Preparation and Properties of Intrinsically Atomic-Oxygen Resistant Polyimide Films Containing Polyhedral Oligomeric Silsesquioxane (POSS) in the Side Chains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
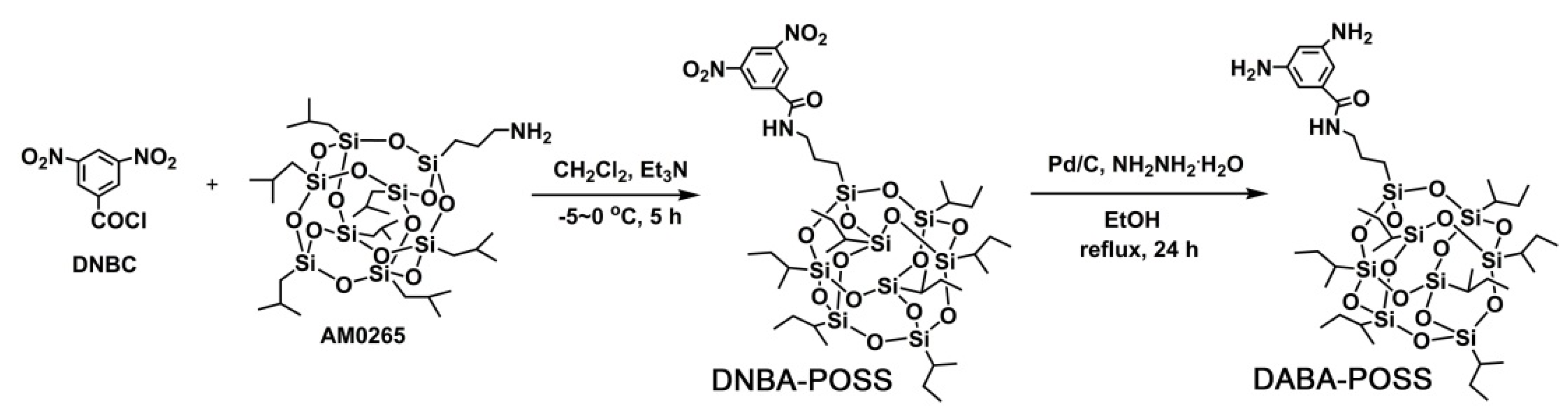
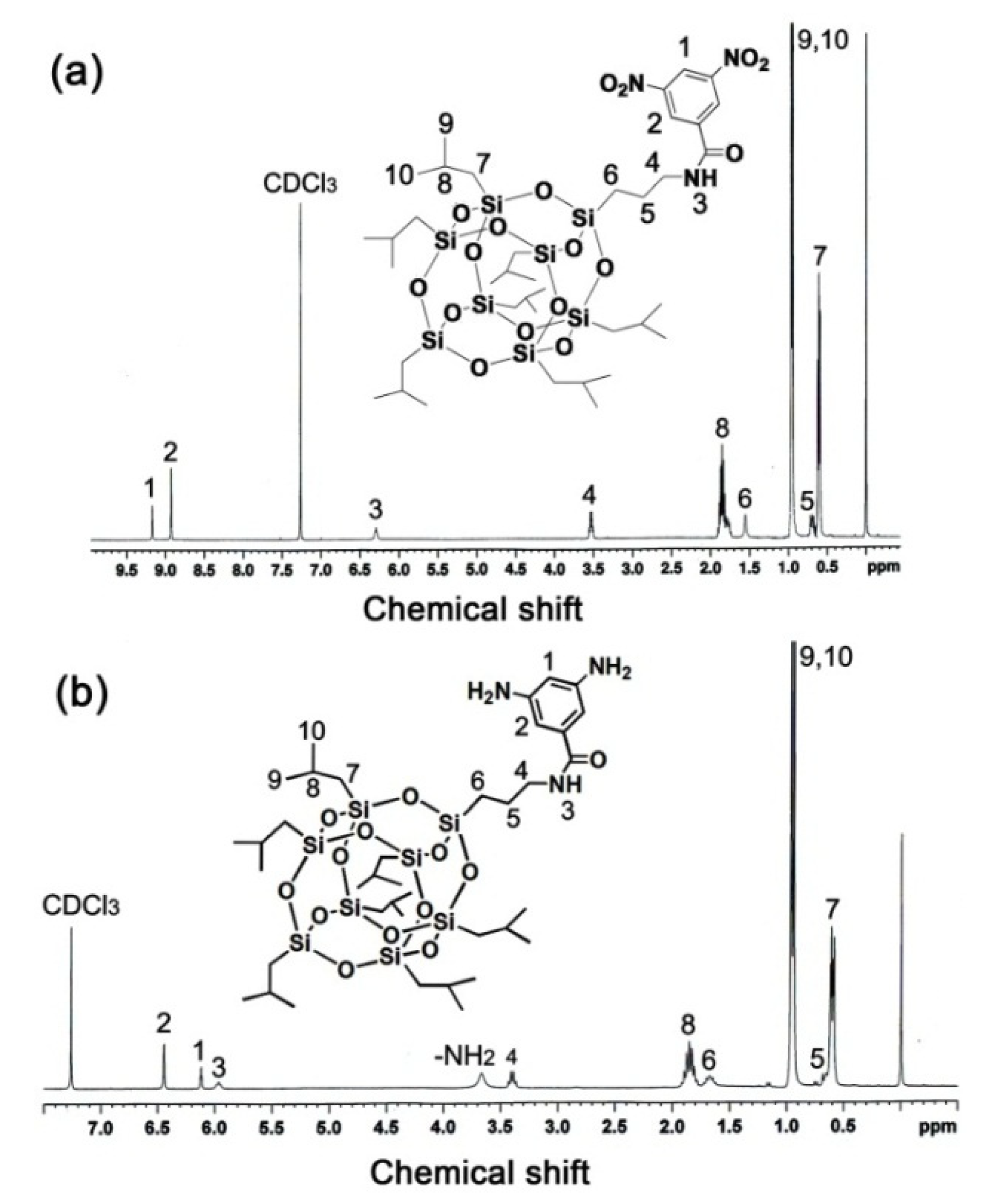
2.3. Monomer Synthesis
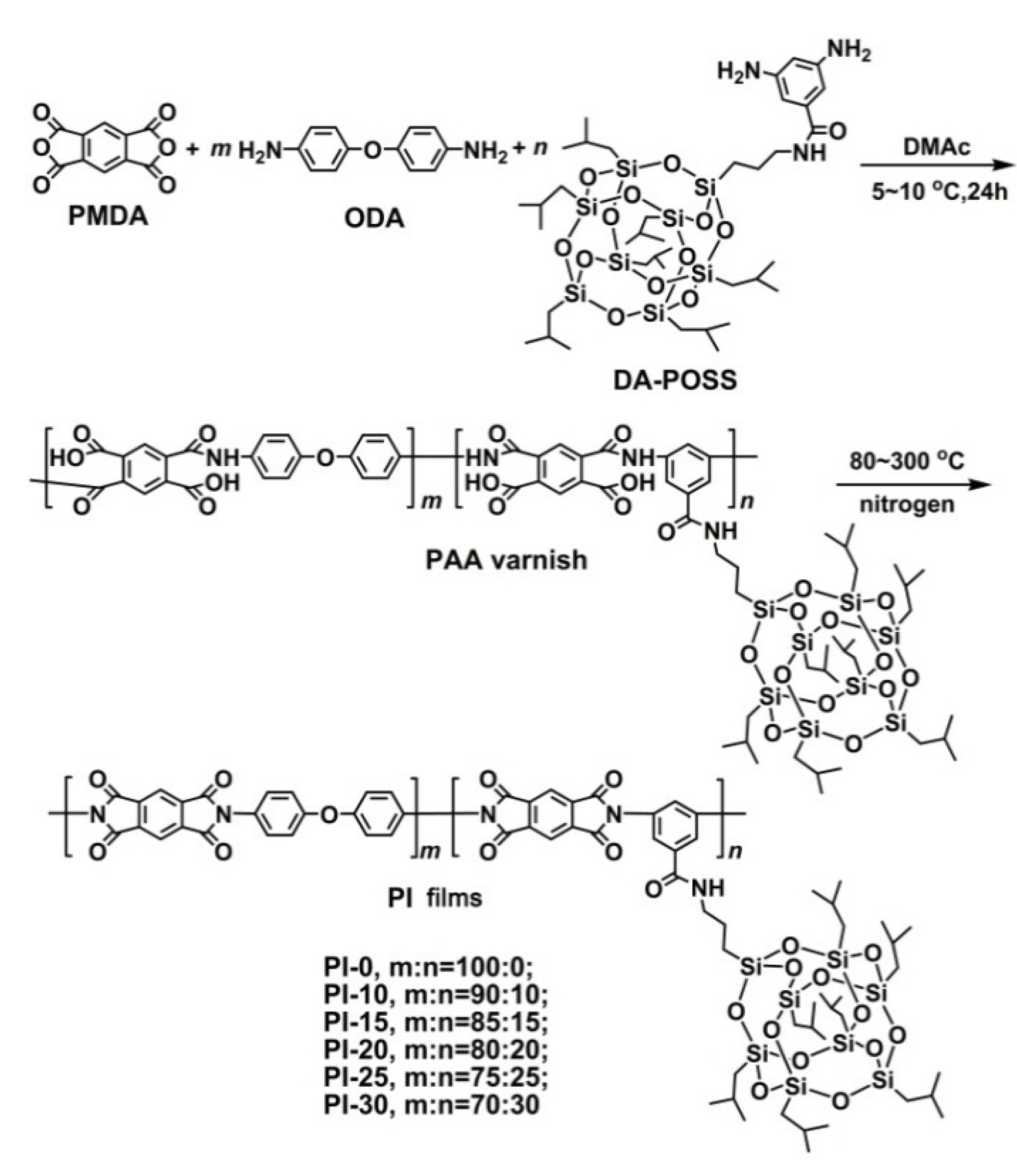
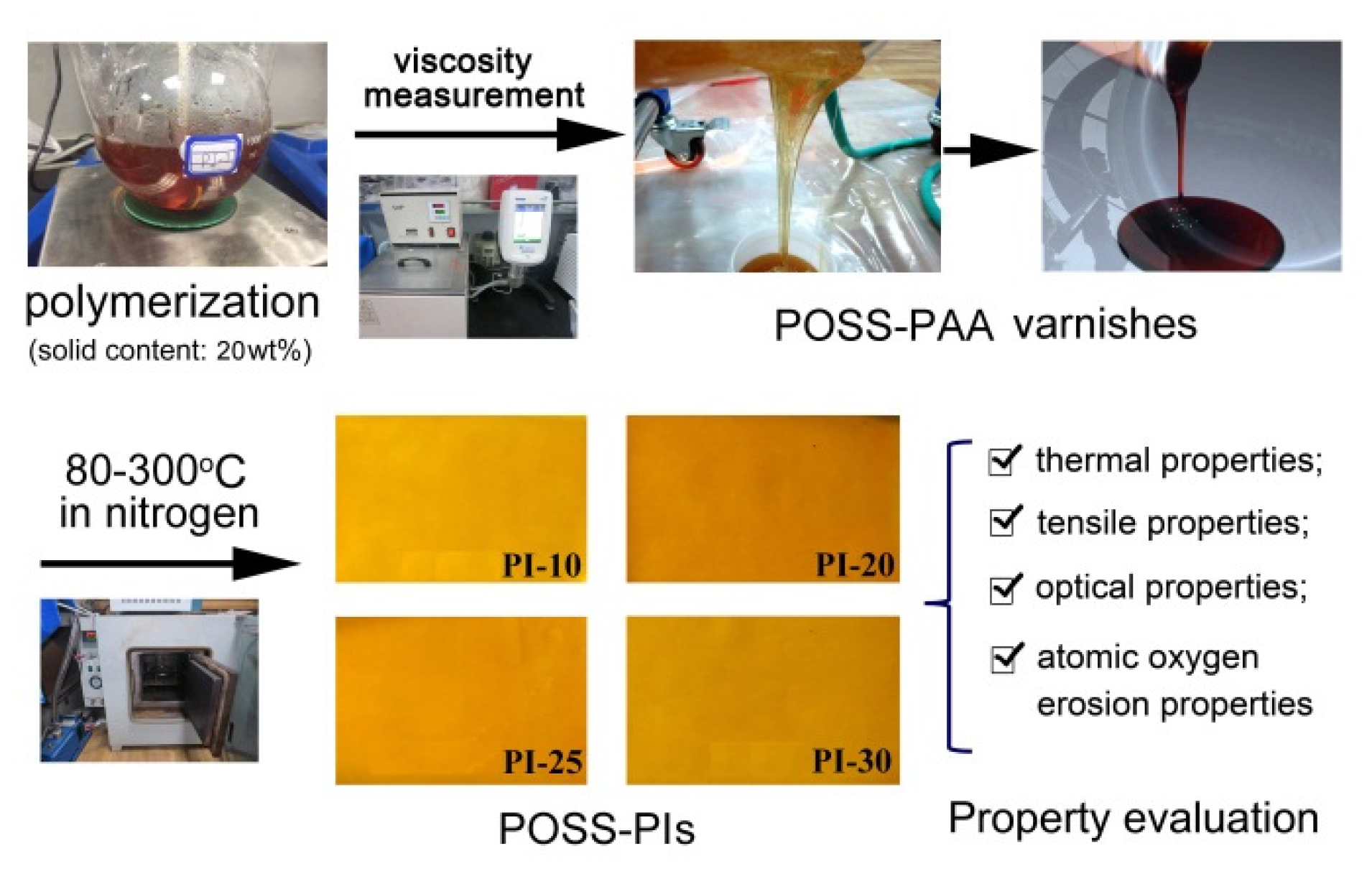
2.4. PAA Synthesis and PI Film Preparation
3. Results and Discussion
3.1. Monomer Synthesis
3.2. PAA Synthesis and Film Preparation
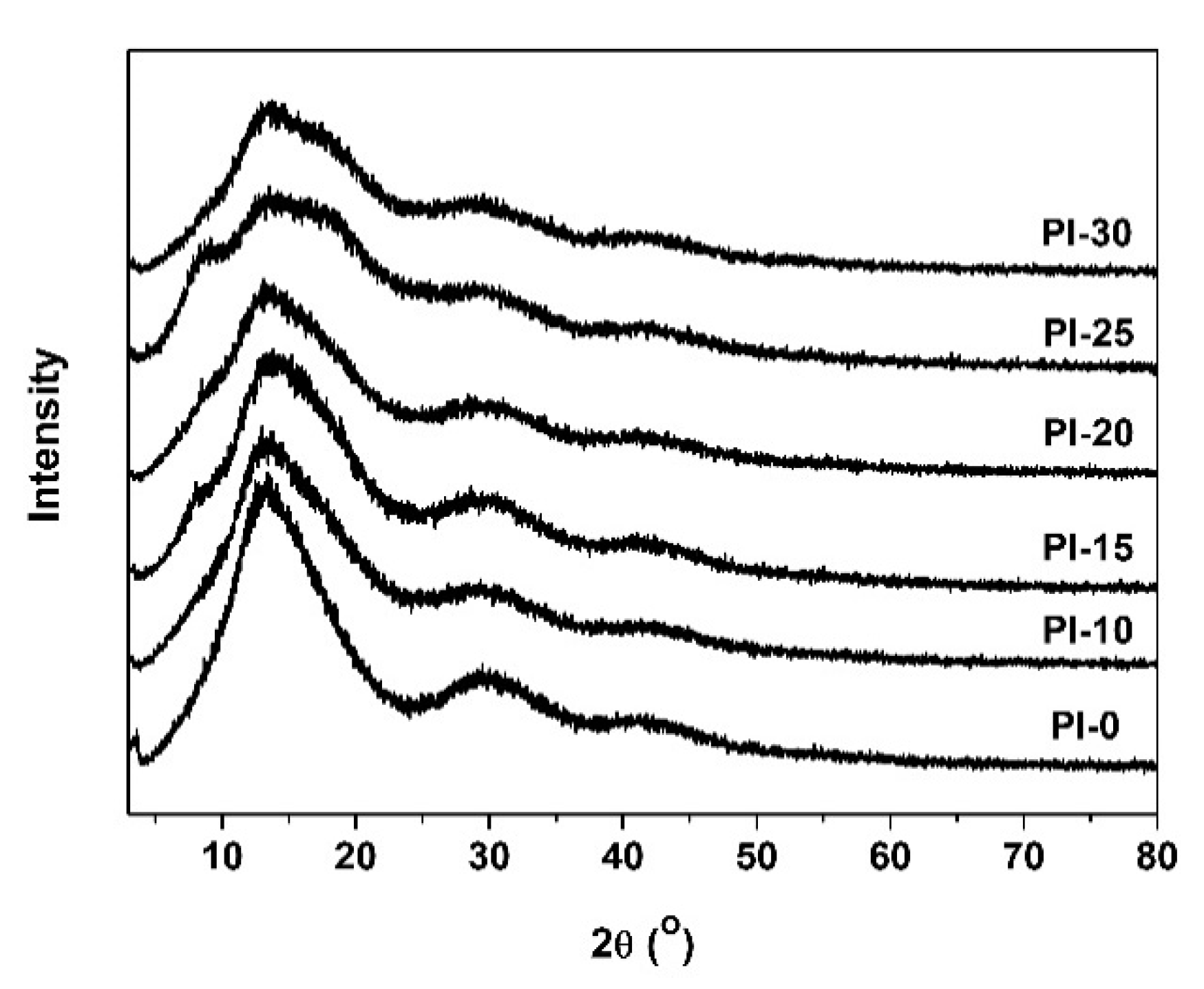
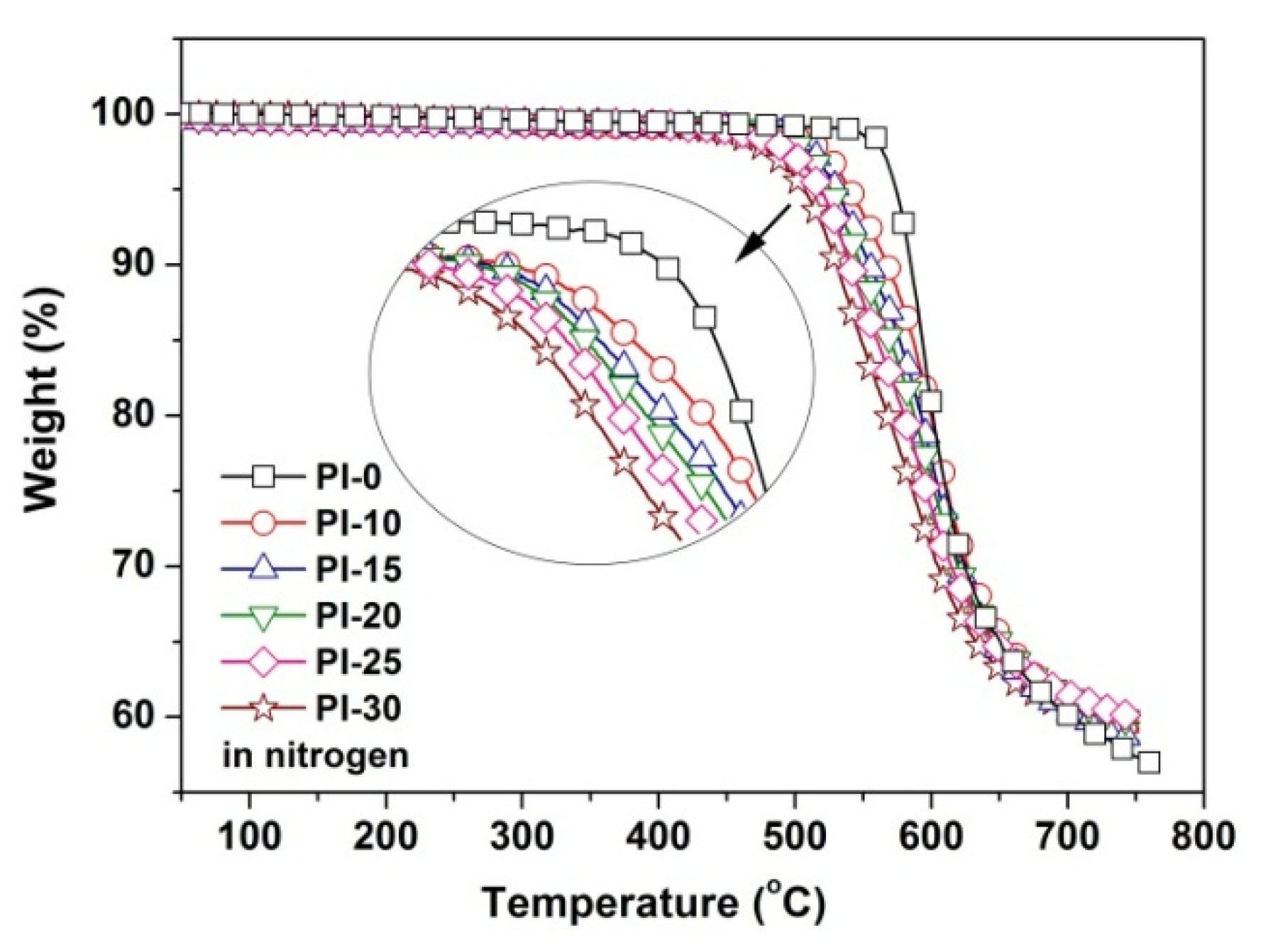
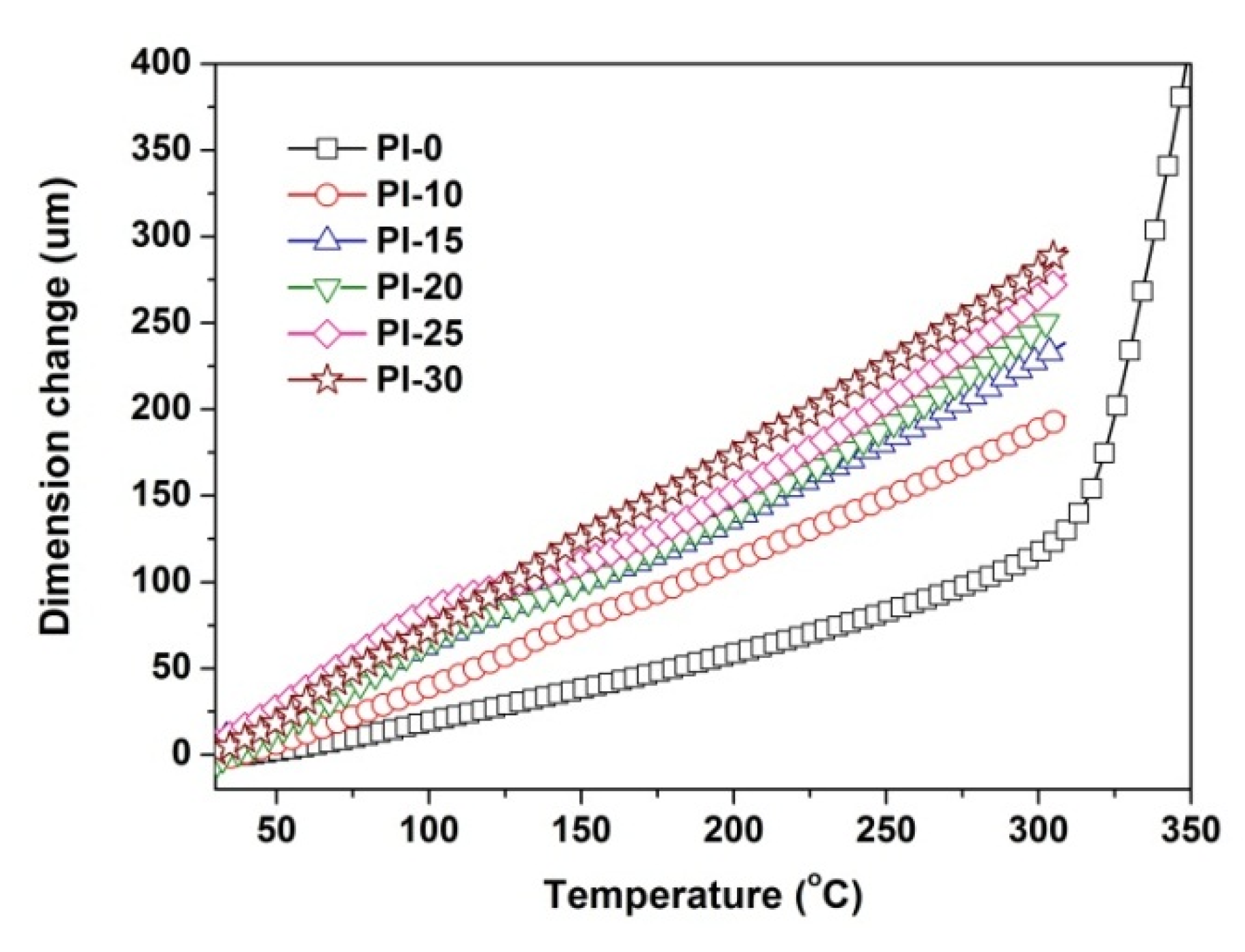
3.3. Thermal and Mechanical Properties
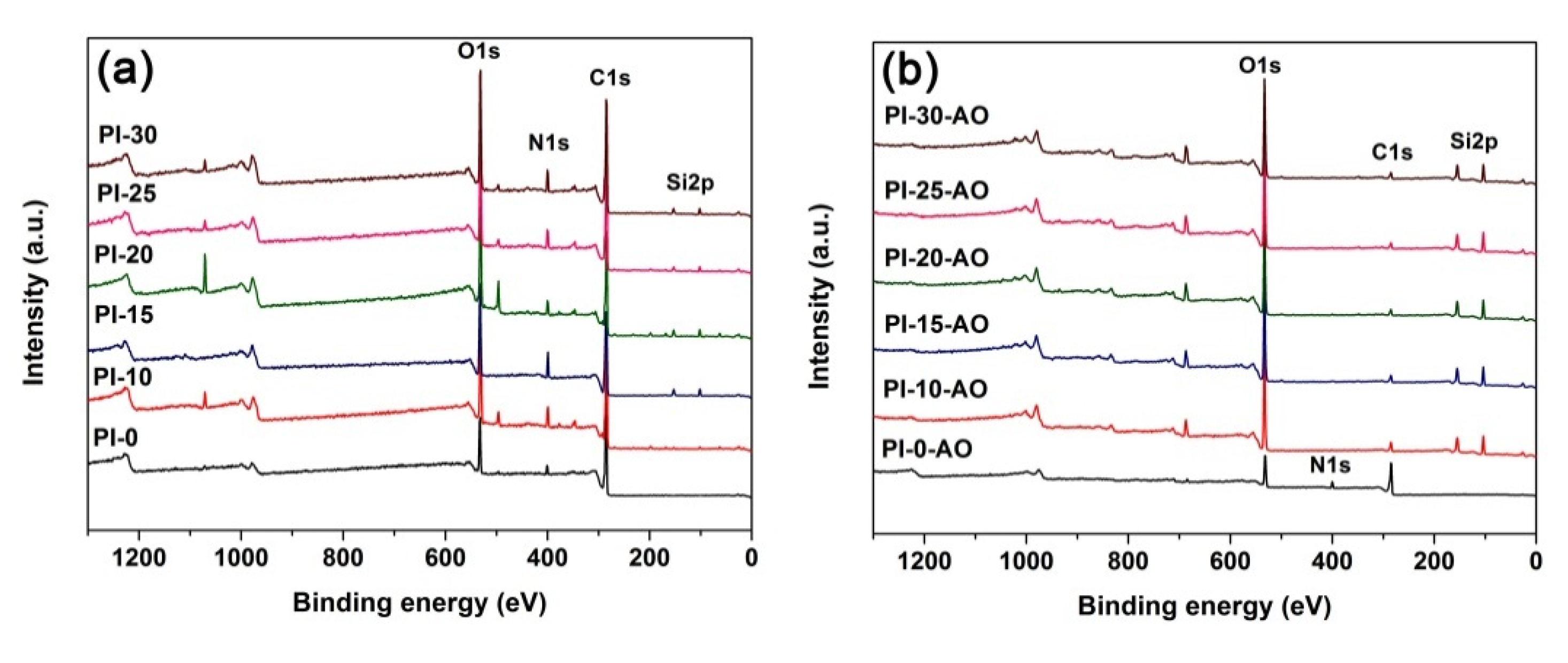
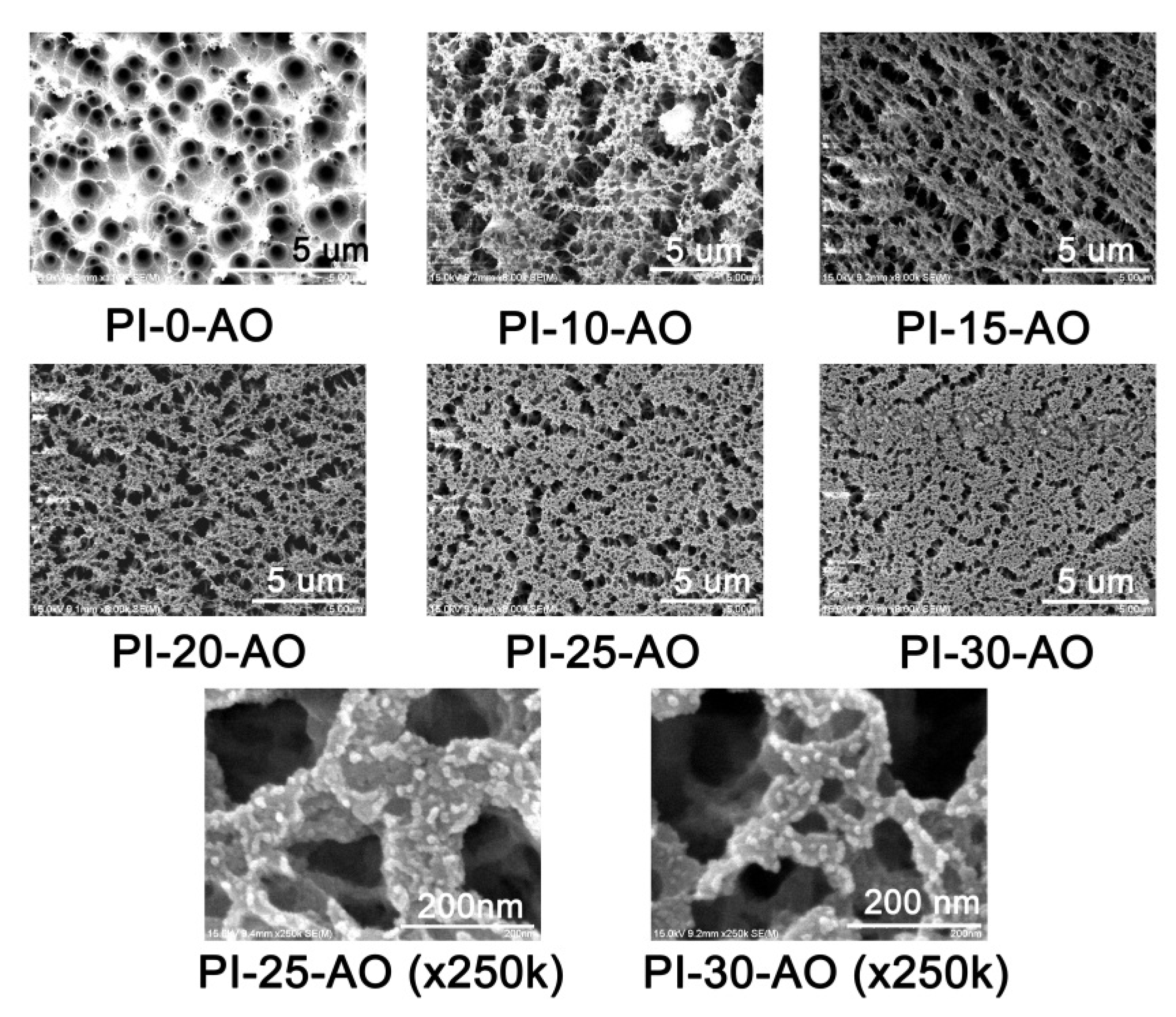
3.4. Atomic Oxygen Resistant Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Synthesis, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Ma, P.; Dai, C.; Wang, H.; Li, Z.; Liu, H.; Li, W.; Yang, C. A review on high temperature resistant polyimide films bearing heterocyclic structures and their applications. Compos. Commun. 2019, 16, 84–93. [Google Scholar] [CrossRef]
- Khomiakova, N.; Hanuš, J.; Kuzminova, A.; Kylián, O. Investigation of wettability, drying and water condensation on polyimide (Kapton) films treated by atmospheric pressure air dielectric barrier discharge. Coatings 2020, 10, 619. [Google Scholar] [CrossRef]
