An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives
Abstract
:1. Introduction
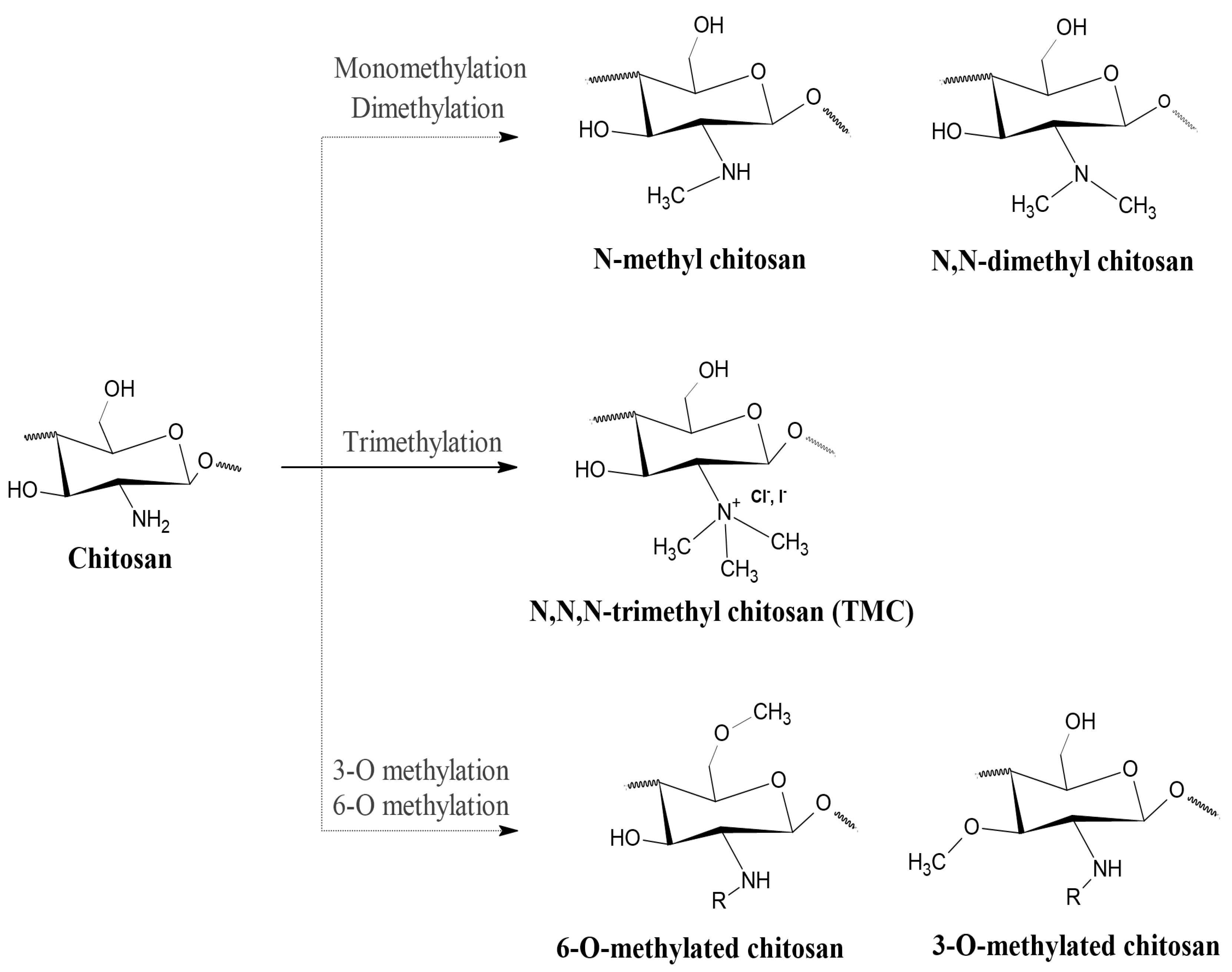
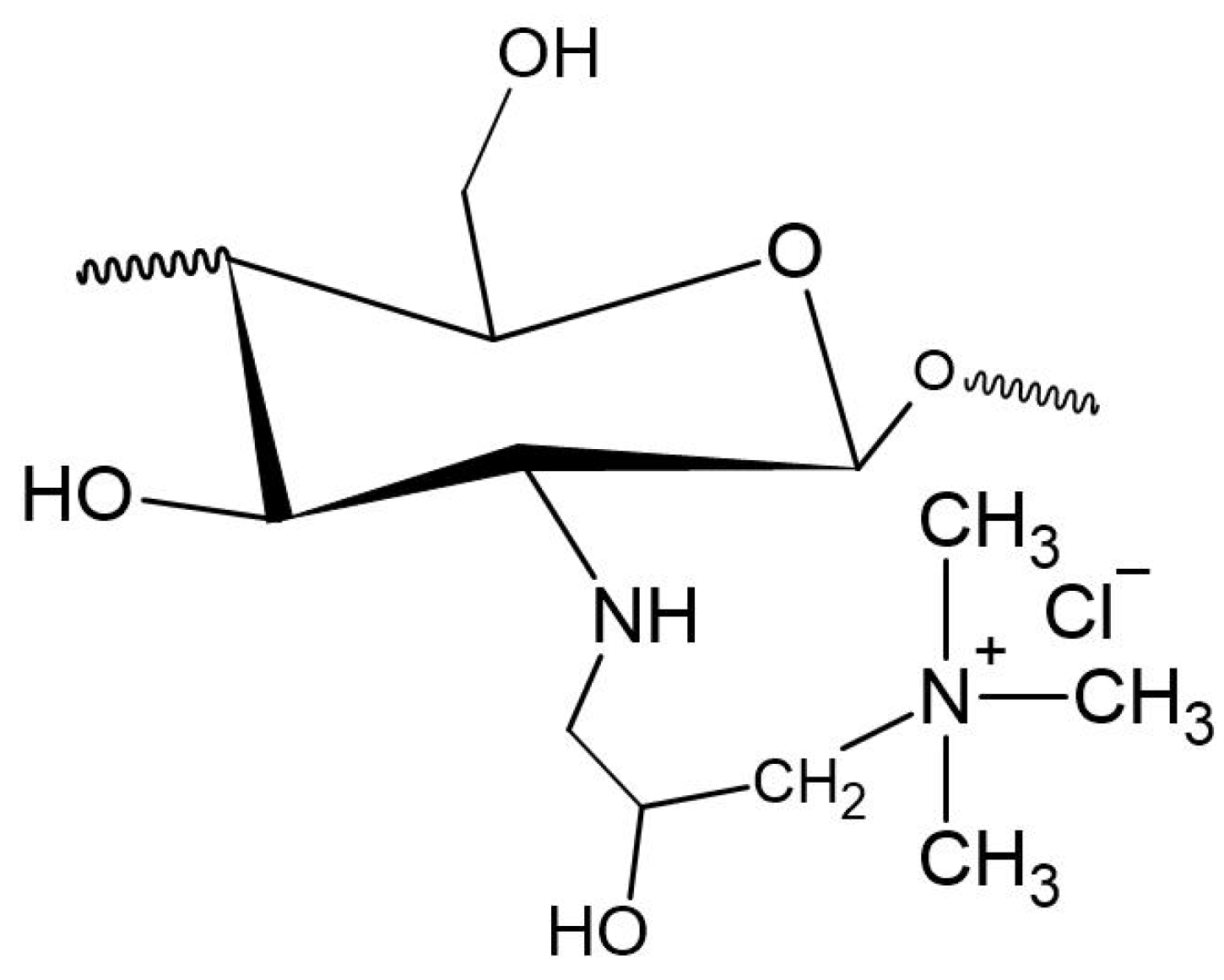
2. TMC
2.1. Structure and Properties of TMC
2.2. Preparation Methods
2.2.1. Conventional Methods
2.2.2. Alternative Methods and Approaches
2.3. Applications of TMC and Its Derivatives
3. HTCC
3.1. Structure and Properties of HTCC
3.2. Preparation Methods
3.3. Applications of HTCC and Similar Compounds
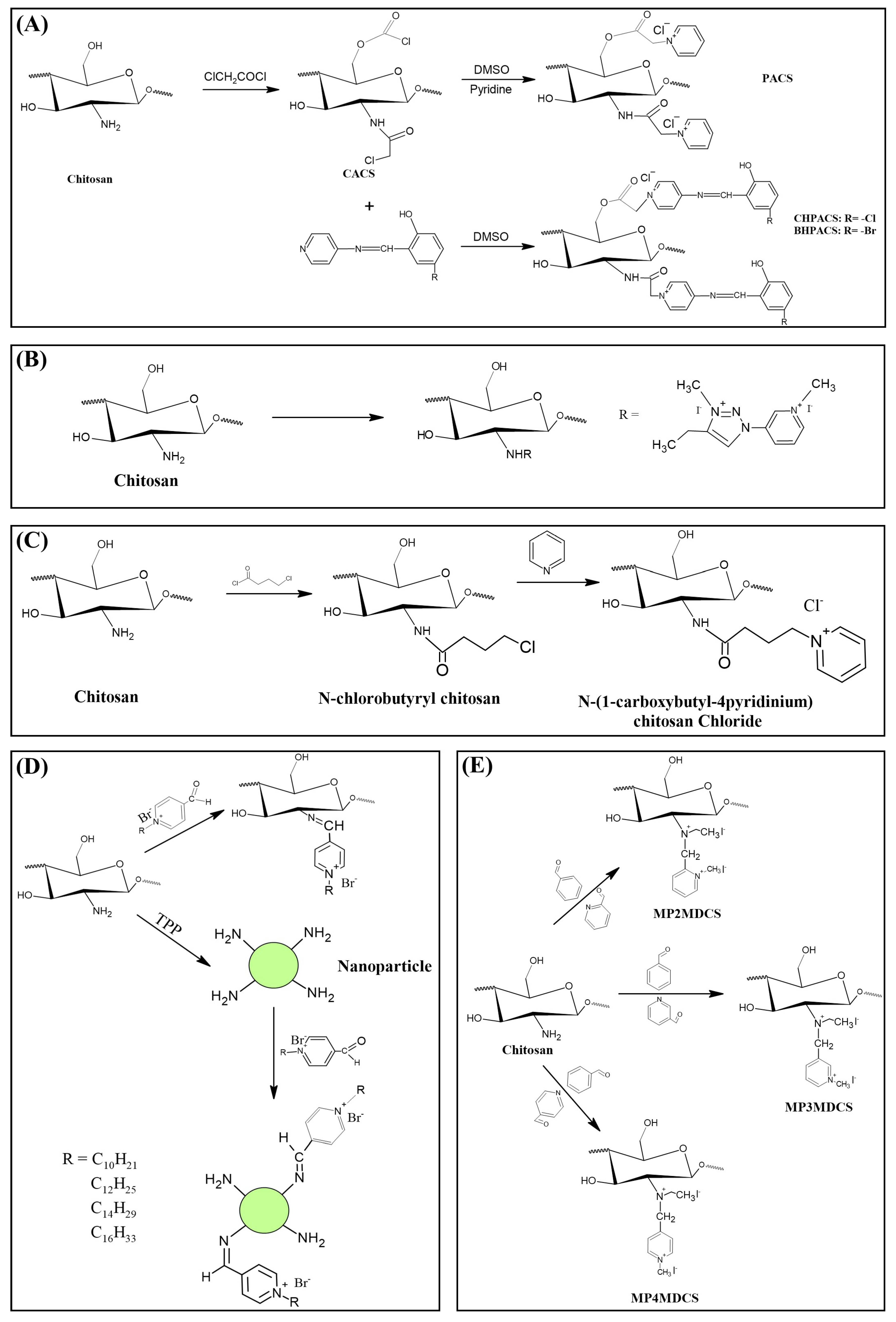
4. Quaternized Chitosan with Pyridinium Salts
5. Quaternized Chitosan with Phosphonium Salts
6. Other Quaternized Chitosan Derivatives
7. Future Prospects
Funding
Acknowledgments
Conflicts of Interest
References
- Muñoz-Bonilla, A.; Cerrada, M.; Fernández-García, M. (Eds.) Polymeric Materials with Antimicrobial Activity; Polymer Chemistry Series; The Royal Society of Chemistry: London, UK, 2014; pp. 1–414. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A.; Deepak; Kumar, R.; Rana, N.K.; Koch, B. Development of a novel chitosan based biocompatible and self-healing hydrogel for controlled release of hydrophilic drug. Int. J. Biol. Macromol. 2018, 116, 37–44. [Google Scholar] [CrossRef]
- Acevedo, C.A.; Olguín, Y.; Briceño, M.; Forero, J.C.; Osses, N.; Díaz-Calderón, P.; Jaques, A.; Ortiz, R. Design of a biodegradable UV-irradiated gelatin-chitosan/nanocomposed membrane with osteogenic ability for application in bone regeneration. Mater. Sci. Eng. C 2019, 99, 875–886. [Google Scholar] [CrossRef]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, P.; Pandey, J. Binary grafted chitosan film: Synthesis, characterization, antibacterial activity and prospects for food packaging. Int. J. Biol. Macromol. 2018, 115, 341–348. [Google Scholar] [CrossRef]
- Kumar, S.; Deepak, V.; Kumari, M.; Dutta, P.K. Antibacterial activity of diisocyanate-modified chitosan for biomedical applications. Int. J. Biol. Macromol. 2016, 84, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dong, P.; Lao, F.; Liu, J.; Liao, X.; Wu, J. Characterization of the major aroma-active compounds in Keitt mango juice: Comparison among fresh, pasteurization and high hydrostatic pressure processing juices. Food Chem. 2019, 289, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, P.; Guo, Z. Cationic chitosan derivatives as potential antifungals: A review of structural optimization and applications. Carbohydr. Polym. 2020, 236, 116002. [Google Scholar] [CrossRef] [PubMed]
- Sajomsang, W.; Gonil, P.; Tantayanon, S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: Preparation and characterization. Int. J. Biol. Macromol. 2009, 44, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ignatova, M.; Manolova, N.; Rashkov, I. Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) prepared by electrospinning. Eur. Polym. J. 2007, 43, 1112–1122. [Google Scholar] [CrossRef]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z.; Tang, T. Quaternized chitosan as an antimicrobial agent: Antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Curti, E.; de Britto, D.; Campana-Filho, S.P. Methylation of Chitosan with Iodomethane: Effect of Reaction Conditions on Chemoselectivity and Degree of Substitution. Macromol. Biosci. 2003, 3, 571–576. [Google Scholar] [CrossRef]
- Liu, B.; Shen, S.; Luo, J.; Wang, X.; Sun, R. One-pot green synthesis and antimicrobial activity of exfoliated Ag NP-loaded quaternized chitosan/clay nanocomposites. RSC Adv. 2013, 3, 9714–9722. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Nevalainen, T.; Hjálmarsdóttir, M.; Järvinen, T.; Loftsson, T.; Einarsson, J.M.; Jónsdóttir, S.; Valdimarsdóttir, M.; Másson, M. Antibacterial activity of methylated chitosan and chitooligomer derivatives: Synthesis and structure activity relationships. Eur. Polym. J. 2007, 43, 2660–2671. [Google Scholar] [CrossRef]
- Hao, J.; Qin, T.; Zhang, Y.; Li, Y.; Zhang, Y. Synthesis, surface properties and antimicrobial performance of novel gemini pyridinium surfactants. Colloids Surf. Biointerfaces 2019, 181, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Fei Liu, X.; Lin Guan, Y.; Zhi Yang, D.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Rathinam, S.; Ólafsdóttir, S.; Jónsdóttir, S.; Hjálmarsdóttir, M.; Másson, M. Selective synthesis of N,N,N-trimethylated chitosan derivatives at different degree of substitution and investigation of structure-activity relationship for activity against P. aeruginosa and MRSA. Int. J. Biol. Macromol. 2020, 160, 548–557. [Google Scholar] [CrossRef]
- Jia, Z.; shen, D.; Xu, W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr. Res. 2001, 333, 1–6. [Google Scholar] [CrossRef]
- Xu, T.; Xin, M.; Li, M.; Huang, H.; Zhou, S.; Liu, J. Synthesis, characterization, and antibacterial activity of N,O-quaternary ammonium chitosan. Carbohydr. Res. 2011, 346, 2445–2450. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef]
- Cho, J.; Grant, J.; Piquette-Miller, M.; Allen, C. Synthesis and physicochemical and dynamic mechanical properties of a water-soluble chitosan derivative as a biomaterial. Biomacromolecules 2006, 7, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Dong, F.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Guo, Z. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr. Polym. 2018, 182, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Duan, Y.; Fang, Q.; Wang, X.; Huang, J. Pyridine-grafted chitosan derivative as an antifungal agent. Food Chem. 2016, 196, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Omidi, S.; Kakanejadifard, A. Modification of chitosan and chitosan nanoparticle by long chain pyridinium compounds: Synthesis, characterization, antibacterial, and antioxidant activities. Carbohydr. Polym. 2019, 208, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, X.; Guo, S.; Peng, Z.; Tang, T. Novel water soluble phosphonium chitosan derivatives: Synthesis, characterization and cytotoxicity studies. Int. J. Biol. Macromol. 2011, 48, 375–380. [Google Scholar] [CrossRef]
