal stem cells | DSPP, DMP-1, Alkaline phosphatase (ALP), osteocalcin (OCN) | The co-culture groups promoted odontoblastic differentiation, proliferation and mineralization. | |
| Mu et al., 2020 [87] | RADA16-I | In vitro | incorporated with stem cell factor | 100 ng/mL was the optimum concentration of the stem cell factor. Nanofibers and pores diameter were (10–30nm and 5–200nm, respectively) | DPSCs and HUVECs | Stem cell factor incorporate RADA16-I holds promise for guided pulp regeneration. | |
| Zhu et al., 2019 [142] | Cells were cultured on Matrigel before being loaded on commercial self-assembling peptide | In vitro In vivo | 300 μL 1% Puramatrix™ (1% w/v) | DPSCs overexpressing Stromal derived factor-(SDF)-1 and vascular endothelial growth factor (VEGF) | SDF-1, VEGF | Combination of VEGF- and SDF-1-overexpressing DPSCs cultured on Matrigel before being loaded on PuramatrixTM enhanced the area of vascularized dental pulp regeneration in vivo. | |
| Xia et al., 2020 [89] | Self-assembling peptide | In vitro In vivo | incorporation of RGD, VEGF mimetic peptide sequence | The nanofibers’ diameters of functionalized peptide were thicker than pure RAD. that the stiffness of RAD/ RGD-mimicking peptide (PRG)/ VEGF-mimicking peptide: (KLT) hydrogels was greater than the others | DPSCs and HUVECs | Modified self-assembling peptide hydrogel effectively stimulated stem cells angiogenic and odontogenic differentiation in vitro and dentin–pulp complex regeneration in vivo. | |
| Poly-dimethylsiloxane Hydrogel | |||||||
| Liu et al., 2017 [263] | Poly-dimethylsiloxane (PDMS) | In vitro | Stiffness for 10:1, 20:1, 30:1 and 40:1 was 135, 54, 16 and 1.4 kPa and roughness was 55.67, 53.38, 50.95, and from 47.32 to 42.50nm. Water contact angle was 65°. | DPSCs | osteopontin (OPN), runt-related transcription factor (RUNX)-2, Bone morphogenetic protein | Osteogenic and odontogenic markers were positively correlated to the substrate stiffness. The results revealed that the mechanical properties promoted the function of DPSCs related to the Wnt/β-catenin pathway. | |
| Poly-N-isopropylacrylamide Gel | |||||||
| Itoh et al., 2018 [267] | Poly-N-isopropylacrylamide (NIPAAm) | In vitro In vivo | NIPAAm crosslinked by PEG-DMA | Decrease in wet weight from 1 to 0.18 at 508 C. Change in surface area from 1 (258 C) to 0.62 (508 C) within 1 h. High wettability. | DPSCs | DSPP in the outer cell layer, Nanog in the center of the constructs | DPSCs in the outer layer of the constructs differentiated into odontoblast-like cells, while DPSCs in the inner layer maintained their stemness. Pulp-like tissues rich in blood vessels were formed in vivo. |
| Polyethylene Glycol | |||||||
| Komabayashi et al., 2013 [275] | PEG | In vitro | PEG–maleate–citrate (PEGMC) (45% w/v), acrylic acid (AA) crosslinker (5% w/v), 2,2′-Azobis (2-methylpropionamidine) dihydrochloride (AAPH) photo-initiator (0.1% w/v), | Optimum cell viability with exposure time of 30 s with a monomer and AAPH concentration of 0.088% and up to 1%, respectively | L929 cells | Cell viability remained up to 80% after 6 h. Controlled Ca2+ release was attained. The viscosity and injection ability into plastic root canal blocks were confirmed in a dental model. | |
| VitroGel 3D | |||||||
| Xiao et al., 2019 [73] | Vitrogel | In vitro In vivo | VitroGel diluted with deionized water 1:2. | SCAP | RUNX-2,DMP-1, DSPP, OCN | VitroGel 3D promoted SCAP proliferation and differentiation. SDFr-1α and BMP-2 co-treatment induced odontogenic differentiation of human SCAP cultured in the VitroGel 3D in vitro and in vivo | |
| Hybrid Hydrogels | |||||||
|---|---|---|---|---|---|---|---|
| Author | Hydrogel Used | Type of Study | Hydrogel Modification | Hydrogel Properties | Cells Used | Upregulated Biological Molecules | Outcomes |
| Alginate/laponite hydrogel | |||||||
| Zhang et al., 2020 [56] | Alginate/laponite hydrogel | In vitro In vivo | Arginine-glycine-aspartic acid (RGD) modified alginate and nano-silicate laponite hydrogel microspheres, encapsulating human dental pulp stem cells (DPSCs) and vascular endothelial growth factor (VEGF). | The RGD-Alg had 55% degradation rate and the RGD- alginate/laponite exhibited 45% while pure alginate degraded only 20% after 28 days. | DPSCs | Alkaline phosphatase (ALP), Dentine matrix protein 1 (DMP-1), collagen I (Col-I) | Hybrid RGD modified alginate/laponite hydrogel microspheres has a promising potential in vascularized dental pulp regeneration. |
