Monomer Selection for In Situ Polymerization Infusion Manufacture of Natural-Fiber Reinforced Thermoplastic-Matrix Marine Composites
Abstract
1. Introduction
2. Resin Infusion under Flexible Tooling (RIFT)
2.1. Polymer Rheology
- Dynamic viscosity (μ): the force required to overcome internal friction. The SI units are Pascal-seconds (Pa·s: identical to 1 kg·m−1·s−1), although the composites industry often uses centimeter–gram–second (CGS) units: centipoise (cP). There is a direct numerical equivalence between millipascal-seconds and centipoise (1 mPa·s = 1 cP).
- Kinematic viscosity (η): the ratio of the viscous force to the inertial force where the latter is a function of the fluid density (ρ). The SI units are m2·s−1, although the parameter is often given in centistokes (CGS units: 1 centistoke is 1 mm2/s). Hence η = μ/ρ.
2.2. Reinforcement Permeability
2.3. Processing Temperature
3. Monomers for Infusion
- Ring-opening polymerization (ROP) in which cyclic molecules are opened into linear monomers or oligomers to produce high molecular weight polymers. The monomers are:
- ○
- Caprolactam (e.g., DSM fiber intermediates APA-6) to produce polyamide-6 (PA6);
- ○
- Laurolactam (e.g., EMS-Grivory APLC12) to produce polyamide-12 (PA12);
- ○
- Cyclic butylene terephthalate (CBT) oligomers (e.g., Cyclics Corporation) to produce polybutylene terephthalate (PBT) polyester;
- ○
- Cyclic bisphenol-A oligomers to produce polycarbonate;
- ○
- L-lactide to produce poly(L-lactide).
- Vinyl polymerization where monomer unsaturation (double bonds) is opened to create free radicals which undergo an addition reaction to form long-chain polymers. The available monomer is:
- ○
- methyl methacrylate (MMA) (e.g., Arkema Elium® acrylic thermoplastic resin formulations specifically designed for RTM/MIFT manufacture of composite parts) to produce polymethyl methacrylate (PMMA).
3.1. Polyamides from Lactams
3.1.1. Polyamide-6 from Caprolactam
3.1.2. Polyamide-12 from Laurolactam
3.1.3. Polyamide-6/12 from Lactams
3.2. Polybutylene Terephthalate from Cyclic Butylene Terephthalate
3.3. Polycarbonate from Cyclic Bisphenol A Oligomer
3.4. Poly(L-lactide) from L-Lactide
3.5. Polymethyl Methacrylate from Methyl Methacrylate (Acrylic) Monomer
4. Properties of Thermoplastic Polymers
5. Monomer Selection Criteria
- Essential criteria
- The viscosity of the monomer must be <1000 mPa·s (NIP) to enable the infusion process.
- The processing temperature must be <200 °C to minimize the thermal degradation of the natural fibers and to reduce the cost of consumables.
- Tg of the cured matrix should be above the maximum use temperature to minimize the creep effect in highly stressed applications.
- Low water sensitivity is needed to maintain proper mechanical and thermal properties in marine environments.
- Desirable criteria
- Monomer/resin should be bio-based or have potential bio-based sources available.
- The open window for infusion should be relatively long to enable the production of a large-scale demonstrator/product with 3D geometry in the future.
- The cost of the monomer/resin should be relatively low.
- Low embodied energy and other environmental burdens of the product across the entire life cycle, and recyclable at end-of-life.
5.1. Essential Criteria
5.1.1. Viscosity
5.1.2. Process Temperature
5.1.3. Glass Transition Temperature
5.1.4. Moisture Content and Depression of Mechanical and Thermal Properties
- non-hydrophilic groups ~generally absorb less than 0.1 w/o (weight percent) of water,
- moderately hydrophilic groups ~generally absorb less than 3 w/o water, and
- strongly hydrophilic groups ~saturated state generally limited to values <10 w/o water.
5.2. Desirable Criteria
5.2.1. Bio-Based Monomer
5.2.2. Open Window for Infusion
Latent Catalysts or Hardeners
5.2.3. Cost
5.2.4. Recyclability
6. Current Challenges and Future Perspectives
- The bio-based MMA monomer being produced on at an industrial scale;
- The modification of lactide monomer resin to reduce processing temperature and enhance the durability of PLA;
- Modified, or new, monomers/polymers to meet the criteria (e.g., reduced moisture absorption of infusible polyamides) in order to introduce more candidates;
- The use of copolymer systems as the matrix.
7. Conclusions
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- Graham-Jones, J.; Summerscales, J. Marine Applications of Advanced Fibre-Reinforced Composites; Woodhead Publishing: Sawston, UK, 2015. [Google Scholar]
- Pemberton, R.; Summerscales, J.; Graham-Jones, J. Marine Composites: Design and Performance; Woodhead Publishing: Sawston, UK, 2019. [Google Scholar]
- Satyanarayana, K.G.; Arizaga, G.G.; Wypych, F. Biodegradable composites based on lignocellulosic fibers—An overview. Prog. Polym. Sci. 2009, 34, 982–1021. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Pickering, K.L.; Efendy, M.A.; Le, T.M. A review of recent developments in natural fibre composites and their mechanical performance. Compos. Part A Appl. Sci. Manuf. 2016, 83, 98–112. [Google Scholar] [CrossRef]
- Moudood, A.; Rahman, A.; Öchsner, A.; Islam, M.; Moudood, A. Flax fiber and its composites: An overview of water and moisture absorption impact on their performance. J. Reinf. Plast. Compos. 2018, 38, 323–339. [Google Scholar] [CrossRef]
- Al-Maharma, A.Y.; Al-Huniti, N. Critical Review of the Parameters Affecting the Effectiveness of Moisture Absorption Treatments Used for Natural Composites. J. Compos. Sci. 2019, 3, 27. [Google Scholar] [CrossRef]
- Célino, A.; Fréour, S.; Jacquemin, F.; Casari, P. The hygroscopic behavior of plant fibers: A review. Front. Chem. 2014, 1, 43. [Google Scholar] [CrossRef]
- Summerscales, J. Virtual Book on Bast Fibres and Their Composites. Available online: https://ecm-academics.plymouth.ac.uk/jsummerscales/mats347/bast_book.htm (accessed on 19 November 2020).