- Samwel, S.W. Low earth orbital atomic oxygen erosion effect on spacecraft material. Space Res. J. 2014, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Buczala, D.M.; Brunsvold, A.L.; Minton, T.K. Erosion of Kapton H® by hyperthermal atomic oxygen. J. Spacecr. Rockets 2006, 43, 421–425. [Google Scholar] [CrossRef]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Qian, M.; Liu, G.; Zhou, B.; Xuan, X.Y.; Niu, Y.P.; Gong, S.Q. Atomic oxygen durable ultra-black polyimide nanocomposite films in solar spectrum. Polym. Degrad. Stab. 2020, 175, 109133. [Google Scholar] [CrossRef]
- Qian, M.; Murray, V.J.; Wei, W.; Marshall, B.C.; Minton, T.K. Resistance of POSS polyimide blends to hyperthermal atomic oxygen attack. ACS Appl. Mater. Interf. 2016, 8, 33982–33992. [Google Scholar] [CrossRef]
- Song, G.; Li, X.; Jiang, Q.; Mu, J.; Jiang, Z. A novel structural polyimide material with synergistic phosphorus and POSS for atomic oxygen resistance. RSC Adv. 2015, 5, 11980–11988. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G. Numerical simulation on atomic oxygen undercutting of Kapton film in low earth orbit. Acta Astronaut. 2010, 67, 388–395. [Google Scholar] [CrossRef]
- Li, X.; Al-Ostaz, A.; Jaradat, M.; Rahmani, F.; Nouranian, S.; Rushing, G.; Manasrah, A.; Alkhateb, H.; Finckenor, M.; Lichtenhan, J. Substantially enhanced durability of polyhedral oligomeric silsequioxane-polyimide nanocomposites against atomic oxygen erosion. Eur. Polym. J. 2017, 92, 233–249. [Google Scholar] [CrossRef]