| PEG-modified natural polymers | |||||||
| Galler et al., 2011 [66] | PEGylated fibrin | In vitro In vivo | PEGylated fibrinogen with added thrombin. | Carboxylated N-hydroxysulfosuccinimide-active ester polyethylene glycol (PEG) (MW = 3400 Da) added to 40 mg/mL of bovine fibrinogen in TRIS- saline at a molar ratio of 10:1. Equal volume of thrombin (200 U/mL in 40 mM CaCl2) was added | Stem cells isolated from human exfoliated deciduous teeth (SHED), DPSCs, periodontal ligament stem cells (PDLSCs), bone marrow mesenchymal stem cells (BMMSCs) | Col I, Col III, matrix metalloproteinase (MMP-2), bone Sialoprotein (BSP), osteocalcin (OCN), runt-related transcription factor -2 (RUNX2), dentin sialophosphoprotein (DSPP), DMP-1 | ALP activity and osteoblastic and odontoblast differentiation genes were higher in dental stem cells than BMMSCs. SHED and PDLSCs exhibited high expression of collagen, while DPSCs and PDLSCs expressed high levels of differentiation late markers. In vivo, fibrin matrix degraded and replaced by vascularized connective tissue. |
| Lu et al., 2015 [313] | PEG fibrinogen | In vitro | PEG fibrinogen with variable amounts of polyethylene glycol diacrylate (PEGDA) | Increase of PEGDA crosslinker allows for higher modulus but longer times of crosslinking and less swelling ratio | DPSCs | Col I, DSPP, DMP-1, OCN | Odontogenic genes expressions and mineralization were correlated to the hydrogel crosslinking degree and matrix stiffness |
| Jones et al., 2016 [314] | HyStem-C is a commercial hydrogel hyaluronic acid (HA)- PEGDA -gelatin | In vitro | Polyethylene glycol diacrylate with an added disulfide bond (PEGSSDA) with an added disulfide bond. | Hydrogel gelation time decreased as the PEGSSDA crosslinker concentration (w/v) increased from (0.5% to 8.0%) | DPSCs | The PEGSSDA-HA-Gn was biocompatible with human DPSCs. Cell proliferation and spreading increased considerably with adding fibronectin to PEGDA-HA-Gn hydrogels. | |
| Feng et al., 2020 [312] | Polyethylene glycol diacrylate\sodium alginate (PEGDA/SA) | In vitro In vivo | PEGDA/SA loaded basic fibroblast growth factor (bFGF) (PEGDA/SA-bFGF) | DPSCs | Reduction of mass ratio of PEGDA/SA to 20:1 ~ 15:1 resulted in the formation of a well-organized pulp structure after implantation | ||
| Comparing Natural and Synthetic Hydrogels | ||||||
|---|---|---|---|---|---|---|
| Galler et al., 2018 [325] | - PEG - Self-assembling peptide (SAPbio) and Puramatrix™) | In vitro In vivo | - PEG was modified to be chemically cured (PEGchem) or light-cured (PEGlight), - Biomimetic hydrogels (PEGbio) modified by cell adhesion motif and MMP-2. - Self-assembling peptide (SAPbio) modified by cell adhesion motif and MMP-2. | DPSCs | TGF-β1 | In vitro viability was higher in natural materials. Scaffold degradation, odontoblast-like cell differentiation, tissue formation and vascularization were higher in natural materials in vivo. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Moshy, S.E.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers 2020, 12, 2935. https://doi.org/10.3390/polym12122935
Abbass MMS, El-Rashidy AA, Sadek KM, Moshy SE, Radwan IA, Rady D, Dörfer CE, Fawzy El-Sayed KM. Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers. 2020; 12(12):2935. https://doi.org/10.3390/polym12122935
Chicago/Turabian StyleAbbass, Marwa M. S., Aiah A. El-Rashidy, Khadiga M. Sadek, Sara El Moshy, Israa Ahmed Radwan, Dina Rady, Christof E. Dörfer, and Karim M. Fawzy El-Sayed. 2020. "Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation" Polymers 12, no. 12: 2935. https://doi.org/10.3390/polym12122935
APA StyleAbbass, M. M. S., El-Rashidy, A. A., Sadek, K. M., Moshy, S. E., Radwan, I. A., Rady, D., Dörfer, C. E., & Fawzy El-Sayed, K. M. (2020). Hydrogels and Dentin–Pulp Complex Regeneration: From the Benchtop to Clinical Translation. Polymers, 12(12), 2935. https://doi.org/10.3390/polym12122935