- Baley, C.; Gomina, M.; Breard, J.; Bourmaud, A.; Davies, P. Variability of mechanical properties of flax fibres for composite reinforcement. A review. Ind. Crops Prod. 2020, 145, 111984. [Google Scholar] [CrossRef]
- Brutch, N.B.; Matvienko, I.; Porokhovinova, E.A.; Pavlov, A.V.; Nôžková, J.; Koshkin, V. Effect of Photoperiod on Linum usitatissimum L. Characters. J. Nat. Fibers 2019, 17, 1345–1354. [Google Scholar] [CrossRef]
- Lee, C.H.; Abdan, K.; Lee, S.H.; Liu, M. A Comprehensive Review on Bast Fiber Retting Process for Optimal Performance in Fibers Reinforced Polymer Composites. Adv. Mater. Sci. Eng. 2020, 13. [Google Scholar] [CrossRef]
- Syduzzaman, M.; Al Faruque, A.; Bilisik, K.; Naebe, M. Plant-Based Natural Fibre Reinforced Composites: A Review on Fabrication, Properties and Applications. Coatings 2020, 10, 973. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Thomas, V.M.; Yoo, C.G.; Ozcan, S.; Deng, Y.; Nelson, K.; Ragauskas, A.J. Recent advancements of plant-based natural fiber–reinforced composites and their applications. Compos. Part B Eng. 2020, 200, 108254. [Google Scholar] [CrossRef]
- Sanivada, U.K.; Mármol, G.; Brito, F.P.; Fangueiro, R. PLA Composites Reinforced with Flax and Jute Fibers—A Review of Recent Trends, Processing Parameters and Mechanical Properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef]
- Vinod, A.; Sanjay, M.; Siengchin, S.; Jyotishkumar, P. Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J. Clean. Prod. 2020, 258, 120978. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, S.; Li, Y.; Wang, Z.; Long, Y.; Yu, T.; Shen, Y. High performances of plant fiber reinforced composites—A new insight from hierarchical microstructures. Compos. Sci. Technol. 2020, 194, 108151. [Google Scholar] [CrossRef]
- Gholampour, A.; Ozbakkaloglu, T. A review of natural fiber composites: Properties, modification and processing techniques, characterization, applications. J. Mater. Sci. 2020, 55, 829–892. [Google Scholar] [CrossRef]
- Pantaloni, D.; Bourmaud, A.; Baley, C.; Clifford, M.J.; Ramage, M.H.; Shah, D.U. A Review of Permeability and Flow Simulation for Liquid Composite Moulding of Plant Fibre Composites. Materials 2020, 13, 4811. [Google Scholar] [CrossRef]
- Chang, B.P.; Mohanty, A.K.; Misra, M. Studies on durability of sustainable biobased composites: A review. RSC Adv. 2020, 10, 17955–17999. [Google Scholar] [CrossRef]
- González-López, M.E.; Del Campo, A.S.M.; Robledo-Ortíz, J.R.; Arellano, M.; Pérez-Fonseca, A.A. Accelerated weathering of poly(lactic acid) and its biocomposites: A review. Polym. Degrad. Stab. 2020, 179, 109290. [Google Scholar] [CrossRef]
- Chaudhary, V.; Ahmad, F. A review on plant fiber reinforced thermoset polymers for structural and frictional composites. Polym. Test. 2020, 91, 106792. [Google Scholar] [CrossRef]
- Gokulkumar, S.; Thyla, P.; Prabhu, L.; Sathish, S. Measuring Methods of Acoustic Properties and Influence of Physical Parameters on Natural Fibers: A Review. J. Nat. Fibers 2020, 17, 1719–1738. [Google Scholar] [CrossRef]
- Milosevic, M.; Valášek, P.; Ruggiero, A. Tribology of Natural Fibers Composite Materials: An Overview. Lubricants 2020, 8, 42. [Google Scholar] [CrossRef]
- Saleem, A.; Medina, L.; Skrifvars, M.; Berglin, L. Hybrid Polymer Composites of Bio-Based Bast Fibers with Glass, Carbon and Basalt Fibers for Automotive Applications—A Review. Molecules 2020, 25, 4933. [Google Scholar] [CrossRef] [PubMed]
- Summerscales, J.; Dissanayake, N.P.; Virk, A.S.; Hall, W. A review of bast fibres and their composites. Part 1—Fibres as reinforcements. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1329–1335. [Google Scholar] [CrossRef]
- Summerscales, J. A review of bast fibres and their composites: Part 4 ∼ organisms and enzyme processes. Compos. Part A Appl. Sci. Manuf. 2020, 140, 106149. [Google Scholar] [CrossRef]
- Summerscales, J.; Gwinnett, C. Forensic Identification of Bast Fibres. In Biocomposites for High-Performance Applications; Ray, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 125–164. [Google Scholar]
- Summerscales, J.; Dissanayake, N.; Virk, A.; Hall, W. A review of bast fibres and their composites. Part 2 —Composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1336–1344. [Google Scholar] [CrossRef]
- Summerscales, J.; Virk, A.; Hall, W. A review of bast fibres and their composites: Part 3—Modelling. Compos. Part A Appl. Sci. Manuf. 2013, 44, 132–139. [Google Scholar] [CrossRef]
- Liu, M.; Thygesen, A.; Summerscales, J.; Meyer, A.S. Targeted pre-treatment of hemp bast fibres for optimal performance in biocomposite materials: A review. Ind. Crops Prod. 2017, 108, 660–683. [Google Scholar] [CrossRef]
- Summerscales, J.; Grove, S. Manufacturing Methods for Natural Fibre Composites. In Natural Fibre Composites: Materials, Processes and Properties; Hodzic, A., Shanks, R., Eds.; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar]
- Dissanayake, N.P.; Summerscales, J. Life Cycle Assessment for Natural Fibre Composites. In Green Composites from Natural Resources; Thakur, V., Ed.; Taylor and Francis Group: Abingdon, UK, 2014; pp. 157–186. [Google Scholar]
- Thomason, J.L.; Jenkins, P.G.; Yang, L. Glass Fibre Strength—A Review with Relation to Composite Recycling. Fibers 2016, 4, 18. [Google Scholar] [CrossRef]
- Hermann, B.; Debeer, L.; De Wilde, B.; Blok, K.; Patel, M.K. To compost or not to compost: Carbon and energy footprints of biodegradable materials waste treatment. Polym. Degrad. Stab. 2011, 96, 1159–1171. [Google Scholar] [CrossRef]
- Bong, C.P.C.; Lim, L.Y.; Ho, W.S.; Lim, J.S.; Klemeš, J.J.; Towprayoon, S.; Ho, C.S.; Lee, C.T. A review on the global warming potential of cleaner composting and mitigation strategies. J. Clean. Prod. 2017, 146, 149–157. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Siddique, N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Wool, R.; Sun, X.S. Bio-Based Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262. [Google Scholar] [CrossRef]
- Hottle, T.A.; Bilec, M.M.; Landis, A.E. Sustainability assessments of bio-based polymers. Polym. Degrad. Stab. 2013, 98, 1898–1907. [Google Scholar] [CrossRef]
- Hancox, N. High performance thermoplastic resins and their composites S. Beland. Mater. Des. 1993, 14, 208. [Google Scholar] [CrossRef]
- Conroy, A.; Halliwell, S.; Reynolds, T. Composite recycling in the construction industry. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1216–1222. [Google Scholar] [CrossRef]
- Stewart, R. Thermoplastic composites—Recyclable and fast to process. Reinf. Plast. 2011, 55, 22–28. [Google Scholar] [CrossRef]
- Sutherland, L. A review of impact testing on marine composite materials: Part I—Marine impacts on marine composites. Compos. Struct. 2018, 188, 197–208. [Google Scholar] [CrossRef]
- Sutherland, L. A review of impact testing on marine composite materials: Part II—Impact event and material parameters. Compos. Struct. 2018, 188, 503–511. [Google Scholar] [CrossRef]
- Sutherland, L. A review of impact testing on marine composite materials: Part III—Damage tolerance and durability. Compos. Struct. 2018, 188, 512–518. [Google Scholar] [CrossRef]