- Zeng, K.; Lin, F.X.; Xie, J.; Wang, M.Z.; Rong, J.L.; Zhao, Y.; You, Y.Z.; Asif, A.; Ge, X.W. Chitosan modified by γ-ray-induced grafting of poly(tributyl-(4-vinylbenzyl)phosphonium) as a biosafe and high-efficiency gene carrier. New J. Chem. 2017, 41, 4182–4189. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.H.; Liu, C.D.; Wang, Y.; Fu, Y.N.; Wang, D.; Sun, H.; Peng, Y.; Jiang, M.; Pu, D.J. The effect of amphiphilic N,N,N-trimethyl-O-octadecyl chitosan on the oral bioavailability of acyclovir. J. Drug Deliv. Sci. Technol. 2019, 51, 244–254. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, H.M.; Yoon, J.H. Synthesis of a Quaternary Ammonium Derivative of Chitosan and Its Application to a Cotton Antimicrobial Finish. Text. Res. J. 1998, 68, 428–434. [Google Scholar] [CrossRef]
- Zhu, Y.; Pei, H.; Hu, W.; Jin, Y.; Xu, H.; Ren, Y.; Xue, D. Effect of chitosan quaternary ammonium salt on the growth and microcystins release of: Microcystis aeruginosa. RSC Adv. 2016, 6, 81028–81036. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Ruktanonchai, U.R.; Petchsangsai, M.; Opanasopit, P.; Puttipipatkhachorn, S. Effect of N-pyridinium positions of quaternized chitosan on transfection efficiency in gene delivery system. Carbohydr. Polym. 2014, 104, 17–22. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Dong, F.; Li, Q.; Guo, Z. Design, synthesis of novel chitosan derivatives bearing quaternary phosphonium salts and evaluation of antifungal activity. Int. J. Biol. Macromol. 2017, 102, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Lang, G.; Wendel, H.; Konrad, E. Process for Making Quaternary Chitosan Derivatives for Cosmetic Agents. U.S. Patent 4,921,949, 1 May 1990. [Google Scholar]
- Mi, F.L.; Shyu, S.S.; Chen, C.T.; Lai, J.Y. Adsorption of indomethacin onto chemically modified chitosan beads. Polymer 2001, 43, 757–765. [Google Scholar] [CrossRef]
- Kamiński, K.; Szczubiałka, K.; Zazakowny, K.; Lach, R.; Nowakowska, M. Chitosan derivatives as novel potential heparin reversal agents. J. Med. Chem. 2010, 53, 4141–4147. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Zhang, W.; Xu, J.; Xue, W.; Liu, Z. Evaluation of N-phosphonium chitosan as a novel vaccine carrier for intramuscular immunization. J. Biomater. Appl. 2017, 32, 677–685. [Google Scholar] [CrossRef]
- Hecq, J.; Siepmann, F.; Siepmann, J.; Amighi, K.; Goole, J. Development and evaluation of chitosan and chitosan derivative nanoparticles containing insulin for oral administration. Drug Dev. Ind. Pharm. 2015, 41, 2037–2044. [Google Scholar] [CrossRef]
- de Britto, D.; Assis, O.B.G. Aspectos químicos, bioquímicos e microbiológicos de sais quaternários de quitosana para revestimento ativo de maçãs fatiadas. Cienc. Tecnol. Aliment. 2012, 32, 599–605. [Google Scholar] [CrossRef] [Green Version]
- Qian, C.; Xu, X.; Shen, Y.; Li, Y.; Guo, S. Synthesis and preliminary cellular evaluation of phosphonium chitosan derivatives as novel non-viral vector. Carbohydr. Polym. 2013, 97, 676–683. [Google Scholar] [CrossRef]
- Wei, L.; Li, Q.; Tan, W.; Dong, F.; Luan, F.; Guo, Z.; Saso, L.; Dux, L.; Wegrzyn, G.; Csont, T. Synthesis, characterization, and the antioxidant activity of double quaternized chitosan derivatives. Molecules 2017, 22, 501. [Google Scholar] [CrossRef] [Green Version]
- Sessarego, S.; Rodrigues, S.C.; Xiao, Y.; Lu, Q.; Hill, J.M. Phosphonium-enhanced chitosan for Cr(VI) adsorption in wastewater treatment. Carbohydr. Polym. 2019, 211, 249–256. [Google Scholar] [CrossRef]
- Mohammad, F.; Arfin, T.; Al-Lohedan, H.A. Enhanced biological activity and biosorption performance of trimethyl chitosan-loaded cerium oxide particles. J. Ind. Eng. Chem. 2017, 45, 33–43. [Google Scholar] [CrossRef]
- Wu, D.; Zhu, L.; Li, Y.; Zhang, X.; Xu, S.; Yang, G.; Delair, T. Chitosan-based Colloidal Polyelectrolyte Complexes for Drug Delivery: A Review. Carbohydr. Polym. 2020, 238, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Terayama, H.; Terayama, E. High molecular antibacterial substances derived from chitin. About the manufacture of Macramin. J. Antibiot. 1948, 2, 44–45. [Google Scholar]
- Hatta, S.; Kuwabara, S.; Miyamoto, H.; Aoyama, K.; Utsunomiya, N.; Tanji, S. Studies on macramin, a new high-molecular antibacterial substance derived from chitin. Jpn. Med J. 1950, 3, 119–123. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Tanfani, F. The N-permethylation of chitosan and the preparation of N-trimethyl chitosan iodide. Carbohydr. Polym. 1985, 5, 297–307. [Google Scholar] [CrossRef]
- Domard, A.; Rinaudo, M.; Terrassin, C. New method for the quaternization of chitosan. Int. J. Biol. Macromol. 1986, 8, 105–107. [Google Scholar] [CrossRef]
- le Dung, P.; Milas, M.; Rinaudo, M.; Desbrières, J. Water soluble derivatives obtained by controlled chemical modifications of chitosan. Carbohydr. Polym. 1994, 24, 209–214. [Google Scholar] [CrossRef]
- Polnok, A.; Borchard, G.; Verhoef, J.C.; Sarisuta, N.; Junginger, H.E. Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride. Eur. J. Pharm. Biopharm. 2004, 57, 77–83. [Google Scholar] [CrossRef]
- Verheul, R.J.; Amidi, M.; van der Wal, S.; van Riet, E.; Jiskoot, W.; Hennink, W.E. Synthesis, characterization and in vitro biological properties of O-methyl free N,N,N-trimethylated chitosan. Biomaterials 2008, 29, 3642–3649. [Google Scholar] [CrossRef]
- Sieval, A.B.; Thanou, M.; Kotzé, A.F.; Verhoef, J.C.; Brussee, J.; Junginger, H.E. Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohydr. Polym. 1998, 36, 157–165. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. Controlled synthesis of N,N,N-trimethyl chitosan for modulated bioadhesion and nasal membrane permeability. Int. J. Biol. Macromol. 2016, 82, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Rossi, S.; Bonferoni, M.C.; Ferrari, F.; Zambito, Y.; Di Colo, G.; Caramella, C. Buccal penetration enhancement properties of N-trimethyl chitosan: Influence of quaternization degree on absorption of a high molecular weight molecule. Int. J. Pharm. 2005, 297, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Snyman, D.; Hamman, J.H.; Kotze, A.F. Evaluation of the mucoadhesive properties of N-trimethyl chitosan chloride. Drug Dev. Ind. Pharm. 2003, 29, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jintapattanakit, A.; Mao, S.; Kissel, T.; Junyaprasert, V.B. Physicochemical properties and biocompatibility of N-trimethyl chitosan: Effect of quaternization and dimethylation. Eur. J. Pharm. Biopharm. 2008, 70, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. 2001, 14, 201–207. [Google Scholar] [CrossRef]
