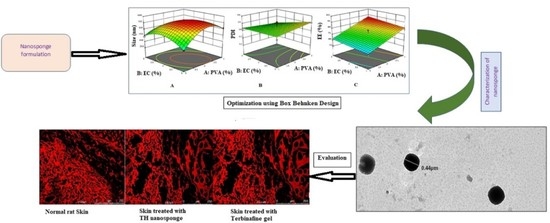
Development and Evaluation of Polymeric Nanosponge Hydrogel for Terbinafine Hydrochloride: Statistical Optimization, In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Experimental Methodologies
2.1. Materials and Animals Used
2.1.1. Materials
2.1.2. Animals
2.2. Development and Characterization of TH-NS
2.2.1. Optimization of TH-NS
2.2.2. Fabrication of TH-NS
2.2.3. Characterization of Prepared TH-NS
2.3. Fabrication of Topical Hydrogel Integrating TH-NS
2.4. Characterisation of TH-NS HG
2.4.1. Visual Observation
2.4.2. Viscosity Determination
2.4.3. pH Determination
2.4.4. Spreadability
2.4.5. Texture Analysis
2.4.6. Determination of Drug Content
2.5. In Vitro Release Study
2.6. In Vitro Permeation Studies
2.7. Confocal Laser Microscopy
2.8. Tape Stripping Technique
2.9. Skin Irritation Study
- Group I: Control (no drug treatment);
- Group II: DS;
- Group III: Placebo HG;
- Group IV: TH-NS HG.
2.10. Antifungal Activity Study (Cylinder Plate Method)
2.11. Statistical Analysis
3. Results and Discussion
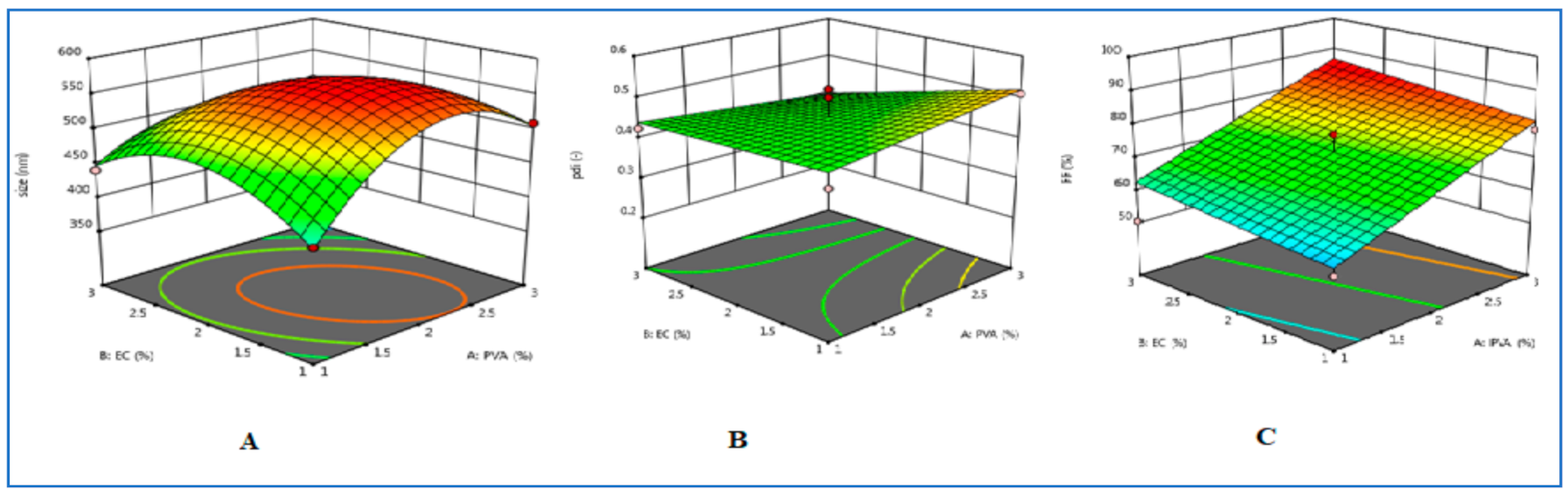
3.1. Optimization of TH-NS
3.2. Characterisation of Optimised TH-NS
3.2.1. Particle Size Determination, Polydispersity Index, Entrapment Efficiency
3.2.2. Thermoanalytical Technique (DSC)
3.3. Preparation and Evaluation of TH-NS HG
3.3.1. Visual Examination
3.3.2. Viscosity
3.3.3. pH Determination
3.3.4. Spreadability
3.3.5. Texture Analysis
3.3.6. Drug Content
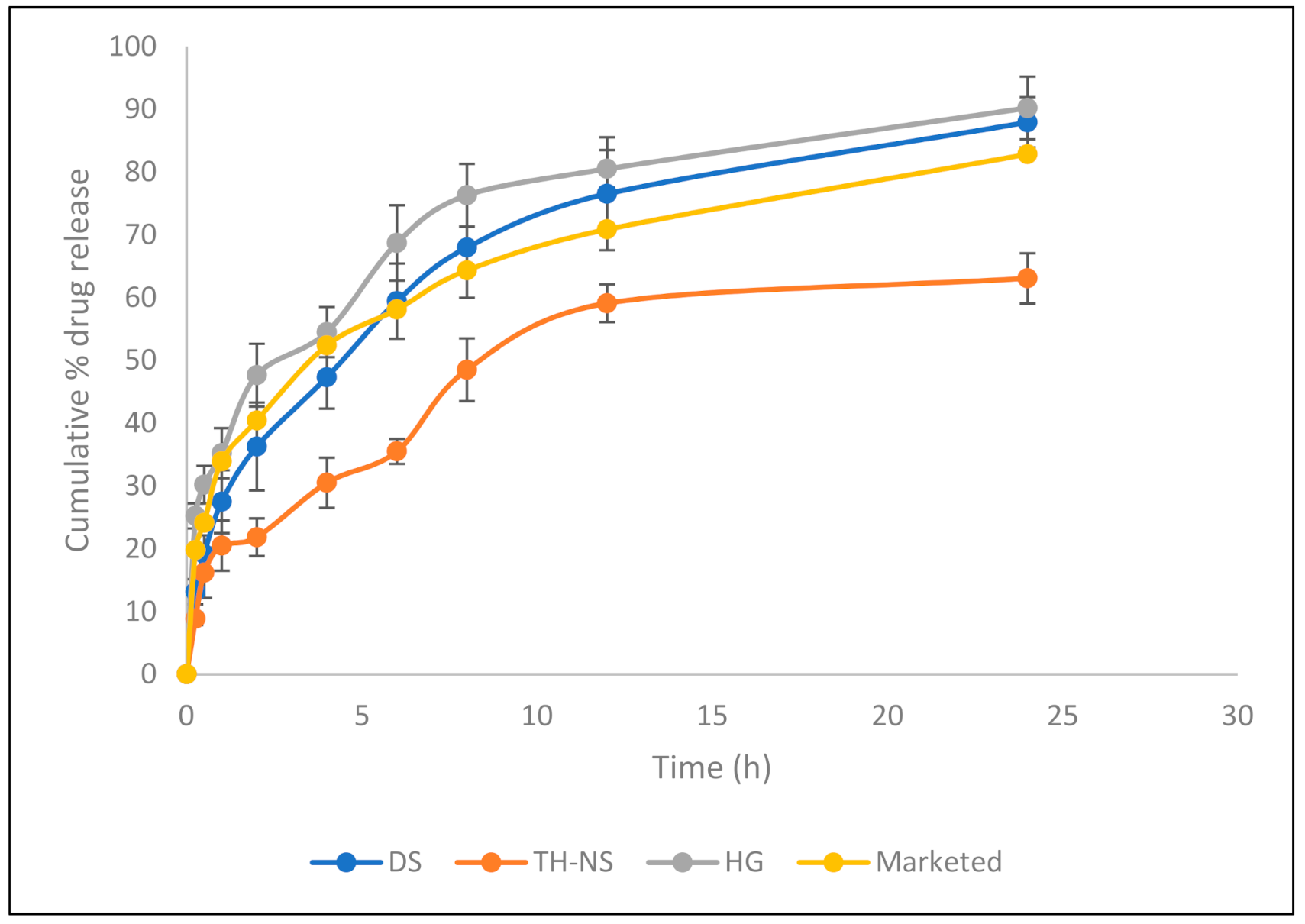
3.4. In Vitro Release Study
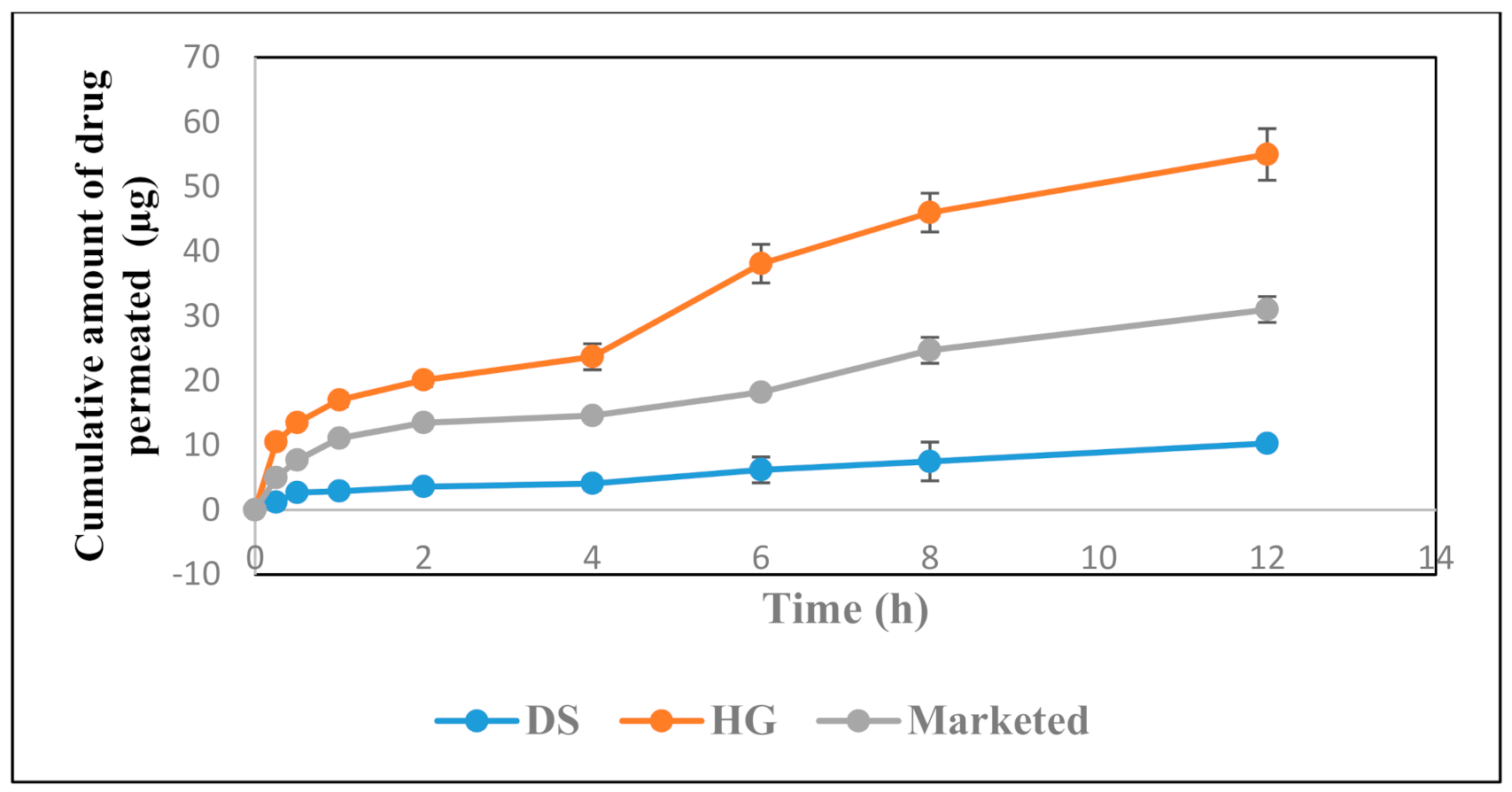
3.5. In Vitro Permeation Studies
3.6. Confocal Study
3.7. Tape Stripping Study
3.8. Skin Irritation Study
3.9. Antifungal Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdellatif, M.M.; Khalil, I.A.; Khalil, M.A. Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: In-vitro, ex-vivo and in-vivo evaluation. Int. J. Pharm. 2017, 527, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Voltan, A.R.; Quindós, G.; Alarcón, K.P.M.; Fusco-Almeida, A.M.; Mendes-Gianinni, M.J.; Chorilli, M. Fungal diseases: Could nanostructured drug delivery systems be a novel paradigm for therapy? Int. J. Nanomed. 