- Hildebrand, M. Local Impact Strength of Various Boat-Building Materials; Technical Research Centre of Finland: Espoo, Finland, 1997. [Google Scholar]
- Otheguy, M.E. Manufacture, Repair and Recycling of Thermoplastic Composite Boats. Ph.D. Thesis, Newcastle University, Newcastle, UK, 2010. [Google Scholar]
- Harten, K. Production by Resin Transfer Moulding. In Composite Materials in Maritime Structures—Fundamental Aspects; Shenoi, R.A., Wellicome, J.F., Eds.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Rudd, C.D.; Long, A.C.; Kendall, K.; Mangin, C. Liquid Moulding Technologies: Resin Transfer Moulding, Structural Reaction Injection Moulding and Related Processing Techniques; Woodhead Publishing: Sawston, UK, 1997. [Google Scholar]
- Potter, K. Resin Transfer Moulding; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Kruckenberg, T.; Paton, R. Resin Transfer Moulding for Aerospace Structures; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Tucker, C.; Dessenberger, R. Resin Transfer Moulding Phenomena in Polymeric Composites. In Flow and Rheology in Polymer Composites Manufacturing; Advani, S.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1994; pp. 257–323. [Google Scholar]
- Benjamin, W.; Beckwith, S. Resin Transfer Moulding; SAMPE Monograph 3; SAMPE: Covina, CA, USA, 1999. [Google Scholar]
- Parnas, R. Liquid Composite Moulding; Hanser Gardner Publications: Cincinnati, OH, USA, 2000. [Google Scholar]
- Pearce, N.; Guild, F.; Summerscales, J. An investigation into the effects of fabric architecture on the processing and properties of fibre reinforced composites produced by resin transfer moulding. Compos. Part A Appl. Sci. Manuf. 1998, 29, 19–27. [Google Scholar] [CrossRef]
- Beckwith, S. Resin infusion technology Part 1—Industry highlights* Part 2—Process definitions and industry variations* Part 3—A detailed overview of RTM and VIP infusion processing. SAMPE J. 2007, 43, 61. [Google Scholar]
- Cripps, D.; Searle, T.; Summerscales, J. Open Mould Techniques for Thermoset Composites. In Comprehensive Composite Materials Encyclopædia; Talreja, R., Månson, J.-A., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 2. [Google Scholar]
- Summerscales, J. Resin Infusion under Flexible Tooling (RIFT). In Encyclopedia of Composites, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 2648–2658. [Google Scholar]
- Summerscales, J.; Searle, T.J. Low-pressure (vacuum infusion) techniques for moulding large composite structures. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2005, 219, 45–58. [Google Scholar] [CrossRef]
- Williams, C.; Summerscales, J.; Grove, S. Resin Infusion under Flexible Tooling (RIFT): A review. Compos. Part A Appl. Sci. Manuf. 1996, 27, 517–524. [Google Scholar] [CrossRef]
- Parton, H.; Verpoest, I. In situ polymerization of thermoplastic composites based on cyclic oligomers. Polym. Compos. 2004, 26, 60–65. [Google Scholar] [CrossRef]
- Hindersmann, A. Confusion about infusion: An overview of infusion processes. Compos. Part A Appl. Sci. Manuf. 2019, 126, 105583. [Google Scholar] [CrossRef]
- Poorzeinolabedin, M.; Parnas, L.; Dashatan, S.H. Resin infusion under flexible tooling process and structural design optimization of the complex composite part. Mater. Des. 2014, 64, 450–455. [Google Scholar] [CrossRef]
- Shojaei, A.; Trochu, F.; Ghaffarian, S.R.; Karimian, S.M.H.; Lessard, L. An Experimental Study of Saturated and Unsaturated Permeabilities in Resin Transfer Molding Based on Unidirectional Flow Measurements. J. Reinf. Plast. Compos. 2004, 23, 1515–1536. [Google Scholar] [CrossRef]
- Sharp, J.J.M.; Simmons, C.T. The compleat Darcy: New lessons learned from the first English translation of les fontaines publiques de la Ville de Dijon. Ground Water 2005, 43, 457–460. [Google Scholar] [CrossRef]
- Becker, D. Tooling for Resin Transfer Moulding; Wichita State University: Wichita, KS, USA, 1991. [Google Scholar]
- Summerscales, J. The Effect of Permeant on the Measured Permeability of a Reinforcement. In Proceedings of the 7th International Conference on Flow Processes in Composite Materials (FPCM-7), Newark, NJ, USA, 7–9 July 2004; pp. 471–476. [Google Scholar]
- Park, C.H.; Krawczak, P. Unsaturated and Saturated Permeabilities of Fiber Reinforcement: Critics and Suggestions. Front. Mater. 2015, 2, 38. [Google Scholar] [CrossRef]
- Steenkamer, D.A.; McKnight, S.H.; Wilkins, D.J.; Karbhari, V.M. Experimental characterization of permeability and fibre wetting for liquid moulding. J. Mater. Sci. 1995, 30, 3207–3215. [Google Scholar] [CrossRef]
- Kim, Y.R.; McCarthy, S.; Fanucci, J.; Nolet, S.; Koppernaes, C. Resin flow through fiber reinforcements during composite processing. SAMPE Q. 1991, 22, 0036–0821. [Google Scholar]
- Diallo, M.; Gauvin, R.; Trochu, F. Key factors affecting the permeability measurement in continuous fiber reinforcements. In Proceedings of the ICCM-11, Gold Coast, Australia, 14–18 July 1997; pp. 441–445. [Google Scholar]
- Lai, Y.-H.; Khomami, B.; Kardos, J.L. Accurate permeability characterization of preforms used in polymer matrix composite fabrication processes. Polym. Compos. 1997, 18, 368–377. [Google Scholar] [CrossRef]
- Francucci, G.; Rodríguez, E.S.; Vázquez, A. Study of saturated and unsaturated permeability in natural fiber fabrics. Compos. Part A Appl. Sci. Manuf. 2010, 41, 16–21. [Google Scholar] [CrossRef]
- Masoodi, R.; Pillai, K.M.; Grahl, N.; Tan, H. Numerical simulation of LCM mold-filling during the manufacture of natural fiber composites. J. Reinf. Plast. Compos. 2012, 31, 363–378. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lagardère, M.; Park, C.H.; Panier, S. Permeability of natural fiber reinforcement for liquid composite molding processes. J. Mater. Sci. 2014, 49, 6449–6458. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, J.; Qin, Y.; Liu, D.; Yan, C.; Zhang, H. Effects of liquid absorption and swelling on the permeability of natural fiber fabrics in liquid composite moulding. Polym. Compos. 2017, 38, 996–1004. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Deléglise, M.; Park, C.H. Modeling of resin flow in natural fiber reinforcement for liquid composite molding processes. Compos. Sci. Technol. 2015, 113, 38–45. [Google Scholar] [CrossRef]
- Chaishome, J.; Rattanapaskorn, S. The influence of alkaline treatment on thermal stability of flax fibres. In Proceedings of the 2nd International Conference on Mining, Material and Metallurgical Engineering, Bangkok, Thailand, 17–18 March 2017. [Google Scholar]
- Gassan, J.; Bledzki, A.K. Thermal degradation of flax and jute fibers. J. Appl. Polym. Sci. 2001, 82, 1417–1422. [Google Scholar] [CrossRef]
- Chaishome, J.; Brown, K.; Brooks, R.; Clifford, M.J. Thermal Degradation of Flax Fibres as Potential Reinforcement in Thermoplastic Composites. Adv. Mater. Res. 2014, 894, 32–36. [Google Scholar] [CrossRef]
- Shahzad, A. A Study in Physical and Mechanical Properties of Hemp Fibres. Adv. Mater. Sci. Eng. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Ouajai, S.; Shanks, R. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym. Degrad. Stab. 2005, 89, 327–335. [Google Scholar] [CrossRef]
- CA Composites. Available online: https://cacomposites.com/ (accessed on 12 February 2020).