- Duo, S.; Chang, Y.; Liu, T.; Zhang, H. Atomic oxygen erosion resistance of polysiloxane/POSS hybrid coatings on Kapton. Phys. Proc. 2013, 50, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Gouzman, I.; Girshevitz, O.; Grossman, E.; Eliaz, N.; Sukenik, C.N. Thin film oxide barrier layers: Protection of Kapton from space environment by liquid phase deposition of titanium oxide. ACS Appl. Mater. Interfaces 2010, 2, 1835–1843. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, D.; Liu, G.; Wei, Q. Atomic oxygen adaptability of flexible Kapton/Al2O3 composite thin films prepared by ion exchange method. Coatings 2019, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ai, L.; Li, X.; Zhang, X.; Lu, Y.; Chen, G.; Fang, X.; Dai, N.; Tan, R.; Song, W. Hollow silica nanosphere/polyimide composite films for enhanced transparency and atomic oxygen resistance. Mater. Chem. Phys. 2019, 222, 384–390. [Google Scholar] [CrossRef]
- Banks, B.A.; Snyder, A.; Miller, S.K.; De Groh, K.K.; Demko, R. Atomic-oxygen undercutting of protected polymers in low earth orbit. J. Spacecr. Rockets 2004, 41, 335–339. [Google Scholar] [CrossRef]
- Lei, X.F.; Chen, Y.; Zhang, H.P.; Li, X.J.; Yao, P.; Zhang, Q.Y. Space survivable polyimides with excellent optical transparency and self-healing properties derived from hyperbranched polysiloxane. ACS Appl. Mater. Interfaces 2013, 5, 10207–10220. [Google Scholar] [CrossRef]