2016, 11, 3715–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, S.A.A.; ElMeshad, A.N.; Shoukri, R.A. Microemulsion loaded hydrogel as a promising vehicle for dermal delivery of the antifungal sertaconazole: Design, optimization and ex vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Liu, T.; Ma, H.; Tian, Y.; Li, L.; Li, Z.; Gao, M.; Zhang, J.; Tang, Z. Preparation of Essential Oil-Based Microemulsions for Improving the Solubility, pH Stability, Photostability, and Skin Permeation of Quercetin. AAPS PharmSciTech 2017, 18, 3097–3104. [Google Scholar] [CrossRef]
- Fachinetti, N.; Rigon, R.B.; Eloy, J.O.; Sato, M.R.; Dos Santos, K.C.; Chorilli, M. Comparative Study of Glyceryl Behenate or Polyoxyethylene 40 Stearate-Based Lipid Carriers for Trans-Resveratrol Delivery: Development, Characterization and Evaluation of the In Vitro Tyrosinase Inhibition. AAPS PharmSciTech 2018, 19, 1401–1409. [Google Scholar] [CrossRef]
- Özcan, I.; Güneri, T.; Abacı, O.; Özer, O.; Uztan, A.H.; Aksu, B.; Hayal Boyacıoğlu, H. Enhanced Topical Delivery of Terbinafine Hydrochloride with Chitosan Hydrogels. AAPS PharmSciTech 2009, 10, 1024–1031. [Google Scholar] [CrossRef] [Green Version]
- Kahraman, E.; Güngör, S.; Ozsoy, Y. Potential enhancement and targeting strategies of polymeric and lipid-based nanocarriers in dermal drug delivery. Ther. Deliv. 2017, 8, 967–985. [Google Scholar] [CrossRef]
- Celebi, N.; Ermiş, S.; Özkan, S. Development of topical hydrogels of terbinafine hydrochloride and evaluation of their antifungal activity. Drug Dev. Ind. Pharm. 2014, 41, 631–639. [Google Scholar] [CrossRef]
- Anandam, S.; Selvamuthukumar, S. Fabrication of cyclodextrin nanosponges for quercetin delivery: Physicochemical characterization, photostability, and antioxidant effects. J. Mater. Sci. 2014, 49, 8140–8153. [Google Scholar] [CrossRef]
- Badr-Eldin, S.M.; Aldawsari, H.M.; Labib, G.S.; El-Kamel, A.H. Design and formulation of a topical hydrogel integrating lemongrass-loaded nanosponges with an enhanced antifungal effect: In vitro/in vivo evaluation. Int. J. Nanomed. 2015, 10, 893–902. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Prajapati, S.K.; Kumari, A.; Mody, N.; Bajpai, M. Engineered nanosponges as versatile biodegradable carriers: An insight. J. Drug Deliv. Sci. Technol. 2020, 57, 101643. [Google Scholar] [CrossRef]
