Donor-Acceptor Polymer Based on Planar Structure of Alkylidene-Fluorene Derivative: Correlation of Power Conversion Efficiency among Polymer and Various Acceptor Units
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Associated Methods
2.2. Instruments and Characterization
2.3. Fabrication of OSCs
3. Results and Discussion
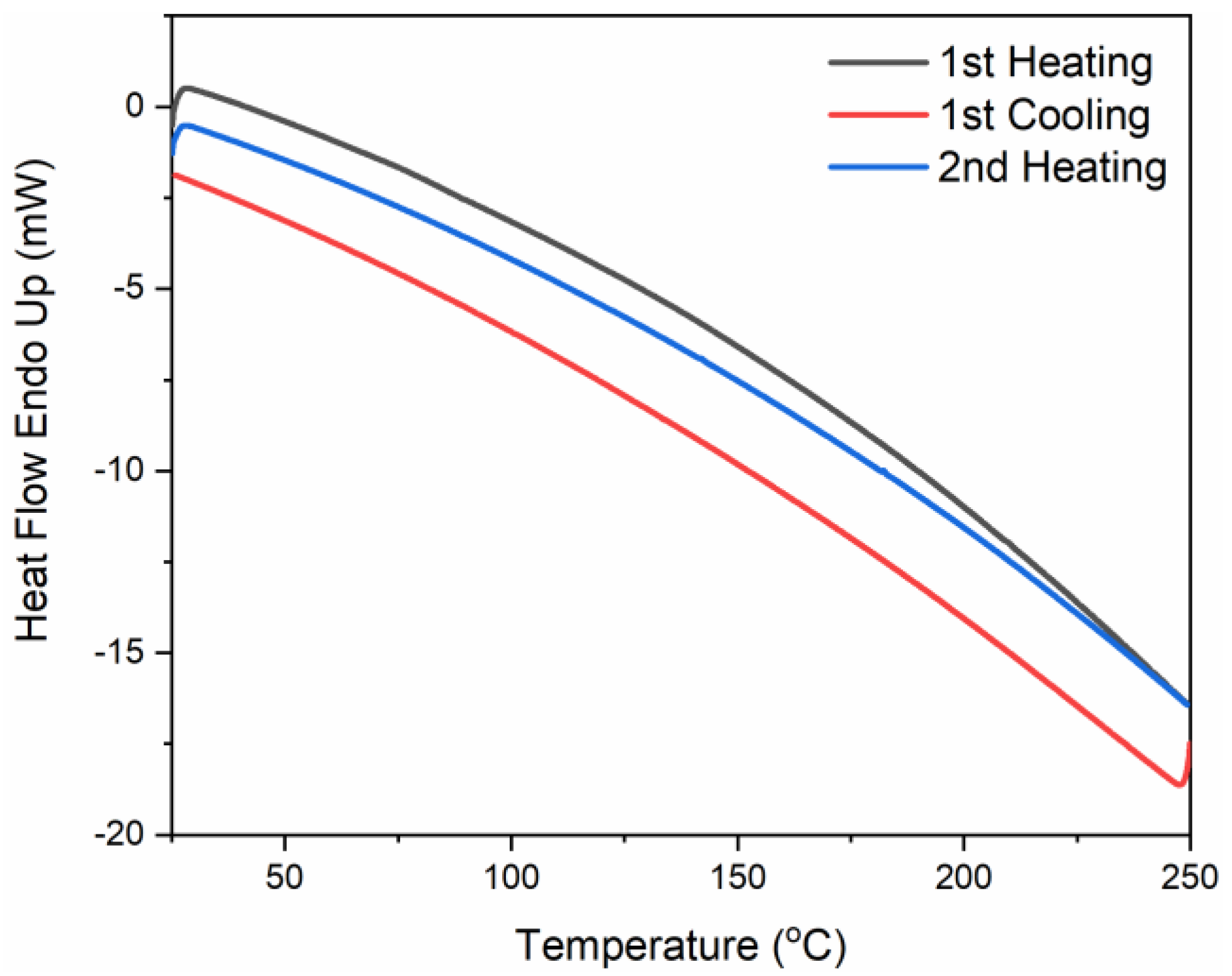