- Thanou, M.M.; Kotzé, A.F.; Scharringhausen, T.; Lueßen, H.L.; De Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Florea, B.I.; Thanou, M.; Junginger, H.E.; Borchard, G. Enhancement of bronchial octreotide absorption by chitosan and N-trimethyl chitosan shows linear in vitro/in vivo correlation. J. Control. Release 2006, 110, 353–361. [Google Scholar] [CrossRef]
- de Britto, D.; Assis, O.B. A novel method for obtaining a quaternary salt of chitosan. Carbohydr. Polym. 2007, 69, 305–310. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial Action of Chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Vidar Rúnarsson, Ö.; Holappa, J.; Malainer, C.; Steinsson, H.; Hjálmarsdóttir, M.; Nevalainen, T.; Másson, M. Antibacterial activity of N-quaternary chitosan derivatives: Synthesis, characterization and structure activity relationship (SAR) investigations. Eur. Polym. J. 2010, 46, 1251–1267. [Google Scholar] [CrossRef]
- Sajomsang, W.; Ruktanonchai, U.R.; Gonil, P.; Warin, C. Quaternization of N-(3-pyridylmethyl) chitosan derivatives: Effects of the degree of quaternization, molecular weight and ratio of N-methylpyridinium and N,N,N-trimethyl ammonium moieties on bactericidal activity. Carbohydr. Polym. 2010, 82, 1143–1152. [Google Scholar] [CrossRef]
- Geng, X.; Yang, R.; Huang, J.; Zhang, X.; Wang, X. Evaluation Antibacterial Activity of Quaternary-Based Chitin/Chitosan Derivatives In Vitro. J. Food Sci. 2013, 78, M90–M97. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Choi, J.W.; Chun, H.J.; Choi, K.S. Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity. Polym. Bull. 1997, 38, 387–393. [Google Scholar] [CrossRef]
- Belalia, R.; Grelier, S.; Benaissa, M.; Coma, V. New Bioactive Biomaterials Based on Quaternized Chitosan. J. Agric. Food Chem. 2008, 56, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.M.; Dorkoosh, F.A.; Avadi, M.R.; Saadat, P.; Rafiee-Tehrani, M.; Junginger, H.E. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 2008, 355, 299–306. [Google Scholar] [CrossRef] [PubMed]
- De Britto, D.; Campana-Filho, S.P. A kinetic study on the thermal degradation of N,N,N-trimethylchitosan. Polym. Degrad. Stab. 2004, 84, 353–361. [Google Scholar] [CrossRef]
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Recent research progress on preparation and application of N,N,N-trimethyl chitosan. Carbohydr. Res. 2016, 434, 27–32. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Ma, L.; Yan, J.; Yan, M.; Gao, C.; Shen, J. The gene transfection efficiency of thermoresponsive N,N,N-trimethyl chitosan chloride-g-poly(N-isopropylacrylamide) copolymer. Biomaterials 2007, 28, 4488–4500. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, W.; Wang, G.; Yin, X.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of N,N,N-trimethyl chitosan salts. Int. J. Biol. Macromol. 2018, 118, 9–14. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Malainer, C.; Holappa, J.; Sigurdsson, S.T.; Másson, M. tert-Butyldimethylsilyl O-protected chitosan and chitooligosaccharides: Useful precursors for N-modifications in common organic solvents. Carbohydr. Res. 2008, 343, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Benediktsdóttir, B.E.; Gaware, V.S.; Rúnarsson, Ö.V.; Jónsdóttir, S.; Jensen, K.J.; Másson, M. Synthesis of N,N,N-trimethyl chitosan homopolymer and highly substituted N-alkyl-N,N-dimethyl chitosan derivatives with the aid of di-tert- butyldimethylsilyl chitosan. Carbohydr. Polym. 2011, 86, 1451–1460. [Google Scholar] [CrossRef]
- Wu, M.; Long, Z.; Xiao, H.; Dong, C. Preparation of N, N, N-trimethyl chitosan via a novel approach using dimethyl carbonate. Carbohydr. Polym. 2017, 169, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, T.; Bangde, P.; Dandekar, P.; Jain, R. Greener approach for synthesis of N,N,N-trimethyl chitosan (TMC) using ternary deep eutectic solvents (TDESs). Carbohydr. Res. 2020, 493, 108033. [Google Scholar] [CrossRef] [PubMed]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lu, C.; Yang, S.; Zhang, J. Bioconjugation of aptamer to fluorescent trimethyl chitosan nanoparticles for bacterial detection. Mater. Lett. 2020, 264, 127330. [Google Scholar] [CrossRef]
- Sahni, J.K.; Chopra, S.; Ahmad, F.J.; Khar, R.K. Potential prospects of chitosan derivative trimethyl chitosan chloride (TMC) as a polymeric absorption enhancer: Synthesis, characterization and applications. J. Pharm. Pharmacol. 2008, 60, 1111–1119. [Google Scholar] [CrossRef]
- Kotzé, A.F.; De Leeuw, B.J.; Lueßen, H.L.; De Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Chitosans for enhanced delivery of therapeutic peptides across intestinal epithelia: In vitro evaluation in Caco-2 cell monolayers. Int. J. Pharm. 1997, 159, 243–253. [Google Scholar] [CrossRef]
- He, W.; Guo, X.; Zhang, M. Transdermal permeation enhancement of N-trimethyl chitosan for testosterone. Int. J. Pharm. 2008, 356, 82–87. [Google Scholar] [CrossRef]
- Jonker, C.; Hamman, J.H.; Kotzé, A.F. Intestinal paracellular permeation enhancement with quaternised chitosan: In situ and in vitro evaluation. Int. J. Pharm. 2002, 238, 205–213. [Google Scholar] [CrossRef]
- Hamman, J.H.; Stander, M.; Kotzé, A.F. Effect of the degree of quaternisation of N-trimethyl chitosan chloride on absorption enhancement: In vivo evaluation in rat nasal epithelia. Int. J. Pharm. 2002, 232, 235–242. [Google Scholar] [CrossRef]
- Di Colo, G.; Burgalassi, S.; Zambito, Y.; Monti, D.; Chetoni, P. Effects of different N-trimethyl chitosans on in vitro/in vivo ofloxacin transcorneal permeation. J. Pharm. Sci. 2004, 93, 2851–2862. [Google Scholar] [CrossRef] [PubMed]
- Benediktsdóttir, B.E.; Gudjónsson, T.; Baldursson, Ó.; Másson, M. N-alkylation of highly quaternized chitosan derivatives affects the paracellular permeation enhancement in bronchial epithelia in vitro. Eur. J. Pharm. Biopharm. 2014, 86, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Zhu, X.; Shan, W.; Li, L.; Zhong, J.; Zhang, Z.; Huang, Y. Efficient mucus permeation and tight junction opening by dissociable “mucus-inert” agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J. Control. Release 2016, 222, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.C.; Chen, C.H.; Lin, C.W.; Ho, Y.C.; Mi, F.L. Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int. J. Biol. Macromol. 2019, 126, 141–150. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Improved oral delivery of resveratrol from N-trimethyl chitosan-g-palmitic acid surface-modified solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2016, 139, 52–61. [Google Scholar] [CrossRef]