- Akhtar, N.; Pathak, K. Cavamax W7 Composite Ethosomal Gel of Clotrimazole for Improved Topical Delivery: Development and Comparison with Ethosomal Gel. AAPS PharmSciTech 2012, 13, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaghasiya, H.; Kumar, A.; Sawant, K. Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride. Eur. J. Pharm. Sci. 2013, 49, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Barot, B.S.; Parejiya, P.B.; Patel, H.K.; Mehta, D.M.; Shelat, P.K. Microemulsion-based antifungal gel delivery to nail for the treatment of onychomycosis: Formulation, optimization, and efficacy studies. AAPS PharmSciTech 2012, 13, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahaparale, P.R.; Ikam, S.A.N.; Chavan, M.S. Development and Evaluation of Terbinafine Hydrochloride Polymeric Microsponges for Topical Drug Delivery. Indian J. Pharm. Sci. 2018, 80, 1086–1092. [Google Scholar] [CrossRef]
- Amer, R.I.; El-Osaily, G.H.; Gad, S.S. Design and optimization of topical terbinafine hydrochloride nanosponges: Application of full factorial design, in vitro and in vivo evaluation. J. Adv. Pharm. Technol. Res. 2020, 11, 13–19. [Google Scholar] [CrossRef]
- Cunha, S.; Costa, C.P.; Loureiro, J.A.; Alves, J.; Peixoto, A.F.; Forbes, B.; Sousa-Lobo, J.; Silva, A. Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics 2020, 12, 599. [Google Scholar] [CrossRef]
- Sharma, R.; Pathak, K. Polymeric nanosponges as an alternative carrier for improved retention of econazole nitrate onto the skin through topical hydrogel formulation. Pharm. Dev. Technol. 2010, 16, 367–376. [Google Scholar] [CrossRef]
- Bachir, Y.N.; Bachir, R.N.; Hadj-Ziane-Zafour, A. Nanodispersions stabilized by β-cyclodextrin nanosponges: Application for simultaneous enhancement of bioactivity and stability of sage essential oil. Drug Dev. Ind. Pharm. 2018, 45, 333–347. [Google Scholar] [CrossRef]
- Anandam, S.; Selvamuthukumar, S. Optimization of microwave-assisted synthesis of cyclodextrin nanosponges using response surface methodology. J. Porous Mater. 2014, 21, 1015–1023. [Google Scholar] [CrossRef]