- Castro Composites. Available online: https://www.castrocompositesshop.com/ (accessed on 12 February 2020).
- Easy Composites. Available online: https://www.easycomposites.co.uk/#!/ (accessed on 12 February 2020).
- Tygavac Advanced Materials. Available online: https://catalogue.tygavac.co.uk/index.php?lang=EN (accessed on 12 February 2020).
- Fibre Glast. Available online: https://www.fibreglast.com/category/Vacuum_Bagging (accessed on 12 February 2020).
- Northern Composites. Available online: https://northerncomposites.com/ (accessed on 12 February 2020).
- Van Rijswijk, K.; Bersee, H. Reactive processing of textile fiber-reinforced thermoplastic composites—An overview. Compos. Part A Appl. Sci. Manuf. 2007, 38, 666–681. [Google Scholar] [CrossRef]
- Prabhakaran, R.; Lystrup, A.; Andersen, T. Attribute Based Selection of Thermoplastic Resin for Vacuum Infusion Process: A Decision Making Methodology. In Dynamic Methods and Process Advancements in Mechanical, Manufacturing, and Materials Engineering; Davim, J.P., Ed.; IGI Global: Hershey, PA, USA, 2013. [Google Scholar]
- Hedrick, R.M.; Richard, J.W.R. Reinforced Polyamides and Process of Preparation Thereof. U.S. Patent 3,419,517, 31 December 1968. [Google Scholar]
- Pillay, S.; Vaidya, U.K.; Janowski, G.M. Liquid Molding of Carbon Fabric-reinforced Nylon Matrix Composite Laminates. J. Thermoplast. Compos. Mater. 2005, 18, 509–527. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, A.; Yang, G. Polyamide single polymer composites prepared via in situ anionic polymerization of ε-caprolactam. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1006–1011. [Google Scholar] [CrossRef]
- Van Rijswijk, K.; Koppes, K.; Bersee, H.; Beukers, A. Processing Window for Vacuum Infusion of Fiber-Reinforced Anionic Polyamide-6. In Proceedings of the FPCM-7 Conference, Newark, DE, USA, 7–9 July 2004. [Google Scholar]
- Van Rijswijk, K.; Bersee, H.; Beukers, A.; Picken, S.; Van Geenen, A. Optimisation of anionic polyamide-6 for vacuum infusion of thermoplastic composites: Influence of polymerisation temperature on matrix properties. Polym. Test. 2006, 25, 392–404. [Google Scholar] [CrossRef]
- Van Rijswijk, K.; Bersee, H.; Jager, W.; Picken, S. Optimisation of anionic polyamide-6 for vacuum infusion of thermoplastic composites: Choice of activator and initiator. Compos. Part A Appl. Sci. Manuf. 2006, 37, 949–956. [Google Scholar] [CrossRef]
- Van Rijswijk, K.; Joncas, S.; Bersee, H.E.N.; Bergsma, O.K.; Beukers, A. Sustainable Vacuum-Infused Thermoplastic Composites for MW-Size Wind Turbine Blades—Preliminary Design and Manufacturing Issues. J. Sol. Energy Eng. 2005, 127, 570–580. [Google Scholar] [CrossRef]
- Bio Based Press. Available online: https://www.biobasedpress.eu/2014/10/bionylon/ (accessed on 1 December 2020).
- Jenkins, S. A Bio-Based Caprolactam Joint-Development Project is Now Underway. Chemical Engineering, 1 March 2018. [Google Scholar]
- Bomgardner, M. Genomatica, Aquafil Partner for Biobased Nylon. Chemical and Engineering News, 23 January 2018. [Google Scholar]
- Lee, Y.; Lin, K.-Y.A.; Kwon, E.E.; Lee, J. Renewable routes to monomeric precursors of nylon 66 and nylon 6 from food waste. J. Clean. Prod. 2019, 227, 624–633. [Google Scholar] [CrossRef]
- Luisier, A.; Bourban, P.; Månson, J. In Situ Polymerization of Polyamide 12 for Thermoplastic Composites. In Proceedings of the 12th International Conference on Composite Materials, Paris, France, 5–9 July 1999; pp. 33–45. [Google Scholar]
- Zingraff, L.; Michaud, V.; Bourban, P.-E.; Månson, J.-A. Resin transfer moulding of anionically polymerised polyamide 12. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1675–1686. [Google Scholar] [CrossRef]
- Chauvel, A.; Lefebvre, G. Petrochemical Processes; OPHRYS Editions: Paris, France, 2001; Volume 80. [Google Scholar]
- Rusu, G.; Rusu, E. Caprolactam-laurolactam (nylon 6/12) copolymers: Synthesis and characterization. High Perform. Polym. 2004, 16, 569–584. [Google Scholar] [CrossRef]
- Wu, W.; Klunker, F.; Xie, L.; Jiang, B.; Ziegmann, G. Simultaneous binding and ex situ toughening concept for textile reinforced pCBT composites: Influence of preforming binders on interlaminar fracture properties. Compos. Part A Appl. Sci. Manuf. 2013, 53, 190–203. [Google Scholar] [CrossRef]
- Agirregomezkorta, A.; Sánchez-Soto, M.; Aretxaga, G.; Sarrionandia, M.; Aurrekoetxea, J. Effects of vacuum infusion processing parameters on the impact behavior of carbon fiber reinforced cyclic butylene terephthalate composites. J. Compos. Mater. 2013, 48, 333–344. [Google Scholar] [CrossRef]
- Baets, J.; Godara, A.; Devaux, J.; Verpoest, I. Toughening of isothermally polymerized cyclic butylene terephthalate for use in composites. Polym. Degrad. Stab. 2010, 95, 346–352. [Google Scholar] [CrossRef]
- Agirregomezkorta, A.; Martínez, A.; Sánchez-Soto, M.; Aretxaga, G.; Sarrionandia, M.; Aurrekoetxea, J. Impact behaviour of carbon fibre reinforced epoxy and non-isothermal cyclic butylene terephthalate composites manufactured by vacuum infusion. Compos. Part B Eng. 2012, 43, 2249–2256. [Google Scholar] [CrossRef]
- Prabhakaran, R.D.; Andersen, T.L.; Lystrup, A. Glass/CBT Laminate Processing and Quality Aspects. In Proceedings of the 10th International Conference on Flow Processes in Composite Materials (FPCM10), Monte Verita, Switzerland, 12–15 July 2010. [Google Scholar]
- Brunelle, D.J.; Bradt, J.E.; Serth-Guzzo, J.; Takekoshi, T.; Evans, T.L.; Pearce, E.J.; Wilson, P.R. Semicrystalline Polymers via Ring-Opening Polymerization: Preparation and Polymerization of Alkylene Phthalate Cyclic Oligomers. Macromolecules 1998, 31, 4782–4790. [Google Scholar] [CrossRef]
- Brunelle, D.J. Cyclic oligomer chemistry. J. Polym. Sci. Part A Polym. Chem. 2007, 46, 1151–1164. [Google Scholar] [CrossRef]
- East, G.; Girshab, A. Cyclic oligomers in poly(1,4-butylene terephthalate). Polymer 1982, 23, 323–324. [Google Scholar] [CrossRef]
- Ishak, Z.M.; Gatos, K.; Karger-Kocsis, J. On the in-situ polymerization of cyclic butylene terephthalate oligomers: DSC and rheological studies. Polym. Eng. Sci. 2006, 46, 743–750. [Google Scholar] [CrossRef]