- Du, Q.; Chen, J.; Yan, G.; Lyu, F.; Huang, J.; Ren, J.; Di, L. Comparison of different aliphatic acid grafted N-trimethyl chitosan surface-modified nanostructured lipid carriers for improved oral kaempferol delivery. Int. J. Pharm. 2019, 568, 118506. [Google Scholar] [CrossRef]
- He, R.; Yin, C. Trimethyl chitosan based conjugates for oral and intravenous delivery of paclitaxel. Acta Biomater. 2017, 53, 355–366. [Google Scholar] [CrossRef]
- Chen, G.; Svirskis, D.; Lu, W.; Ying, M.; Huang, Y.; Wen, J. N-trimethyl chitosan nanoparticles and CSKSSDYQC peptide: N-trimethyl chitosan conjugates enhance the oral bioavailability of gemcitabine to treat breast cancer. J. Control. Release 2018, 277, 142–153. [Google Scholar] [CrossRef]
- Kontogiannidou, E.; Meikopoulos, T.; Virgiliou, C.; Bouropoulos, N.; Gika, H.; Vizirianakis, I.S.; Müllertz, A.; Fatouros, D.G. Towards the development of Self-Nano-Emulsifying Drug Delivery Systems (SNEDDS) containing trimethyl chitosan for the oral delivery of amphotericin B: In vitro assessment and cytocompatibility studies. J. Drug Deliv. Sci. Technol. 2020, 56, 101524. [Google Scholar] [CrossRef]
- Pardeshi, C.V.; Belgamwar, V.S. N,N,N‑trimethyl chitosan modified flaxseed oil based mucoadhesive neuronanoemulsions for direct nose to brain drug delivery. Int. J. Biol. Macromol. 2018, 120, 2560–2571. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Agnihotri, V.V.; Patil, K.Y.; Pardeshi, S.R.; Surana, S.J. Mannose-anchored N,N,N-trimethyl chitosan nanoparticles for pulmonary administration of etofylline. Int. J. Biol. Macromol. 2020, 165, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, X.; Yang, Y.; Zhang, L.; Liu, R.; Li, Z. Trimethyl chitosan nanoparticles for ocular baicalein delivery: Preparation, optimization, in vitro evaluation, in vivo pharmacokinetic study and molecular dynamics simulation. Int. J. Biol. Macromol. 2020, 156, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Hakimi, S.; Esmaeily, A.; Samadi, F.Y.; Mortazavian, E.; Nazari, M.; Mohammadi, Z.; Tehrani, N.R.; Tehrani, M.R. Novel chitosan based nanoparticles as gene delivery systems to cancerous and noncancerous cells. Int. J. Pharm. 2019, 560, 306–314. [Google Scholar] [CrossRef]
- Baghaei, M.; Tekie, F.S.M.; Khoshayand, M.R.; Varshochian, R.; Hajiramezanali, M.; Kachousangi, M.J.; Dinarvand, R.; Atyabi, F. Optimization of chitosan-based polyelectrolyte nanoparticles for gene delivery, using design of experiment: in vitro and in vivo study. Mater. Sci. Eng. C 2021, 118, 111036. [Google Scholar] [CrossRef]
- Slütter, B.; Plapied, L.; Fievez, V.; Alonso Sande, M.; des Rieux, A.; Schneider, Y.J.; Van Riet, E.; Jiskoot, W.; Préat, V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release 2009, 138, 113–121. [Google Scholar] [CrossRef]
- Hagenaars, N.; Verheul, R.J.; Mooren, I.; de Jong, P.H.; Mastrobattista, E.; Glansbeek, H.L.; Heldens, J.G.; van den Bosch, H.; Hennink, W.E.; Jiskoot, W. Relationship between structure and adjuvanticity of N,N,N-trimethyl chitosan (TMC) structural variants in a nasal influenza vaccine. J. Control. Release 2009, 140, 126–133. [Google Scholar] [CrossRef]
- Verheul, R.J.; Hagenaars, N.; Van Es, T.; Van Gaal, E.V.; De Jong, P.H.; Bruijns, S.; Mastrobattista, E.; Slütter, B.; Que, I.; Heldens, J.G.; et al. A step-by-step approach to study the influence of N-acetylation on the adjuvanticity of N,N,N-trimethyl chitosan (TMC) in an intranasal nanoparticulate influenza virus vaccine. Eur. J. Pharm. Sci. 2012, 45, 467–474. [Google Scholar] [CrossRef]
- Sayin, B.; Somavarapu, S.; Li, X.W.; Sesardic, D.; Şenel, S.; Alpar, O.H. TMC-MCC (N-trimethyl chitosan-mono-N-carboxymethyl chitosan) nanocomplexes for mucosal delivery of vaccines. Eur. J. Pharm. Sci. 2009, 38, 362–369. [Google Scholar] [CrossRef]
- Cevher, E.; Salomon, S.K.; Somavarapu, S.; Brocchini, S.; Alpar, H.O. Development of chitosan–pullulan composite nanoparticles for nasal delivery of vaccines: in vivo studies. J. Microencapsul. 2015, 32, 769–783. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Khalil, Z.G.; Hussein, W.M.; Powell, J.; Batzloff, M.R.; Capon, R.J.; Good, M.F.; Skwarczynski, M.; Toth, I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018, 80, 278–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabaghian, M.; Latifi, A.M.; Tebianian, M.; NajmiNejad, H.; Ebrahimi, S.M. Nasal vaccination with r4M2e.HSP70c antigen encapsulated into N-trimethyl chitosan (TMC) nanoparticulate systems: Preparation and immunogenicity in a mouse model. Vaccine 2018, 36, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.M.; Slütter, B.; Verheul, R.; Bouwstra, J.A.; Jiskoot, W. Adjuvanted, antigen loaded N-trimethyl chitosan nanoparticles for nasal and intradermal vaccination: Adjuvant- and site-dependent immunogenicity in mice. Eur. J. Pharm. Sci. 2012, 45, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Schipper, P.; van der Maaden, K.; Groeneveld, V.; Ruigrok, M.; Romeijn, S.; Uleman, S.; Oomens, C.; Kersten, G.; Jiskoot, W.; Bouwstra, J. Diphtheria toxoid and N-trimethyl chitosan layer-by-layer coated pH-sensitive microneedles induce potent immune responses upon dermal vaccination in mice. J. Control. Release 2017, 262, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Abkar, M.; Fasihi-Ramandi, M.; Kooshki, H.; Lotfi, A.S. Intraperitoneal immunization with Urease loaded N-trimethyl Chitosan nanoparticles elicits high protection against Brucella melitensis and Brucella abortus infections. Immunol. Lett. 2018, 199, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yan, D.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Biomaterials based on N,N,N-trimethyl chitosan fibers in wound dressing applications. Int. J. Biol. Macromol. 2016, 89, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Patrulea, V.; Laurent-Applegate, L.A.; Ostafe, V.; Borchard, G.; Jordan, O. Polyelectrolyte nanocomplexes based on chitosan derivatives for wound healing application. Eur. J. Pharm. Biopharm. 2019, 140, 100–108. [Google Scholar] [CrossRef]