- Zakaria, A.S.; Afifi, S.A.; Elkhodairy, K.A. Newly Developed Topical Cefotaxime Sodium Hydrogels: Antibacterial Activity andIn VivoEvaluation. BioMed Res. Int. 2016, 2016, 6525163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, A.; Samad, A.; Ramzan, M.; Ahsan, M.N.; Rehman, Z.U.; Ahmad, F.J. Elastic liposome-based gel for topical delivery of 5-fluorouracil: In vitro and in vivo investigation. Drug Deliv. 2016, 23, 1115–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.X.; Alexander, K.S.; Baki, G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J. Pharm. 2016, 2016, 5754349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karavana, Y.S.; Güneri, P.; Ertan, G. Benzydamine hydrochloride buccal bioadhesive gels designed for oral ulcers: Preparation, rheological, textural, mucoadhesive and release properties. Pharm. Dev. Technol. 2009, 14, 623–631. [Google Scholar] [CrossRef]
- Pons, M.; Fiszman, S. Instrumental Texture Profile Analysis with Particular Reference to Gelled Systems. J. Texture Stud. 1996, 27, 597–624. [Google Scholar] [CrossRef]
- Chauhan, S.; Gulati, N.; Nagaich, U. Fabrication and evaluation of ultra deformable vesicles for atopic dermatitis as topical delivery. Int. J. Polym. Mater. 2018, 68, 266–277. [Google Scholar] [CrossRef]
- Lee, S.G.; Kang, J.B.; Kim, S.R.; Kim, C.J.; Yeom, D.W.; Yoon, H.Y.; Kwak, S.S.; Choi, Y.W. Enhanced topical delivery of tacrolimus by a carbomer hydrogel formulation with Transcutol P. Drug Dev. Ind. Pharm. 2016, 42, 1–29. [Google Scholar] [CrossRef]
- Alvarez-Román, R.; Naik, A.; Kalia, Y.N.; Guy, R.H.; Fessi, H. Enhancement of Topical Delivery from Biodegradable Nanoparticles. Pharm. Res. 2004, 21, 1818–1825. [Google Scholar] [CrossRef]
- Jacobs, A.G.; Gerber, M.; Malan, M.M.; Preez, L.J.; Lizelle, T. Topical delivery of acyclovir and ketoconazole. Drug Deliv. 2016, 23, 631–641. [Google Scholar] [CrossRef]
- Gupta, M.; Vyas, S.P. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem. Phys. Lipids 2012, 165, 454–461. [Google Scholar] [CrossRef]
- Venugopal, V.; Kumar, K.J.; Muralidharan, S.; Parasuraman, S.; Raj, P.V.; Kumar, K.V. Optimization and in-vivo evaluation of isradipine nanoparticles using Box-Behnken design surface response methodology. OpenNano 2016, 1, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Rehman, S.; Nabi, B.; Baboota, S.; Ali, J. Tailoring lipid nanoconstructs for the oral delivery of paliperidone: Formulation, optimization and in vitro evaluation. Chem. Phys. Lipids 2020, 105005. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Rehman, S.; Nabi, B.; Baboota, S.; Ali, J. Harnessing nanotechnology for enhanced topical delivery of clindamycin phosphate. J. Drug Deliv. Sci. Technol. 2019, 54, 101253. [Google Scholar] [CrossRef]
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery. Saudi Pharm. J. 2012, 20, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Salam, F.S.; Mahmoud, A.A.; Ammar, H.O.; Elkheshen, S.A. Nanostructured lipid carriers as semisolid topical delivery formulations for diflucortolone valerate. J. Liposome Res. 2016, 27, 41–55. [Google Scholar] [CrossRef]
- Verma, A.; Pathak, K. Topical Delivery of Drugs for the Effective Treatment of Fungal Infections of Skin. Curr. Pharm. Des. 2015, 21, 2892–2913. [Google Scholar] [CrossRef]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.-J.; Fluhr, J. The tape stripping procedure—Evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef]
- Moreno, E.; Calvo, A.; Schwartz, J.; Navarro-Blasco, I.; González-Peñas, E.; Sanmartín, C.; Irache, J.M.; Espuelas, S. Evaluation of Skin Permeation and Retention of Topical Dapsone in Murine Cutaneous Leishmaniasis Lesions. Pharmaceutics 2019, 11, 607. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.; Patel, D.H. Development and validation of RP-HPLC method for simultaneous estimation of Terbinafine hydrochloride and mometasone furoate in combined dosage form. Int. J. Pharm. Pharm. Sci. 2014, 6, 106–109. [Google Scholar]
- Elkomy, M.H.; El Menshawe, S.F.; Eid, H.M.; Ali, A.M.A. Development of a nanogel formulation for transdermal delivery of tenoxicam: A pharmacokinetic-pharmacodynamic modeling approach for quantitative prediction of skin absorption. Drug Dev. Ind. Pharm. 2016, 43, 531–544. [Google Scholar] [CrossRef]
- Iqbal, B.; Ali, J.; Baboota, S. Silymarin loaded nanostructured lipid carrier: From design and dermatokinetic study to mechanistic analysis of epidermal drug deposition enhancement. J. Mol. Liq. 2018, 255, 513–529. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Raman, S.K.; Kuppusamy, G.; Sanapalli, B.K.R.; Wadhwani, A.; Patel, V.; Malayandi, R. In vitro Antifungal Activity of a Novel Allylamine Antifungal Nanoemulsion Gel. J. Nanosci. Curr. Res. 2018, 3, 1–5. [Google Scholar] [CrossRef]