- Steeg, M.; Mitschang, P.; Chakraborty, P.; Hartmann, T. Modeling the viscosity and conversion of in-situ polymerizing PBT using empirical data. In Proceedings of the 17th ICCM, Edinburgh, UK, 27–31 July 2009. [Google Scholar]
- Pang, K.; Kotek, R.; Tonelli, A. Review of conventional and novel polymerization processes for polyesters. Prog. Polym. Sci. 2006, 31, 1009–1037. [Google Scholar] [CrossRef]
- Abt, T.; Sánchez-Soto, M. A Review of the Recent Advances in Cyclic Butylene Terephthalate Technology and its Composites. Crit. Rev. Solid State Mater. Sci. 2016, 42, 173–217. [Google Scholar] [CrossRef]
- Baets, J.; Dutoit, M.; Devaux, J.; Verpoest, I. Toughening of glass fiber reinforced composites with a cyclic butylene terephthalate matrix by addition of polycaprolactone. Compos. Part A Appl. Sci. Manuf. 2008, 39, 13–18. [Google Scholar] [CrossRef]
- Brunelle, D.J.; Shannon, T.G. Preparation and polymerization of bisphenol A cyclic oligomeric carbonates. Macromolecules 1991, 24, 3035–3044. [Google Scholar] [CrossRef]
- Salem, A.; Stewart, K.; Gifford, S.; Berenbaum, A. Fabrication of thermoplastic matrix structural composites by resin transfer molding of cyclic bisphenol-A polycarbonate oligomers. SAMPE J. 1991, 27, 17–22. [Google Scholar]
- Gonçalves, C.; Gonçalves, I.C.; Magalhães, F.D.; Pinto, A.M. Poly (lactic acid) Composites Containing Carbon-Based Nanomaterials: A Review. Polymers 2017, 9, 269. [Google Scholar] [CrossRef]
- Avinc, O.; Khoddami, A. Overview of Poly (lactic acid) (PLA) Fibre. Fibre Chem. 2009, 41, 391–401. [Google Scholar] [CrossRef]
- Kim, H.; Kobayashi, S.; Abdurrahim, M.A.; Zhang, M.J.; Khusainova, A.; Hillmyer, M.A.; Abdala, A.A.; Macosko, C.W. Graphene/polyethylene nanocomposites: Effect of polyethylene functionalization and blending methods. Polymer 2011, 52, 1837–1846. [Google Scholar] [CrossRef]
- Zhuang, W.; Liu, J.; Zhang, J.; Hu, B.X.; Shen, J. Preparation, characterization, and properties of TiO2/PLA nanocomposites by in situ polymerization. Polym. Compos. 2009, 30, 1074–1080. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, S.-H.; Lee, Y.-D. Preparation and characterization of poly(l-lactide)–graphene composites using the in situ ring-opening polymerization of PLLA with graphene as the initiator. J. Mater. Chem. 2012, 22, 10805. [Google Scholar] [CrossRef]
- Louisy, E.; Samyn, F.; Bourbigot, S.; Fontaine, G.; Bonnet, F. Preparation of Glass Fabric/Poly(l-lactide) Composites by Thermoplastic Resin Transfer Molding. Polymers 2019, 11, 339. [Google Scholar] [CrossRef]
- ARKEMA. Elium® Resin for Composites. Available online: https://www.arkema.com/global/en/products/product-finder/product-range/incubator/elium_resins/ (accessed on 1 December 2020).
- ARKEMA. Liquid Thermoplastic Resin for Tougher Composites. Available online: https://www.arkema.com/export/shared/.content/media/downloads/products-documentations/incubator/brochure-elium-2017.pdf (accessed on 24 October 2019).
- Raponi, O.D.A.; De Souza, B.R.; Barbosa, L.C.M.; Ancelotti, A.C., Jr. Thermal, rheological, and dielectric analyses of the polymerization reaction of a liquid thermoplastic resin for infusion manufacturing of composite materials. Polym. Test. 2018, 71, 32–37. [Google Scholar] [CrossRef]
- Bhudolia, S.K.; Perrotey, P.; Joshi, S.C. Mode I fracture toughness and fractographic investigation of carbon fibre composites with liquid Methylmethacrylate thermoplastic matrix. Compos. Part B Eng. 2018, 134, 246–253. [Google Scholar] [CrossRef]
- Obande, W.; Mamalis, D.; Ray, D.; Yang, L.; Brádaigh, C.M. Mechanical and thermomechanical characterisation of vacuum-infused thermoplastic- and thermoset-based composites. Mater. Des. 2019, 175, 107828. [Google Scholar] [CrossRef]
- Bhudolia, S.K.; Perrotey, P.; Joshi, S.C. Optimizing Polymer Infusion Process for Thin Ply Textile Composites with Novel Matrix System. Materials 2017, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Monti, A.; El Mahi, A.; Jendli, Z.; Guillaumat, L. Mechanical behaviour and damage mechanisms analysis of a flax-fibre reinforced composite by acoustic emission. Compos. Part A Appl. Sci. Manuf. 2016, 90, 100–110. [Google Scholar] [CrossRef]
- Haggui, M.; Jendli, Z.; Akrout, A.; El Mahi, A.; Haddar, M. Damage identification in flax fibre composite with thermoplastic matrix under quasi-static loading. In Proceedings of the International Conference on Advanced Materials, Mechanics and Manufacturing A3M, Hammamet, Tunisia, 19–21 December 2016. [Google Scholar]
- Tison, F. Thermoplastic composites production by room-temperature vacuum infusion. JEC Compos. 2016, 102, 57–58. [Google Scholar]
- GVR. Synthetic and Bio-Based Methyl Methacrylate (MMA) Market Size, Application Analysis, Regional Outlook, Competitive Strategies and Forecasts, 2014 to 2020; Grand View Research Report GVR1280; GVR: San Francisco, CA, USA, 2014. [Google Scholar]
- Youk, J.H.; Kambour, R.P.; Macknight, W.J. Polymerization of Ethylene Terephthalate Cyclic Oligomers with Antimony Trioxide. Macromolecules 2000, 33, 3594–3599. [Google Scholar] [CrossRef]
- Youk, J.H.; Boulares, A.; Kambour, R.P.; Macknight, W.J. Polymerization of Ethylene Terephthalate Cyclic Oligomers with a Cyclic Dibutyltin Initiator. Macromolecules 2000, 33, 3600–3605. [Google Scholar] [CrossRef][Green Version]
- Nagahata, R.; Sugiyama, J.; Goyal, M.; Goto, M.; Honda, K.; Asai, M.; Ueda, M.; Takeuchi, K. Thermal polymerization of uniform macrocyclic ethylene terephthalate dimer. Polymer 2001, 42, 1275–1279. [Google Scholar] [CrossRef]
- Nagahata, R.; Sugiyama, J.I.; Goyal, M.; Asai, M.; Ueda, M.; Takeuchi, K. Solid-phase thermal polymerization of macrocyclic ethylene terephthalate dimer using various transesterification catalysts. J. Polym. Sci. Pol. Chem. 2000, 38, 3360–3368. [Google Scholar] [CrossRef]
- Pini, N.; Zaniboni, C.; Busato, S.; Ermanni, P. Perspectives for Reactive Molding of PPA as Matrix for High-performance Composite Materials. J. Thermoplast. Compos. Mater. 2006, 19, 207–216. [Google Scholar] [CrossRef]
- Goodfellow. PLLA. Available online: http://www.goodfellow.com/E/Poly-L-lactic-acid-Biopolymer.html (accessed on 24 October 2019).