- Romero, R.; Chubb, L.; Travers, J.K.; Gonzales, T.R.; Ehrhart, N.P.; Kipper, M.J. Coating cortical bone allografts with periosteum-mimetic scaffolds made of chitosan, trimethyl chitosan, and heparin. Carbohydr. Polym. 2015, 122, 144–151. [Google Scholar] [CrossRef]
- Tabriz, A.; Ur Rehman Alvi, M.A.; Khan Niazi, M.B.; Batool, M.; Bhatti, M.F.; Khan, A.L.A.U.; Khan, A.L.A.U.; Jamil, T.; Ahmad, N.M. Quaternized trimethyl functionalized chitosan based antifungal membranes for drinking water treatment. Carbohydr. Polym. 2019, 207, 17–25. [Google Scholar] [CrossRef]
- Bigogno, R.G.; Rodríguez, R.J.S.; Abreu, M.d.F. Quaternized Chitosan for Ecological Treatment of Bauxite Mining Effluents. J. Polym. Environ. 2018, 26, 4169–4175. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; ElHafeez, E.A.; Goda, E.S.; Lee, S.; Yoon, K.R. Smart bactericidal filter containing biodegradable polymers for crystal violet dye adsorption. Cellulose 2019, 26, 9179–9206. [Google Scholar] [CrossRef]
- Lang, G.; Konrad, E.; Wendel, H. Chitosan derivatives: water-soluble products by reaction with epoxides. In Proceedings of the Third International Conference on Chitin and Chitosan; Muzzarelli, R., Jeuniaux, C., Gooday, G.W., Eds.; Plenum Press: Senigallia, Italy, 1986; pp. 303–306. [Google Scholar]
- Loubaki, E.; Ourevitch, M.; Sicsic, S.; Dunant, R.H. Chemical Modification of Chitosan By Glycidyl Trimethylammonium chloride. Eur. Polym. J. 1991, 27, 311–317. [Google Scholar] [CrossRef]
- Stefan, J.; Lorkowska-Zawicka, B.; Kaminski, K.; Szczubialka, K.; Nowakowska, M.; Korbut, R. The current view on biological potency of cationically modified chitosan. J. Physiol. Pharmacol. 2014, 65, 341–347. [Google Scholar] [PubMed]
- Seong, H.S.; Whang, H.S.; Ko, S.W. Synthesis of a Quaternary Ammonium Derivative of Chito-oligosaccharide as Antimicrobial Agent for Cellulosic Fibers. J. Appl. Polym. Sci. 2000, 76, 2009–2015. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, C.; Qiao, C.; Mu, X.; Li, T.; Xu, J.; Shi, L.; Zhang, D. A simple and convenient method to synthesize N-[(2-hydroxyl)-propyl-3-trimethylammonium] chitosan chloride in an ionic liquid. Carbohydr. Polym. 2015, 130, 325–332. [Google Scholar] [CrossRef]
- Jiang, T.; James, R.; Kumbar, S.G.; Laurencin, C.T. Chitosan as a Biomaterial: Structure, Properties, and Applications in Tissue Engineering and Drug Delivery, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 91–113. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Li, Q.; Shen, Y.; Ge, Z.; Zhang, W.; Chen, S. Enhanced water-solubility, antibacterial activity and biocompatibility upon introducing sulfobetaine and quaternary ammonium to chitosan. Carbohydr. Polym. 2016, 143, 246–253. [Google Scholar] [CrossRef]
- Shagdarova, B.; Lunkov, A.; Il’ina, A.; Varlamov, V. Investigation of the properties of N-[(2-hydroxy-3-trimethylammonium) propyl] chloride chitosan derivatives. Int. J. Biol. Macromol. 2019, 124, 994–1001. [Google Scholar] [CrossRef]
- Wang, L.; Qin, C.; Wang, W.; Li, W. Effect of orally administered N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan on the levels of iron, zinc, copper, calcium and lead in mice. Carbohydr. Polym. 2011, 84, 1289–1292. [Google Scholar] [CrossRef]
- Xiao, B.; Wan, Y.; Wang, X.; Zha, Q.; Liu, H.; Qiu, Z.; Zhang, S. Synthesis and characterization of N-(2-hydroxy)propyl-3-trimethyl ammonium chitosan chloride for potential application in gene delivery. Colloids Surf. B Biointerfaces 2012, 91, 168–174. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, Z.H.; Xie, H.H.; Gong, J.Y.; Lou, J.; Ge, Q.; Wang, Y.J.; Wu, Y.F.; Liu, S.W.; Sun, P.L.; et al. Effect of quaternization degree on physiochemical and biological activities of chitosan from squid pens. Int. J. Biol. Macromol. 2014, 70, 545–550. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Liu, X.; Mao, J.; Gu, S.; Xu, W. Potential of quaternization-functionalized chitosan fiber for wound dressing. Int. J. Biol. Macromol. 2013, 52, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, X.; Qiao, C.; Li, Y.; Li, T.; Xu, C. Effects of chitosan quaternary ammonium salt on the physicochemical properties of sodium carboxymethyl cellulose-based films. Carbohydr. Polym. 2018, 184, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Fan, Q.Z.; Liu, Y.; Yue, H.; Ma, X.W.; Wu, J.; Ma, G.H.; Su, Z.G. Improving adjuvanticity of quaternized chitosan–based microgels for H5N1 split vaccine by tailoring the particle properties to achieve antigen dose sparing effect. Int. J. Pharm. 2016, 515, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Nam, C.W.; Choi, J.W.; Jang, J. Durable antimicrobial treatment of cotton fabrics using N-(2-hydroxy)propyl-3-trimethylammonium chitosan chloride and polycarboxylic acids. J. Appl. Polym. Sci. 2003, 88, 1567–1572. [Google Scholar] [CrossRef]
- Chi, W.; Qin, C.; Zeng, L.; Li, W.; Wang, W. Microbiocidal activity of chitosan-N-2-hydroxypropyl trimethyl ammonium chloride. J. Appl. Polym. Sci. 2007, 103, 3851–3856. [Google Scholar] [CrossRef]
- Qin, C.; Xiao, Q.; Li, H.; Fang, M.; Liu, Y.; Chen, X.; Li, Q. Calorimetric studies of the action of chitosan-N-2-hydroxypropyl trimethyl ammonium chloride on the growth of microorganisms. Int. J. Biol. Macromol. 2004, 34, 121–126. [Google Scholar] [CrossRef]
- Hoque, J.; Adhikary, U.; Yadav, V.; Samaddar, S.; Konai, M.M.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Sanyal, K.; Haldar, J. Chitosan Derivatives Active against Multidrug-Resistant Bacteria and Pathogenic Fungi: In Vivo Evaluation as Topical Antimicrobials. Mol. Pharm. 2016, 13, 3578–3589. [Google Scholar] [CrossRef]
- Milewska, A.; Ciejka, J.; Kaminski, K.; Karewicz, A.; Bielska, D.; Zeglen, S.; Karolak, W.; Nowakowska, M.; Potempa, J.; Bosch, B.J.; et al. Novel polymeric inhibitors of HCoV-NL63. Antivir. Res. 2013, 97, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Milewska, A.; Kaminski, K.; Ciejka, J.; Kosowicz, K.; Zeglen, S.; Wojarski, J.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. HTCC: Broad range inhibitor of coronavirus entry. PLoS ONE 2016, 11, e0156552. [Google Scholar] [CrossRef] [Green Version]