| QTPP | Target | Justification |
|---|---|---|
| Drug delivery system | NS | The system offers augmented skin retentivity in comparison to other nanosystems |
| Dosage type | Controlled release | This will enable amplified drug absorption profile |
| Route of administration | Topical | They offer ease of applicability, non-invasive nature, and on-site delivery |
| Drug release (%) | More than 80% | It is a pre-requisite for optimal therapeutic and pharmacological activity |
| Variables | Levels of Variables | |
|---|---|---|
| Independent variables | Low | High |
| PVA (%) | 1 | 3 |
| EC (%) | 1 | 3 |
| T80 (%) | 1 | 5 |
| Dependent variables | Goals | |
| Particle mean size (nm) | Minimize | |
| PDI | Minimize | |
| EE (%) | Maximize | |
| Formulation Code | PVA Conc (X1) | EC Conc (X2) | T-80 Conc (X3) | Particle Size (nm) (Y1) | PDI (Y2) | EE (Y3) |
|---|---|---|---|---|---|---|
| N1 | 3 | 2 | 1 | 448.4 | 0.3 | 85.45 |
| N2 | 1 | 3 | 3 | 440.7 | 0.425 | 50.5 |
| N3 | 2 | 1 | 5 | 520.4 | 0.47 | 73.6 |
| N4 | 2 | 1 | 1 | 560.8 | 0.509 | 65.9 |
| N5 | 1 | 1 | 3 | 425.7 | 0.42 | 54.72 |
| N6 | 1 | 2 | 1 | 476.89 | 0.411 | 58.2 |
| N7 | 1 | 2 | 5 | 490.1 | 0.48 | 59.6 |
| N8 | 2 | 3 | 5 | 530.25 | 0.48 | 81.4 |
| N9 | 2 | 2 | 3 | 571.4 | 0.482 | 77.2 |
| N10 | 2 | 2 | 3 | 573.7 | 0.501 | 77.9 |
| N11 | 3 | 3 | 3 | 360.9 | 0.41 | 80.1 |
| N12 | 2 | 3 | 1 | 452.62 | 0.32 | 83.77 |
| N13 | 3 | 2 | 5 | 555.2 | 0.564 | 76 |
| N14 | 2 | 2 | 3 | 571.4 | 0.501 | 77.2 |
| N15 | 3 | 1 | 3 | 509.12 | 0.51 | 78.61 |
| N16 | 1 | 2 | 3 | 500.2 | 0.43 | 72.21 |
| N17 | 3 | 1 | 1 | 489.12 | 0.5 | 75.67 |
| Compositions (% w/w) | Formulation Code | |||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |
| Carbopol 940 | 0.2 | 0.5 | 0.8 | 1 | 1.2 | 1.5 |
| TH-loaded NS | 2 | 2 | 2 | 2 | 2 | 2 |
| Ethanol (95% v/v) | 10 | 10 | 10 | 10 | 10 | 10 |
| TEA (2%) | 2 | 2 | 2 | 2 | 2 | 2 |
| Distilled water | q.s | q.s | q.s | q.s | q.s | q.s |