- Lei, X.; Yao, P.; Qiao, M.; Sun, W.; Zhang, H.; Zhang, Q. Atomic oxygen resistance of polyimide/silicon hybrid thin films with different compositions and architectures. High. Perform. Polym. 2014, 26, 712–724. [Google Scholar] [CrossRef]
- Thompson, C.M.; Smith, J.G.; Connell, J.W. Polyimides prepared from 4,4′-(2-diphenylphosphinyl-1,4-phenylenedioxy)diphthalic anhydride for potential space applications. High. Perform. Polym. 2003, 15, 181–195. [Google Scholar] [CrossRef]
- Watson, K.A.; Palmieri, F.L.; Connell, J.W. Space environmentally stable polyimides and copolyimides derived from [2,4-bis(3-aminophenoxy)phenyl]diphenylphosphine oxide. Macromolecules 2002, 35, 4968–4974. [Google Scholar] [CrossRef]
- Li, Z.; Song, H.; He, M.; Liu, J.; Yang, S. Atomic oxygen-resistant and transparent polyimide coatings from [3,5-bis(3-aminophenoxy)phenyl]diphenylphosphine oxide and aromatic dianhydrides: Preparation and characterization. Prog. Org. Coat. 2012, 75, 49–58. [Google Scholar] [CrossRef]
- Brunsvold, A.L.; Minton, T.K.; Gouzman, I.; Grossman, E.; Gonzalez, R. An investigation of the resistance of polyhedral oligomeric silsesquioxane polyimide to atomic-oxygen attack. High. Perform. Polym. 2004, 16, 303–318. [Google Scholar] [CrossRef]
- Wright, M.E.; Petteys, B.J.; Guenthner, A.J.; Fallis, S.; Yandek, G.R.; Tomczak, S.J.; Minton, T.K.; Brunsvold, A. Chemical modification of fluorinated polyimides: New thermally curing hybrid polymers with POSS. Macromolecules 2006, 39, 4710–4718. [Google Scholar] [CrossRef]