- Milewska, A.; Chi, Y.; Szczepanski, A.; Barreto-Duran, E.; Liu, K.; Liu, D.; Guo, X.; Ge, Y.; Li, J.; Cui, L.; et al. HTCC as a highly effective polymeric inhibitor of SARS-CoV-2 and MERS-CoV. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.J.; Kim, Y.H. Synthesis and thermo-responsive properties of chitosan-g-poly (N-isopropylacrylamide) and HTCC-g-poly(N-isopropylacrylamide) copolymers. Fibers Polym. 2010, 11, 164–169. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Xiao, B.; Zan, X.; Gao, J.; Wan, Y. N-(2-hydroxypropyl)-3-trimethylammonium chitosan-poly(ϵ-caprolactone) copolymers and their antibacterial activity. Carbohydr. Polym. 2011, 83, 824–830. [Google Scholar] [CrossRef]
- Mivehi, L.; Bahrami, S.H.; Malek, R.M.A. Properties of Polyacrylonitrile-N-(2-hydroxy) propyl-3-trimethylammonium Chitosan Chloride Blend Films and Fibers. J. Appl. Polym. Sci. 2008, 109, 545–554. [Google Scholar] [CrossRef]
- Li, S.D.; Li, P.W.; Yang, Z.M.; Peng, Z.; Quan, W.Y.; Yang, X.H.; Yang, L.; Dong, J.J. Synthesis and characterization of chitosan quaternary ammonium salt and its application as drug carrier for ribavirin. Drug Deliv. 2014, 21, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Spinelli, V.A.; Laranjeira, M.C.; Fávere, V.T. Preparation and characterization of quaternary chitosan salt: Adsorption equilibrium of chromium(VI) ion. React. Funct. Polym. 2004, 61, 347–352. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Lu, S.; Huang, F.; Li, G. Preparation of quaternary ammonium salt of chitosan nanoparticles and their textile properties on Antheraea pernyi silk modification. Text. Res. J. 2014, 84, 2115–2124. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, S.; Hou, L.; Wei, W.; Zhou, M.; Su, Z.; Wu, J.; Chen, W.; Ma, G. Novel thermal-sensitive hydrogel enhances both humoral and cell-mediated immune responses by intranasal vaccine delivery. Eur. J. Pharm. Biopharm. 2012, 81, 486–497. [Google Scholar] [CrossRef]
- Nam, C.W.; Kim, Y.H.; Ko, S.W. Modification of polyacrylonitrile (PAN) fiber by blending with N-(2-hydroxy)propyl-3-trimethyl-ammonium chitosan chloride. J. Appl. Polym. Sci. 1999, 74, 2258–2265. [Google Scholar] [CrossRef]
- Ruihua, H.; Bingchao, Y.; Zheng, D.; Wang, B. Preparation and characterization of a quaternized chitosan. J. Mater. Sci. 2012, 47, 845–851. [Google Scholar] [CrossRef]
- Song, H.; Wu, H.; Li, S.J.; Tian, H.; Li, Y.R.; Wang, J.G. Homogeneous synthesis of cationic chitosan via new avenue. Molecules 2018, 23, 1921. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Duan, Y.; Huang, J.; Zheng, Q. Synthesis, antioxidant and cathepsin D inhibition activity of quaternary ammonium chitosan derivatives. Carbohydr. Polym. 2016, 136, 884–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Geng, X.; Jiang, H.; Li, J.; Huang, J. Synthesis and characteristics of chitin and chitosan with the (2-hydroxy-3-trimethylammonium)propyl functionality, and evaluation of their antioxidant activity in vitro. Carbohydr. Polym. 2012, 89, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Zheng, H.Q.; Fu, T.; He, Z.J.; Hong, Y. N-(2-hydroxy) propyl-3-trimethylammonium chitosan chloride: An immune-enhancing adjuvant for hepatitis E virus recombinant polypeptide vaccine in mice. Hum. Vaccines Immunother. 2017, 13, 1818–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.A.; Singh, R.P. Synthesis and characterization of a modified chitosan. Macromol. Symp. 2009, 277, 1–7. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, H.; Xie, T.; Chen, L.; Hu, S.; Tian, H.; Wang, Y.; Wang, J. Characterization and antibacterial effect of quaternized chitosan anchored cellulose beads. Int. J. Biol. Macromol. 2020, 155, 1325–1332. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, A. Preparation of Nanoparticles Composed of Chitosan and Its Derivatives as Delivery Systems for Macromolecules Yan. J. Appl. Polym. Sci. 2007, 105, 552–561. [Google Scholar] [CrossRef]
- Soares, P.I.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar] [CrossRef]
- Wang, F.; Yao, J.; Russel, M.; Chen, H.; Chen, K.; Zhou, Y.; Ceccanti, B.; Zaray, G.; Choi, M.M. Development and analytical application of a glucose biosensor based on glucose oxidase/O-(2-hydroxyl)propyl-3-trimethylammonium chitosan chloride nanoparticle-immobilized onion inner epidermis. Biosens. Bioelectron. 2010, 25, 2238–2243. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, S.; Lu, L.; Song, X.; Li, P.; Wang, F. Curdlan sulfate-O-linked quaternized chitosan nanoparticles: Potential adjuvants to improve the immunogenicity of exogenous antigens via intranasal vaccination. Int. J. Nanomed. 2018, 13, 2377–2394. [Google Scholar] [CrossRef] [Green Version]
- Cheah, W.Y.; Show, P.L.; Ng, I.S.; Lin, G.Y.; Chiu, C.Y.; Chang, Y.K. Antibacterial activity of quaternized chitosan modified nanofiber membrane. Int. J. Biol. Macromol. 2019, 126, 569–577. [Google Scholar] [CrossRef]
- Guang, W.Y. The Effect of Chitosan and Its Derivatives on the Dyeability of Silk. Ph.D. Thesis, The Hong Kong Polytechnic University, Hong Kong, China, 2003. [Google Scholar]
- Gruškiene, R.; Deveikyte, R.; Makuška, R. Quaternization of chitosan and partial destruction of the quaternized derivatives making them suitable for electrospinning. Chemija 2013, 24, 325–334. [Google Scholar]
- Wan, A.; Xu, Q.; Sun, Y.; Li, H. Antioxidant activity of high molecular weight chitosan and N,O-quaternized chitosans. J. Agric. Food Chem. 2013, 61, 6921–6928. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Pei, H.; Hu, W.; Zhu, Y.; Xu, H.; Ma, C.; Sun, J.; Li, H. A promising application of chitosan quaternary ammonium salt to removal of Microcystis aeruginosa cells from drinking water. Sci. Total. Environ. 2017, 583, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, P.D.; Pandima, D.M.K.; Arumugam, P.; Sudharsan, K.; Anruradha, V. Larvicidal Activities of N-(2-Hydroxyl) Propyl-3-Trimethyl Ammonium Chitosan Chloride (HTCC) and Silver Nanoparticles against Two Mosquito Species, Aedes and Culex: A Comparative Study. Res. Rev. J. Zool. Sci. 2017, 5, 1–8. [Google Scholar]
- Mi, F.L.; Shyu, S.S.; Chen, C.T.; Schoung, J.Y. Porous chitosan microsphere for controlling the antigen release of Newcastle disease vaccine: Preparation of antigen-adsorbed microsphere and in vitro release. Biomaterials 1999, 20, 1603–1612. [Google Scholar] [CrossRef]