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Tham, W.L.; Poh, B.T.; Ishak, Z.A.M.; Chow, W.S. Characterisation of water absorption of biodegradable poly(lactic acid)/halloysite nanotube nanocomposites at different temperatures. J. Eng. Sci. 2016, 12, 13. [Google Scholar]
- Zhang, L.; Lv, S.; Sun, C.; Wan, L.; Tan, H.; Zhang, Y. Effect of MAH-g-PLA on the Properties of Wood Fiber/Polylactic Acid Composites. Polymers 2017, 9, 591. [Google Scholar] [CrossRef] [PubMed]
- Polymer Properties Database. Polyurethane. Available online: https://polymerdatabase.com/polymers/hexamethylenediisocyanatediethyleneglycol.html (accessed on 24 October 2019).
- Goodfellow. PEK. Available online: http://www.goodfellow.com/catalogue/GFCat4.php?ewd_token=hoDZrxdrFfmNKKrxxD1M5IWFzhfmZo&n=1QxXsTWTaq3A26aehcP1wUGGfOrqUF&ewd_urlNo=GFCatSeab1&CatLook=PEK (accessed on 24 October 2019).
- PerkinElmer. DSC Transitions of Common Thermoplastics. Available online: https://thermalsupport.com/wp-content/uploads/2018/05/PETech-44.pdf (accessed on 29 June 2020).
- RTP Imagineering Plastics. RTP2201A Polyetherketone (PEK) Glass Fiber Product Data Sheet & General Processing Conditions. Available online: http://web.rtpcompany.com/info/data/2200A/RTP2201A.htm (accessed on 29 June 2020).
- Davies, P. Environmental degradation of composites for marine structures: New materials and new applications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150272. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Arhant, M. Fatigue Behaviour of Acrylic Matrix Composites: Influence of Seawater. Appl. Compos. Mater. 2018, 26, 507–518. [Google Scholar] [CrossRef]
- Ishak, Z.A.M.; Lim, N.C. Effect of moisture absorption on the tensile properties of short glass fiber reinforced poly(butylene terephthalate). Polym. Eng. Sci. 1994, 34, 1645–1655. [Google Scholar] [CrossRef]
- Tanaka, K.; Fukushima, Y.; Kashihara, K.; Katayama, T. Effect of water absorption on the mechanical properties of continuous carbon fibre reinforced polycarbonate composites. High Perform. Struct. Mater. V 2010, 112, 153–162. [Google Scholar] [CrossRef]
- Prabhakaran, R.D.; Andersen, T.L.; Lystrup, A. Influence of moisture absorption on properties of fiber reinforced polyamide 6 composites. In Proceedings of the 2nd Joint US-Canada Conference on Composites, Montreal, QC, Canada, 26–28 September 2011; pp. 500–510. [Google Scholar]
- Colin, X.; Verdu, J. Humid Ageing of Organic Matrix Composites. In Durability of Composites in a Marine Environment; Davies, P., Rajapakse, Y., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 47–114. [Google Scholar]
- Xie, Y.; Hill, C.A.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Mohit, H.; Selvan, V.A.M. A comprehensive review on surface modification, structure interface and bonding mechanism of plant cellulose fiber reinforced polymer based composites. Compos. Interfaces 2018, 25, 629–667. [Google Scholar] [CrossRef]
- Graupner, N.; Rößler, J.; Ziegmann, G.; Müssig, J. Fibre/matrix adhesion of cellulose fibres in PLA, PP and MAPP: A critical review of pull-out test, microbond test and single fibre fragmentation test results. Compos. Part A Appl. Sci. Manuf. 2014, 63, 133–148. [Google Scholar] [CrossRef]
- Anbupalani, M.S.; Venkatachalam, C.D.; Rathanasamy, R. Influence of coupling agent on altering the reinforcing efficiency of natural fibre-incorporated polymers—A review. J. Reinf. Plast. Compos. 2020, 39, 520–544. [Google Scholar] [CrossRef]
- Wright, W. The effect of diffusion of water into epoxy resins and their carbon-fibre reinforced composites. Composites 1981, 12, 201–205. [Google Scholar] [CrossRef]
- Smith, L.; Schmitz, V. The effect of water on the glass transition temperature of poly(methyl methacrylate). Polymer 1988, 29, 1871–1878. [Google Scholar] [CrossRef]
- Passerini, N.; Craig, D.Q. An investigation into the effects of residual water on the glass transition temperature of polylactide microspheres using modulated temperature DSC. J. Control. Release 2001, 73, 111–115. [Google Scholar] [CrossRef]
- Summerscales, J. Durability of Composites in the Marine Environment. In Durability of Composites in a Marine Environment; Davies, P., Rajapakse, Y., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–13. [Google Scholar]
- Parodi, E.; Peters, G.; Govaert, L.E. Structure–Properties Relations for Polyamide 6, Part 1: Influence of the Thermal History during Compression Moulding on Deformation and Failure Kinetics. Polymers 2018, 10, 710. [Google Scholar] [CrossRef]
- Greene, J. PLA and PHA Biodegradation in the Marine Environment; CalRecycle: Sacramento, CA, USA, 2012.