| Formulation Code | Visual Characteristics | Viscociy (Centipoises) (n = 20) ± SD | pH (n = 3) ± SD | Spreadability (g·cm/s) (n = 3) ± SD | Texture Analysis | Drug Content (%) ± SD | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Colour | Homogenity | Grittiness | Hardness | Adhesiveness | Elasticity | Cohesiveness | |||||
| G1 | Whitish | - | - | 0.384 ± 0.0004 | 5.72 ± 0.196 | 2.00 ± 0.04 | 198.10 | −210.233 | 0.372 | 0.563 | 82.6 ± 0.002 |
| G2 | Whitish | - | - | 0.465 ± 0.0003 | 5.76 ± 0.05 | 1.8 ± 0.03 | 222.283 | −228.233 | 0.793 | 0.762 | 83.4 ± 0.002 |
| G3 | Whitish | - | - | 0.789 ± 0.0002 | 5.90 ± 0.005 | 1.8 ± 0.02 | 240.10 | −232.22 | 0.801 | 0.77 | 84.4 ± 0.002 |
| G4 | Whitish | - | - | 1.177 ± 0.0002 | 5.91 ± 0.02 | 1.00 ± 0.02 | 254.999 | −247.275 | 0.822 | 0.8 | 86.5 ± 0.002 |
| G5 | Whitish | Slightly clumpy | - | 1.878 ± 0.0004 | 5.95 ± 0.02 | 0.8 ± 0.05 | 260.002 | −259.087 | 0.822 | 0.801 | 84.1 ± 0.001 |
| G6 | Whitish | clumpy | - | 2.999 ± 0.0003 | 5.99 ± 0.05 | 0.4 ± 0.03 | 286.666 | −272.794 | 0.824 | 0.802 | 84.4 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghose, A.; Nabi, B.; Rehman, S.; Md, S.; Alhakamy, N.A.; Ahmad, O.A.A.; Baboota, S.; Ali, J. Development and Evaluation of Polymeric Nanosponge Hydrogel for Terbinafine Hydrochloride: Statistical Optimization, In Vitro and In Vivo Studies. Polymers 2020, 12, 2903. https://doi.org/10.3390/polym12122903
Ghose A, Nabi B, Rehman S, Md S, Alhakamy NA, Ahmad OAA, Baboota S, Ali J. Development and Evaluation of Polymeric Nanosponge Hydrogel for Terbinafine Hydrochloride: Statistical Optimization, In Vitro and In Vivo Studies. Polymers. 2020; 12(12):2903. https://doi.org/10.3390/polym12122903
Chicago/Turabian StyleGhose, Aditee, Bushra Nabi, Saleha Rehman, Shadab Md, Nabil A. Alhakamy, Osama A. A. Ahmad, Sanjula Baboota, and Javed Ali. 2020. "Development and Evaluation of Polymeric Nanosponge Hydrogel for Terbinafine Hydrochloride: Statistical Optimization, In Vitro and In Vivo Studies" Polymers 12, no. 12: 2903. https://doi.org/10.3390/polym12122903
APA StyleGhose, A., Nabi, B., Rehman, S., Md, S., Alhakamy, N. A., Ahmad, O. A. A., Baboota, S., & Ali, J. (2020). Development and Evaluation of Polymeric Nanosponge Hydrogel for Terbinafine Hydrochloride: Statistical Optimization, In Vitro and In Vivo Studies. Polymers, 12(12), 2903. https://doi.org/10.3390/polym12122903