- Xu, Y.; Du, Y.; Huang, R.; Gao, L. Preparation and modification of N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a protein carrier. Biomaterials 2003, 24, 5015–5022. [Google Scholar] [CrossRef]
- Zhao, S.H.; Wu, X.T.; Guo, W.C.; Du, Y.M.; Yu, L.; Tang, J. N-(2-hydroxyl) propyl-3-trimethyl ammonium chitosan chloride nanoparticle as a novel delivery system for Parathyroid Hormone-Related Protein 1-34. Int. J. Pharm. 2010, 393, 269–273. [Google Scholar] [CrossRef]
- Lorkowska-Zawicka, B.; Kamiński, K.; Ciejka, J.; Szczubiałka, K.; Białas, M.; Okoń, K.; Adamek, D.; Nowakowska, M.; Jawień, J.; Olszanecki, R.; et al. Inactivation of heparin by cationically modified chitosan. Mar. Drugs 2014, 12, 3953–3969. [Google Scholar] [CrossRef] [Green Version]
- Rosa, S.; Laranjeira, M.C.; Riela, H.G.; Fávere, V.T. Cross-linked quaternary chitosan as an adsorbent for the removal of the reactive dye from aqueous solutions. J. Hazard. Mater. 2008, 155, 253–260. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, C.; Zhao, Y.; Lu, X. One-pot synthesis and characterization of cross-linked quaternized chitosan microspheres as protein adsorbent. Int. J. Biol. Macromol. 2011, 49, 688–692. [Google Scholar] [CrossRef]
- Ciejka, J.; Wolski, K.; Nowakowska, M.; Pyrc, K.; Szczubiałka, K. Biopolymeric nano/microspheres for selective and reversible adsorption of coronaviruses. Mater. Sci. Eng. C 2017, 76, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Du, Y.; Wu, X.; Zhan, H. Effect of molecular weight and degree of substitution of quaternary chitosan on its adsorption and flocculation properties for potential retention-aids in alkaline papermaking. Colloids Surf. A Physicochem. Eng. Asp. 2004, 242, 1–8. [Google Scholar] [CrossRef]
- Li, H.; Du, Y.; Xu, Y.; Zhan, H.; Kennedy, J.F. Interactions of cationized chitosan with components in a chemical pulp suspension. Carbohydr. Polym. 2004, 58, 205–214. [Google Scholar] [CrossRef]
- Wan, Y.; Peppley, B.; Creber, K.A.; Bui, V.T.; Halliop, E. Quaternized-chitosan membranes for possible applications in alkaline fuel cells. J. Power Sources 2008, 185, 183–187. [Google Scholar] [CrossRef]
- An, Y.; Jiang, G.; Ren, Y.; Zhang, L.; Qi, Y.; Ge, Q. An environmental friendly and biodegradable shale inhibitor based on chitosan quaternary ammonium salt. J. Pet. Sci. Eng. 2015, 135, 253–260. [Google Scholar] [CrossRef]
- Grant, J.; Lee, H.; Soo, P.L.; Cho, J.; Piquette-Miller, M.; Allen, C. Influence of molecular organization and interactions on drug release for an injectable polymer-lipid blend. Int. J. Pharm. 2008, 360, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Peng, Z.; Li, Q.; Xu, X.; Guo, S.; Tang, T. The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus. Biomaterials 2012, 33, 365–377. [Google Scholar] [CrossRef]
- Huang, T.W.; Ho, Y.C.; Tsai, T.N.; Tseng, C.L.; Lin, C.; Mi, F.L. Enhancement of the permeability and activities of epigallocatechin gallate by quaternary ammonium chitosan/fucoidan nanoparticles. Carbohydr. Polym. 2020, 242, 116312. [Google Scholar] [CrossRef]
- Fortage, J.; Tuyèras, F.; Peltier, C.; Dupeyre, G.; Calboréan, A.; Bedioui, F.; Ochsenbein, P.; Puntoriero, F.; Campagna, S.; Ciofini, I.; et al. Tictoid expanded pyridiniums: Assessing structural, electrochemical, electronic, and photophysical features. J. Phys. Chem. A 2012, 116, 7880–7891. [Google Scholar] [CrossRef]
- Madaan, P.; Tiyagi, V.K. Quaternary pyridinium salts: A review. J. Oleo Sci. 2008, 57, 197–215. [Google Scholar] [CrossRef] [Green Version]
- Vogel, A. Vogel-A Text-Book of Practical Organic Chemistry; Longman Scientific & Technical: London, UK, 1989; p. 815. [Google Scholar]
- Haldar, J.; Kondaiah, P.; Bhattacharya, S. Synthesis and antibacterial properties of novel hydrolyzable cationic amphiphiles. Incorporation of multiple head groups leads to impressive antibacterial activity. J. Med. Chem. 2005, 48, 3823–3831. [Google Scholar] [CrossRef] [PubMed]
- Ilangovan, A.; Venkatesan, P.; Sundararaman, M.; Kumar, R.R. Synthesis, characterization and antimicrobial activity of 4-amino-1-alkyl pyridinium salts. Med. Chem. Res. 2012, 21, 694–702. [Google Scholar] [CrossRef]
- Sowmiah, S.; Esperança, J.M.; Rebelo, L.P.; Afonso, C.A. Pyridinium salts: From synthesis to reactivity and applications. Org. Chem. Front. 2018, 5, 453–493. [Google Scholar] [CrossRef]
- Li, R.; Guo, Z.; Jiang, P. Synthesis, characterization, and antifungal activity of novel quaternary chitosan derivatives. Carbohydr. Res. 2010, 345, 1896–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Chen, R.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. Novel derivatives of chitosan and their antifungal activities in vitro. Carbohydr. Res. 2006, 341, 351–354. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Gao, Z.; Qiu, S.; Dong, F.; Guo, Z. Design, synthesis of novel starch derivative bearing 1,2,3-triazolium and pyridinium and evaluation of its antifungal activity. Carbohydr. Polym. 2017, 157, 236–243. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Tan, W.; Gu, G.; Guo, Z. Novel amino-pyridine functionalized chitosan quaternary ammonium derivatives: Design, synthesis, and antioxidant activity. Molecules 2017, 22, 156. [Google Scholar] [CrossRef] [Green Version]
- Vetter, A.C.; Nikitin, K.; Gilheany, D.G. Long sought synthesis of quaternary phosphonium salts from phosphine oxides: Inverse reactivity approach. Chem. Commun. 2018, 54, 5843–5846. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Kandil, S. CHAPTER 3 Synthesis, Antimicrobial Activity and Applications of Polymers with Ammonium and Phosphonium Groups. In Polymeric Materials with Antimicrobial Activity: From Synthesis to Applications; The Royal Society of Chemistry: London, UK, 2014; pp. 54–74. [Google Scholar] [CrossRef]
- Guo, A.; Wang, F.; Lin, W.; Xu, X.; Tang, T.; Shen, Y.; Guo, S. Evaluation of antibacterial activity of N-phosphonium chitosan as a novel polymeric antibacterial agent. Int. J. Biol. Macromol. 2014, 67, 163–171. [Google Scholar] [CrossRef]