- Pelegrini, K.; Donazzolo, I.; Brambilla, V.; Grisa, A.M.C.; Piazza, D.; Zattera, A.J.; Brandalise, R.N. Degradation of PLA and PLA in composites with triacetin and buriti fiber after 600 days in a simulated marine environment. J. Appl. Polym. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Soroudi, A.; Jakubowicz, I. Recycling of bioplastics, their blends and biocomposites: A review. Eur. Polym. J. 2013, 49, 2839–2858. [Google Scholar] [CrossRef]
- Álvarez-Chávez, C.R.; Edwards, S.E.; Moure-Eraso, R.; Geiser, K. Sustainability of bio-based plastics: General comparative analysis and recommendations for improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Yates, M.R.; Barlow, C.Y. Life cycle assessments of biodegradable, commercial biopolymers—A critical review. Resour. Conserv. Recycl. 2013, 78, 54–66. [Google Scholar] [CrossRef]
- Murphy, R.; Bartle, I. Biodegradable Polymers and Sustainability: Insights from Life Cycle Assessment. In Proceedings of the National Non-Food Crops Centre Seminar, London, UK, 25 May 2004. [Google Scholar]
- Krzan, A.; Hemjinda, S.; Miertus, S.; Corti, A.; Chiellini, E. Standardization and certification in the area of environmentally degradable plastics. Polym. Degrad. Stab. 2006, 91, 2819–2833. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials Under Controlled Composting Conditions, Incorporating Thermophilic Temperatures; D5338-15; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM. Standard Specification for Labeling of Plastics Designed to be Aerobically Composted in Municipal or Industrial Facilities; D6400-19; ASTM: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM. Standard Specification for Labeling of End Items that Incorporate Plastics and Polymers as Coatings or Additives with Paper and Other Substrates Designed to be Aerobically Composted in Municipal or Industrial Facilities; D6868-19; ASTM: West Conshohocken, PA, USA, 2019. [Google Scholar]
- BSI. Packaging. Requirements for Packaging Recoverable Through Composting and Biodegradation. Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging; BS EN 13432; BSI: London, UK, 2000. [Google Scholar]
- ISO. Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Analysis of Evolved Carbon Dioxide; ISO 14852; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- ASTM. Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in the Marine Environment by a Defined Microbial Consortium or Natural Sea Water Inoculum; D6691-17; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM. Standard Test Method for Weight Attrition of Plastic Materials in the Marine Environment by Open System Aquarium Incubations; D7473-12; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ASTM. Standard Test Method for Determining Aerobic Biodegradation of Plastics Buried in Sandy Marine Sediment under Controlled Laboratory Conditions; D7991-15; ASTM: West Conshohocken, PA, USA, 2015. [Google Scholar]
- BSI. Plastics. Determination of the Degree of Disintegration of Plastic Materials in Marine Habitats under Real Field Conditions; BS ISO 22766; BSI: London, UK, 2000. [Google Scholar]
- Zumstein, M.T.; Narayan, R.; Kohler, H.-P.E.; McNeill, K.; Sander, M. Dos and Do Nots When Assessing the Biodegradation of Plastics. Environ. Sci. Technol. 2019, 53, 9967–9969. [Google Scholar] [CrossRef] [PubMed]
- Naumann, S.; Buchmeiser, M.R. Latent and Delayed Action Polymerization Systems. Macromol. Rapid Commun. 2014, 35, 682–701. [Google Scholar] [CrossRef]
- Endo, T.; Sanda, F. Design of latent catalysts and their application to polymer synthesis. Macromol. Symp. 1996, 107, 237–242. [Google Scholar] [CrossRef]
- Groote, R.R.; Jakobs, R.T.M.; Sijbesma, R.P. Mechanocatalysis: Forcing latent catalysts into action. Polym. Chem. 2013, 4, 4846–4859. [Google Scholar] [CrossRef]
- Alibaba. Available online: https://www.alibaba.com/ (accessed on 7 July 2020).
- Goodship, V. Management, Recycling and Reuse of Waste Composites; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- ARKEMA. Recycling Composite Parts Made of Elium® Resin is Possible! Available online: https://www.arkema.com/en/products/product-finder/range-viewer/Elium-resins-for-composites/ (accessed on 24 October 2019).
- Cousins, D.S.; Suzuki, Y.; Murray, R.E.; Samaniuk, J.R.; Stebner, A.P. Recycling glass fiber thermoplastic composites from wind turbine blades. J. Clean. Prod. 2019, 209, 1252–1263. [Google Scholar] [CrossRef]
- Silcock, A.; Hinkley, P. Report on the Spread of Fire at Summerland in Douglas on the Isle of Man, 2 August 1973; BRE Current Paper CP74/74; Building Research Establishment: Watford, UK, 1974. [Google Scholar]
- Le Duigou, A.; Pillin, I.; Bourmaud, A.; Davies, P.; Baley, C. Effect of recycling on mechanical behaviour of biocompostable flax/poly(l-lactide) composites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1471–1478. [Google Scholar] [CrossRef]
- Patel, M. Cumulative energy demand (CED) and cumulative CO2 emissions for products of the organic chemical industry. Energy 2003, 28, 721–740. [Google Scholar] [CrossRef]
- Morão, A.; De Bie, F. Life Cycle Impact Assessment of Polylactic Acid (PLA) Produced from Sugarcane in Thailand. J. Polym. Environ. 2019, 27, 2523–2539. [Google Scholar] [CrossRef]
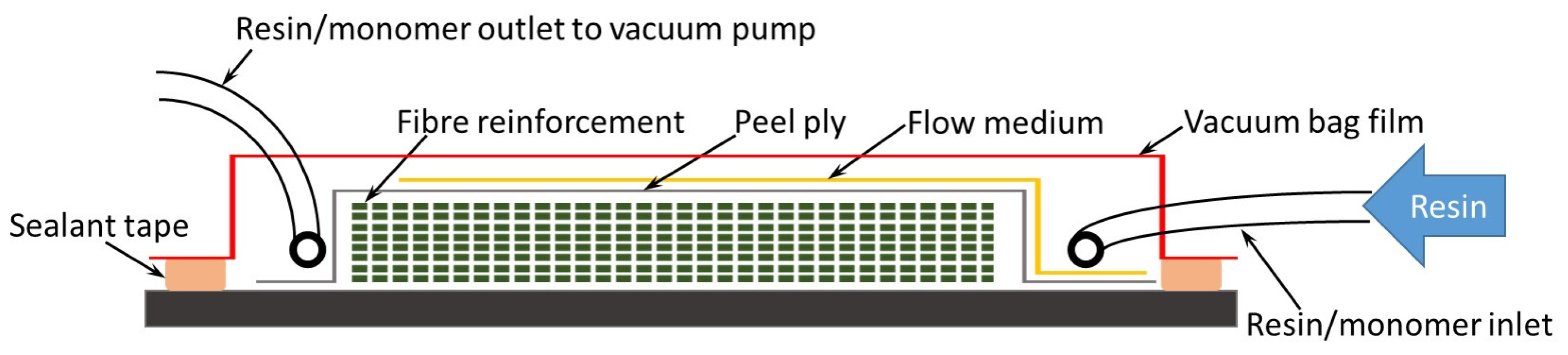
| Consumables | Materials | Maximum Temperature (°C) |
|---|---|---|
| Tape | Polyamide (PA) film tape rubber adhesive | 204 |
| Polyester tape with rubber/acrylic/silicone adhesive | 177–220 | |
| Polytetrafluoroethylene (PTFE) tape with rubber/acrylic/silicone adhesive | 204, 260 | |
| Fluoropolymer tape with rubber/silicone adhesive | 204, 260 | |
| Polytetrafluoroethylene (PTFE) coated glass fabric tape with silicone adhesive | 260 | |
| Polyimide (PI) tape with silicone adhesive | 399 | |
| Bagging film | Polyethylene (PE) | 48, 82 |
| Polypropylene (PP) | 135 | |
| Polyvinyl chloride (PVC) | 121 | |