- Tomczak, S.J.; Wright, M.E.; Guenthner, A.J.; Pettys, B.J.; Brunsvold, A.L.; Knight, C.; Minton, T.K.; Vij, V.; McGrath, L.M.; Mabry, J.M. Space survivability of main-chain and side-chain POSS-Kapton polyimides. AIP Confer. Proc. 2009, 1087, 505–518. [Google Scholar]
- Vij, V.; Haddad, T.S.; Yandek, G.R.; Ramirez, S.M.; Mabry, J.M. Synthesis of aromatic polyhedral oligomeric silsesquioxane (POSS) dianilines for use in high-temperature polyimides. Silicon 2012, 4, 267–280. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Liu, J.; Ni, H.; Yang, H.; Yang, S. Intrinsically atomic-oxygen-resistant POSS-containing polyimide aerogels: Synthesis and characterization. Chem. Lett. 2015, 44, 1083–1085. [Google Scholar] [CrossRef]
- Atar, N.; Grossman, E.; Gouzman, I.; Bolker, A.; Murray, V.J.; Marshall, B.C.; Qian, M.; Minton, T.K.; Hanein, Y. Atomic-oxygen-durable and electrically-conductive CNT-POSS-Polyimide flexible films for space applications. ACS Appl. Mater. Interfaces 2015, 7, 12047–12056. [Google Scholar] [CrossRef]
- Minton, T.K.; Wright, M.E.; Tomczak, S.J.; Marquez, S.A.; Shen, L.; Brunsvold, A.L.; Cooper, R.; Zhang, J.; Vij, V.; Guenthner, A.J.; et al. Atomic oxygen effects on POSS polyimides in low earth orbit. ACS Appl. Mater. Interfaces 2012, 4, 492–502. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Y.; Yang, D.; Yang, Y.; Yu, Q.; Che, L.; Liu, J. Self-healing anti-atomic-oxygen phosphorus-containing polyimide film via molecular level incorporation of nanocage trisilanolphenyl POSS: Preparation and characterization. Polymers 2019, 11, 1013. [Google Scholar] [CrossRef] [Green Version]
- De Groh, K.K.; Banks, B.A.; Mccarthy, C.E.; Rucker, R.N.; Roberts, L.M.; Berger, L.A. MISSE 2 PEACE polymers atomic oxygen erosion experiment on the International Space Station. High. Perform. Polym. 2008, 20, 388–409. [Google Scholar] [CrossRef]
- Lei, X.F.; Qiao, M.T.; Tian, L.D.; Yao, P.; Ma, Y.; Zhang, H.P.; Zhang, Q.Y. Improved space survivability of polyhedral oligomeric silsesquioxane (POSS) polyimides fabricated via novel POSS-diamine. Corros. Sci. 2015, 90, 223–238. [Google Scholar] [CrossRef]
- Leu, C.M.; Chang, Y.T.; Wei, K.H. Synthesis and dielectric properties of polyimide-tethered polyhedral oligomeric silsesquioxane (POSS) nanocomposites via POSS-diamine. Macromolecules 2003, 36, 9122–9127. [Google Scholar] [CrossRef]



| PAA | [η]inh a (dL/g) | Mnb (×104 g/mol) | Mwb (×104 g/mol) | PDI b |
|---|---|---|---|---|
| PAA-0 | 1.33 | 10.70 | 17.11 | 1.59 |
| PAA-10 | 1.28 | 9.87 | 15.88 | 1.61 |
| PAA-15 | 1.20 | 9.61 | 16.29 | 1.69 |
| PAA-20 | 1.13 | 9.57 | 15.60 | 1.63 |
| PAA-25 | 1.04 | 8.56 | 14.34 | 1.68 |
| PAA-30 | 0.93 | 7.43 | 13.63 | 1.83 |