- Zhu, D.; Cheng, H.; Li, J.; Zhang, W.; Shen, Y.; Chen, S.; Ge, Z.; Chen, S. Enhanced water-solubility and antibacterial activity of novel chitosan derivatives modified with quaternary phosphonium salt. Mater. Sci. Eng. C 2016, 61, 79–84. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Dong, F.; Chen, Q.; Guo, Z. Preparation and characterization of novel cationic chitosan derivatives bearing quaternary ammonium and phosphonium salts and assessment of their antifungal properties. Molecules 2017, 22, 1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Cai, Z.S.; Zhu, X.M.; Shang, S.B.; Pei, L.J. Synthesis, characterization, and performance of a novel polymeric cationic surfactant based on low molecular weight chitosan and 3-chloro-2-hydroxypropyl dimethyl dehydroabietyl ammonium chloride (CHPDMDHA). J. Surfactants Deterg. 2015, 18, 463–470. [Google Scholar] [CrossRef]
- Holappa, J.; Hjálmarsdóttir, M.; Másson, M.; Rúnarsson, Ö.; Asplund, T.; Soininen, P.; Nevalainen, T.; Järvinen, T. Antimicrobial activity of chitosan N-betainates. Carbohydr. Polym. 2006, 65, 114–118. [Google Scholar] [CrossRef]
- Holappa, J.; Nevalainen, T.; Safin, R.; Soininen, P.; Asplund, T.; Luttikhedde, T.; Másson, M.; Järvinen, T. Novel water-soluble quaternary piperazine derivatives of chitosan: Synthesis and characterization. Macromol. Biosci. 2006, 6, 139–144. [Google Scholar] [CrossRef]
- Korjamo, T.; Holappa, J.; Taimisto, S.; Savolainen, J.; Järvinen, T.; Mönkkönen, J. Effect of N-betainate and N-piperazine derivatives of chitosan on the paracellular transport of mannitol in Caco-2 cells. Eur. J. Pharm. Sci. 2008, 35, 226–234. [Google Scholar] [CrossRef]
- Zambito, Y.; Uccello-Barretta, G.; Zaino, C.; Balzano, F.; Di Colo, G. Novel transmucosal absorption enhancers obtained by aminoalkylation of chitosan. Eur. J. Pharm. Sci. 2006, 29, 460–469. [Google Scholar] [CrossRef]
- Zambito, Y.; Zaino, C.; Burchielli, S.; Carelli, V.; Serafini, M.F.; Di Colo, G. Novel quaternary ammonium chitosan derivatives for the promotion of intraocular drug absorption. J. Drug Deliv. Sci. Technol. 2007, 17, 19–24. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, W.; Xiong, J.; Qu, N.; Li, H.; Yao, G. Synthesis and properties of N,N-dimethyl-O-quaternary ammonium chitosan. Adv. Mater. Res. 2011, 152–153, 1337–1341. [Google Scholar] [CrossRef]
- Li, Z.; Yang, F.; Yang, R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015, 75, 378–387. [Google Scholar] [CrossRef]
- Pan, H.; Zhao, T.; Xu, L.; Shen, Y.; Wang, L.; Ding, Y. Preparation of novel chitosan derivatives and applications in functional finishing of textiles. Int. J. Biol. Macromol. 2020, 153, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Dang, Q.; Liu, C.; Wang, T.; Fan, B.; Yan, J.; Xu, Y. Preparation, characterization and antibacterial activity of O-acetyl-chitosan-N-2-hydroxypropyl trimethyl ammonium chloride. Int. J. Biol. Macromol. 2015, 80, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Ding, D.; Ren, J.; Zhu, X.; Yao, Y. Synthesis, characterization, and drug-release behavior of amphiphilic quaternary ammonium chitosan derivatives. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Pedro, R.D.O.; Schmitt, C.C.; Neumann, M.G. Syntheses and characterization of amphiphilic quaternary ammonium chitosan derivatives. Carbohydr. Polym. 2016, 147, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lin, Q. Synthesis, characterization, and antibacterial activity of quaternized of N-aromatic chitosan derivatives. Appl. Mech. Mater. 2012, 138-139, 1202–1208. [Google Scholar] [CrossRef]
- Badawy, M.E.; Rabea, E.I. Synthesis and antifungal property of N-(aryl) and quaternary N-(aryl) chitosan derivatives against Botrytis cinerea. Cellulose 2014, 21, 3121–3137. [Google Scholar] [CrossRef]
- Sang, W.; Tang, Z.; He, M.Y.; Hua, Y.P.; Xu, Q. Synthesis and preservative application of quaternized carboxymethyl chitosan containing guanidine groups. Int. J. Biol. Macromol. 2015, 75, 489–494. [Google Scholar] [CrossRef]
- Rahimi, M.; Ahmadi, R.; Samadi Kafil, H.; Shafiei-Irannejad, V. A novel bioactive quaternized chitosan and its silver-containing nanocomposites as a potent antimicrobial wound dressing: Structural and biological properties. Mater. Sci. Eng. C 2019, 101, 360–369. [Google Scholar] [CrossRef]





| Quaternized Chitosan Derivatives | Structural Formula | Application/Potential Applications | Source |
|---|---|---|---|
| N,N,N-trimethyl O-(2-hydroxy-3- trimethylammonium propyl) chitosan |  | Antibacterial agent | [20] |
| LWCS-g-CHPDMDHA |  | Polymeric surfactants for medicines, emulsions and treatment of wastewater | [190] |
| N-betainate chitosan |  | Antibacterial agent | [192] |
| N-piperazine chitosan |  | Pharmaceutical applications | [191] |
| N,O-[N,N-diethylaminomethyl (diethyldimethylene ammonium)]methyl chitosans |  | Intraocular drug delivery | [195] |
| N,N-dimethyl-O-quaternary ammonium chitosan |  | Moisturizer for cosmetics, biomedical materials, and antibacterial agents | [196] |
| OQCATUCS |  | Bactericide in the fields of medicine and food | [197] |
| NMA-HDCC |  | Functional finishing textiles | [198] |
| O-acetyl-chitosan- N-2-hydroxypropyl trimethyl ammonium chloride (C) |  | Food preservative | [199] |
| 2-N-carboxymethyl-6-O -diethylaminoethyl chitosan |  | Drug delivery | [200] |
| BPTADDACH |  | Drug delivery | [201] |
| Quaternized N-(aryl) chitosans |  | Antibacterial agent | [202] |
| N,N,N-(diethylaryl) chitosans chloride |  | Fungicide | [203] |
| QGCMCH |  | Food preservative | [204] |
| QC-IMDZ |  | Wound dressings | [205] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, E.D.; Moura Jr., C.F.; Kerwald, J.; Beppu, M.M. An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives. Polymers 2020, 12, 2878. https://doi.org/10.3390/polym12122878
Freitas ED, Moura Jr. CF, Kerwald J, Beppu MM. An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives. Polymers. 2020; 12(12):2878. https://doi.org/10.3390/polym12122878
Chicago/Turabian StyleFreitas, Emanuelle Dantas, Celso Fidelis Moura Jr., Jonas Kerwald, and Marisa Masumi Beppu. 2020. "An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives" Polymers 12, no. 12: 2878. https://doi.org/10.3390/polym12122878
APA StyleFreitas, E. D., Moura Jr., C. F., Kerwald, J., & Beppu, M. M. (2020). An Overview of Current Knowledge on the Properties, Synthesis and Applications of Quaternary Chitosan Derivatives. Polymers, 12(12), 2878. https://doi.org/10.3390/polym12122878