| Polyolefin | 121, 140 | |
| Polyamide (PA) and polyolefin multilayer | 155 | |
| Polyamide (PA) + polyethylene (PE) + polyamide (PA) multilayer | 170 | |
| Polyurethane (PU) | 135, 176 | |
| Polyamide (PA) and polypropylene (PP) multilayer | 180 | |
| Polymethyl pentene (PMP) and polyamide (PA) multilayer | 190 | |
| Thermoplastic elastomer (TPE) | 121–195 | |
| Ethylene tetrafluoroethylene (ETFE) and polyamide (PA) multilayer | 230 | |
| Polyamide (PA) | 120–246 | |
| Polytetrafluoroethylene (PTFE) | 315 | |
| Polyimide (PI) | 399, 426 | |
| Peel ply | Polyester | 121–249 |
| Polyamide (PA) | 160–260 | |
| Fiberglass coated with polytetrafluoroethylene (PTFE) | 288, 316 | |
| Fiberglass coated with silicone | 427 | |
| Flow medium | Low-density polyethylene (LDPE) | 65 |
| Low-density polyethylene (LDPE) and high-density polyethylene (HDPE) | 65, 100 | |
| Polyethylene (PE) | 120 | |
| High-density polyethylene (HDPE) | 93, 125 | |
| Polypropylene (PP) | 100, 150 | |
| Polyester | 170, 200 | |
| Polyamide (PA) | 177–216 | |
| Release film | Polypropylene (PP) | 100 |
| Polyolefin and high-density polyethylene (HDPE) | 120 | |
| Polyolefin | 121–157 | |
| Polyethylene (PE) | 125 | |
| Polymethyl pentene (PMP) | 193, 200 | |
| Fluorinated ethylene propylene (FEP) | 210, 260 | |
| Ethylene tetrafluoroethylene (ETFE) | 220, 260 | |
| Fluoropolymer | 260, 315 | |
| Polyimide (PI) | 405 | |
| Sealant tape | Synthetic Rubber | 90–232 |
| Silicone | 400, 427 | |
| Hose/Pipe | Polyvinyl chloride (PVC) | 50 |
| Polyurethane (PU) | 60 | |
| Low-density polyethylene (LDPE) | 90 | |
| High-density polyethylene (HDPE) | 90 | |
| Polyethylene (PE) | 90, 121 | |
| Silicone | 230, 260 | |
| Spiral | Polyethylene (PE) | 80, 121 |
| Polypropylene (PP) | 60, 120 |
| Polymer | Monomer Viscosity (mPa·s) | Processing Temperature (°C) | Bio-Based Monomer Available? | Ref. |
|---|---|---|---|---|
| Polyamide-6 (PA6) | ~5 | 130–200 | Yes | [94,95,96,97] |
| Polyamide-12 (PA12) | 23 | 180–240 | No | [92,106] |
| Polybutylene terephthalate (PBT) | 20–150 | 180–260 | No | [64,92,109,110,111,112,113,115,117] |
| Polycarbonate (PC) | 1000 | 250–300 | No | [123] |
| Polylactide (PLA) | - | 150–185 | Yes | [127,128,129] |
| Polymethyl methacrylate (PMMA, Elium®) | 100 | 20–100 | Yes * | [131] |
| Thermoplastic polyurethane (TPU) | 800 | 300 | No | [93] |
| Polyether ketone (PEK) | 80 | 340–390 | No | [92] |
| Polyethylene terephthalate (PET) | 30 | 250–325 | No | [140,141,142,143] |
| Polyphthalamide (PPA) | 1000 | 200–290 | No | [93,144] |
| Polymer | Density (g/cm3) | Tensile Strength (MPa) | Tensile Modulus (GPa) | Strain to Failure (%) | Tg (°C) | Tm (°C) | Moisture Absorption (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| PA6 | 1.13 | 85 | 2.0–3.8 | 19 | 40–60 | 219–230 | 6–11 | [93] |
| PA12 | 1.04 | 50–60 | 1.4 | 300 | 40–50 | 180 | 1.1–1.8 | [93,144] |
| PBT | 1.31 | 85 | 1.8–2.7 | 30 | 25–60 | 225 | 0.09 | [93] |
| PC | 1.20 | 60 | 2.2 | >100 | 150 | 300 | 0.16 | [93] |
| PLA | 1.25 | 70 | 3.6 | 2.4 | 55–65 | 170–200 | <2 | [145,146,147,148] |
| PMMA (Elium®) | 1.20 | 66 | 3.17 | 2.6 | 107 | - | 0.5 | [131,135,138] |
| TPU | 1.20 | 40 | 0.2–2.3 | >500 | −8–17 | 140 | 0.1 | [93,149] |
| PEK | 1.30 | 115 | 3.7 | 20 | 228 | - | 0.07 | [92,150] |
| PET | 1.38 | 69 | 3 | 13 | 72 | 255 | 0.5 | [93] |
| PPA | 1.18 | 90 | 2.5–3.5 | 6 | 121–138 | 310–330 | 0.36 | [93,144] |
| Polymer | Essential Criteria | Desirable Criteria | Pass/Fail | ||||
|---|---|---|---|---|---|---|---|
| Monomer Process Viscosity (mPa·s) | Process Temperature (°C) | Tg (°C) | Moisture Absorption (%) | Bio-Based | Recyclable | ||
| PA6 | ~5 | 130–200 | 40–60 | 6–11 | ✓ 196k | Tm = 219–230 °C | ✕ |
| PA12 | 23 | 180–240 | 40–50 | <2 | ✓ 136k | Tm = 180 °C | ✕ |
| PBT | 20–150 | 180–260 | 25–60 | 0.09 | ✓ 1110k | Tm = 225 °C | ✕ |
| PC | 250–300 | 250–300 | 150 | 0.16 | ✓ 286 M | amorphous (Tp *~235 °C) | ✕ |
| PLA | - | 150–185 | 55–65 | <2 | ✓ 22 M | Tm = 170–200 °C | ✓ |
| PMMA (Elium®) | 100 | 20–100 | 107 | 0.5 | ✓ 220k | depolymerize | ✓ |
| TPU | 800 | 300 | −8–17 | 0.1 | ✓ 1360k | Tm = 140 °C | ✕ |
| PEK | 340–390 | 340–390 | 228 | 0.07 | ✓ 1140k | Tm = 385–413 °C | ✕ |
| PET | 250–325 | 250–325 | 73 | 0.5 | ✓ 217 M | Tm = 255 °C | ✕ |
| PPA | 1000 | 200–290 | 121–138 | 0.36 | ✓ 1230k | Tm = 310–330 °C | ✕ |
| Polymer | CPR (GJ/ton) | CED (GJ/ton) | CCO2 (kg CO2/ton) |
|---|---|---|---|
| Thermoplastics | |||
| PA6 | 90.7 | 122.7 | 4680 |
| PC | 49.3 | 80.3 | 3110 |
| PET | 33.4 | 59.4 | 2070 |
| PLA | ~ | 89.2 | 501 (2334 *) |
| PMA ** | 55.6 | 82.6 | 3740 |
| PU | 48.5 | 75.5 | 3050 |
| Thermosets | |||
| Epoxy | 73.6 | 107.1 | 4680 |
| Polyester | 37.5 | 64.5 | 2390 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Summerscales, J.; Graham-Jones, J.; Meng, M.; Pemberton, R. Monomer Selection for In Situ Polymerization Infusion Manufacture of Natural-Fiber Reinforced Thermoplastic-Matrix Marine Composites. Polymers 2020, 12, 2928. https://doi.org/10.3390/polym12122928
Qin Y, Summerscales J, Graham-Jones J, Meng M, Pemberton R. Monomer Selection for In Situ Polymerization Infusion Manufacture of Natural-Fiber Reinforced Thermoplastic-Matrix Marine Composites. Polymers. 2020; 12(12):2928. https://doi.org/10.3390/polym12122928
Chicago/Turabian StyleQin, Yang, John Summerscales, Jasper Graham-Jones, Maozhou Meng, and Richard Pemberton. 2020. "Monomer Selection for In Situ Polymerization Infusion Manufacture of Natural-Fiber Reinforced Thermoplastic-Matrix Marine Composites" Polymers 12, no. 12: 2928. https://doi.org/10.3390/polym12122928
APA StyleQin, Y., Summerscales, J., Graham-Jones, J., Meng, M., & Pemberton, R. (2020). Monomer Selection for In Situ Polymerization Infusion Manufacture of Natural-Fiber Reinforced Thermoplastic-Matrix Marine Composites. Polymers, 12(12), 2928. https://doi.org/10.3390/polym12122928