| PI | CIE Lab Parameters a | Thermal Properties b | Tensile Properties c | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | haze (%) | T5% (oC) | Rw750 (%) | CTE (×10−6/K) | TS (MPa) | TM (GPa) | Eb (%) | |
| PI-0 | 89.93 | −11.09 | 84.23 | 0.68 | 574 | 57.4 | 28.9 | 131.0 | 1.90 | 73.2 |
| PI-10 | 87.76 | −7.48 | 85.41 | 4.78 | 534 | 59.1 | 45.6 | 111.7 | 1.87 | 29.4 |
| PI-15 | 87.05 | −5.86 | 86.64 | 7.65 | 528 | 58.5 | 50.4 | 108.1 | 1.77 | 28.9 |
| PI-20 | 85.84 | −4.87 | 89.46 | 9.55 | 524 | 59.6 | 56.1 | 97.6 | 1.69 | 28.0 |
| PI-25 | 85.05 | −2.66 | 94.41 | 14.53 | 519 | 59.9 | 55.0 | 93.3 | 1.64 | 25.6 |
| PI-30 | 81.79 | −0.49 | 96.42 | 31.70 | 512 | 59.3 | 54.6 | 75.0 | 1.55 | 16.1 |
| Sample | W1a (mg) | W2a (mg) | ΔW a (mg) | Esb (10−25 cm3/atom) |
|---|---|---|---|---|
| PI-0 (Kapton) | 4535 | 900 | 3635 | 30.0 |
| PI-10 | 2775 | 2460 | 315 | 2.6 |
| PI-15 | 2225 | 1970 | 255 | 2.1 |
| PI-20 | 2335 | 2130 | 205 | 1.7 |
| PI-25 | 2670 | 2515 | 155 | 1.3 |
| PI-30 | 2980 | 2845 | 135 | 1.1 |
| Samples | Unexposed Samples | AO Exposed Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| Si2p | C1s | O1s | N1s | Si2p | C1s | O1s | N1s | |
| PI-10 | 3.92 | 67.92 | 21.07 | 6.86 | 24.27 | 12.04 | 53.52 | 0.41 |
| PI-15 | 3.23 | 65.27 | 22.95 | 6.68 | 24.30 | 10.24 | 54.32 | 0.43 |
| PI-20 | 3.63 | 59.95 | 25.55 | 4.25 | 24.50 | 8.86 | 54.76 | 0.35 |
| PI-25 | 2.29 | 67.68 | 21.87 | 5.67 | 24.29 | 8.54 | 55.02 | 0.34 |
| PI-30 | 3.08 | 64.63 | 23.63 | 6.14 | 23.42 | 9.51 | 53.71 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhang, Y.; Guo, Y.-D.; Qi, H.-R.; An, Y.-C.; Jia, Y.-J.; Tan, Y.-Y.; Liu, J.-G.; Wu, B.-H. Preparation and Properties of Intrinsically Atomic-Oxygen Resistant Polyimide Films Containing Polyhedral Oligomeric Silsesquioxane (POSS) in the Side Chains. Polymers 2020, 12, 2865. https://doi.org/10.3390/polym12122865
Wu H, Zhang Y, Guo Y-D, Qi H-R, An Y-C, Jia Y-J, Tan Y-Y, Liu J-G, Wu B-H. Preparation and Properties of Intrinsically Atomic-Oxygen Resistant Polyimide Films Containing Polyhedral Oligomeric Silsesquioxane (POSS) in the Side Chains. Polymers. 2020; 12(12):2865. https://doi.org/10.3390/polym12122865
Chicago/Turabian StyleWu, Hao, Yan Zhang, Yi-Dan Guo, Hao-Ran Qi, Yuan-Cheng An, Yan-Jiang Jia, Yao-Yao Tan, Jin-Gang Liu, and Bo-Han Wu. 2020. "Preparation and Properties of Intrinsically Atomic-Oxygen Resistant Polyimide Films Containing Polyhedral Oligomeric Silsesquioxane (POSS) in the Side Chains" Polymers 12, no. 12: 2865. https://doi.org/10.3390/polym12122865
APA StyleWu, H., Zhang, Y., Guo, Y.-D., Qi, H.-R., An, Y.-C., Jia, Y.-J., Tan, Y.-Y., Liu, J.-G., & Wu, B.-H. (2020). Preparation and Properties of Intrinsically Atomic-Oxygen Resistant Polyimide Films Containing Polyhedral Oligomeric Silsesquioxane (POSS) in the Side Chains. Polymers, 12(12), 2865. https://doi.org/10.3390/polym12122865