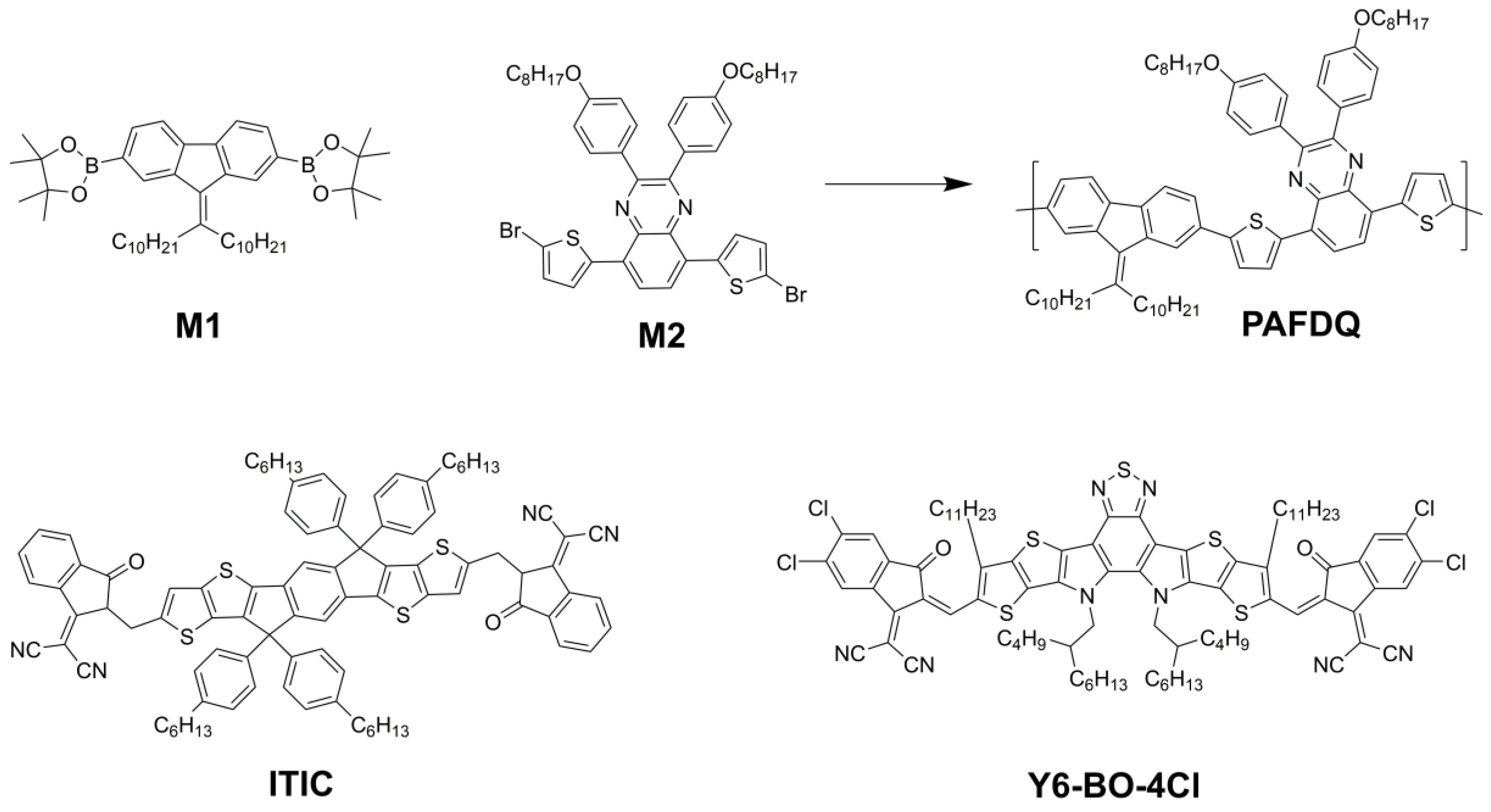
3.1. Characterization of the Polymers
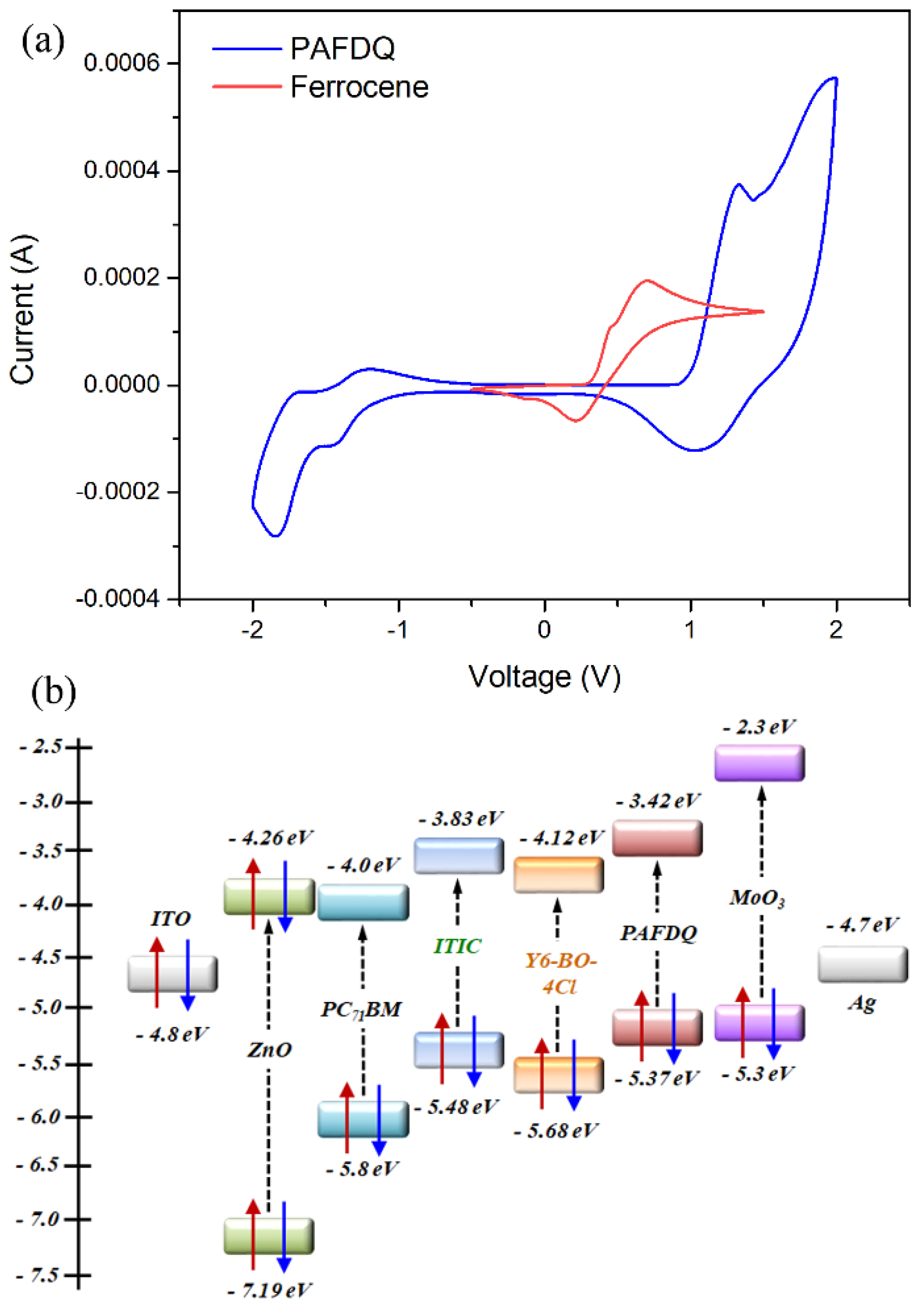
3.2. Optical and Electrochemical Properties
3.3. Orientation Analysis
3.4. Photovoltaic Characteristics
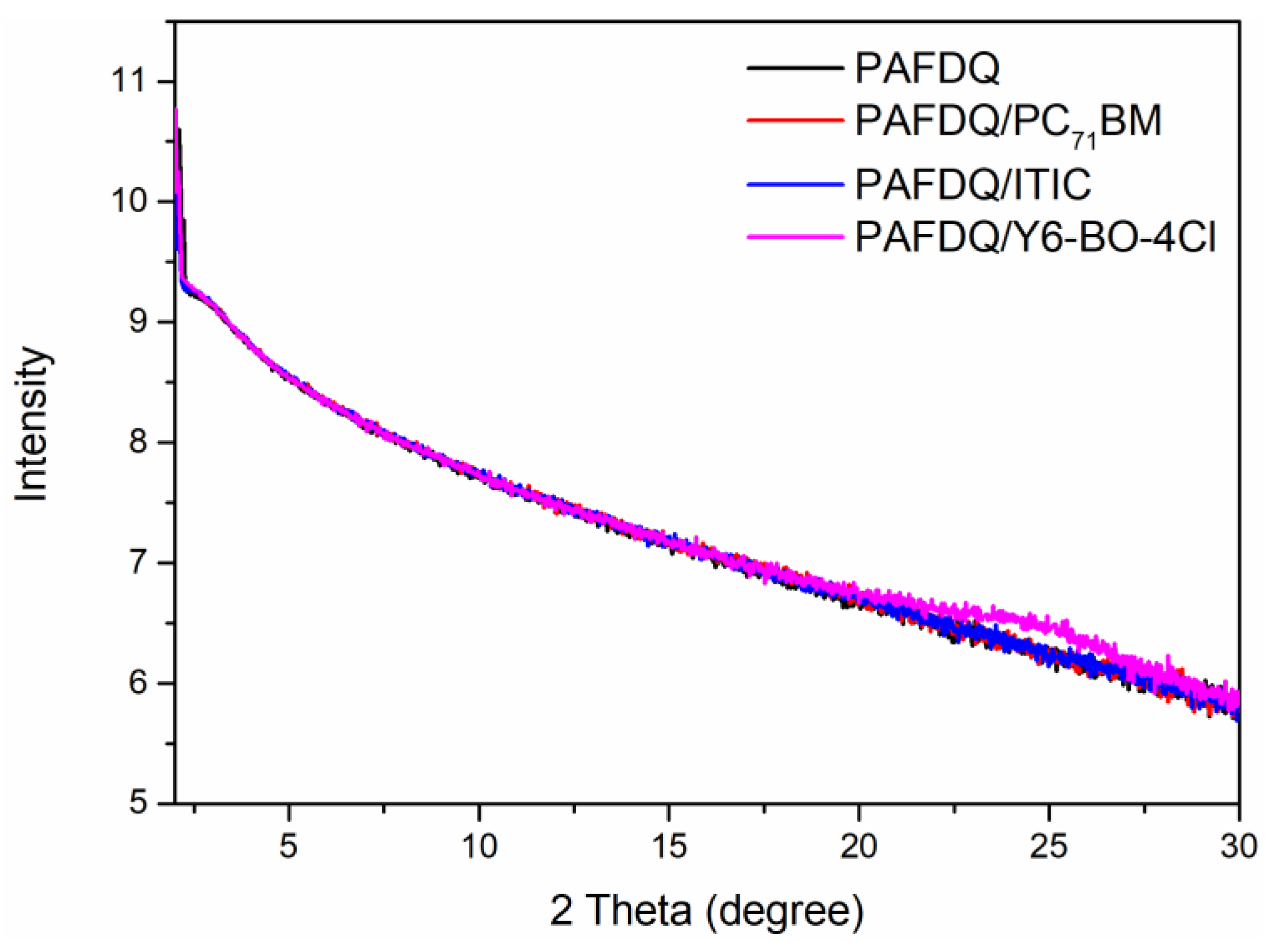
3.5. Morphology Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Dos Santos, D.A.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Breen, C.A.; Tischler, J.R.; Bulović, V.; Swager, T.M. Highly efficient blue electroluminescence from poly(phenylene ethynylene) via energy transfer from a hole-transport matrix. Adv. Mater. 2005, 17, 1981–1985. [Google Scholar] [CrossRef]
- Song, H.J.; Lee, J.Y.; Song, I.S.; Moon, D.K.; Haw, J.R. Synthesis and electroluminescence properties of fluorene-anthracene based copolymers for blue and white emitting diodes. J. Ind. Eng. Chem. 2011, 17, 352–357. [Google Scholar] [CrossRef]
- Krebs, F.C. Polymer solar cell modules prepared using roll-to-roll methods: Knife-over-edge coating, slot-die coating and screen printing. Sol. Energy Mater. Sol. Cells 2009, 93, 465–475. [Google Scholar] [CrossRef]
- Subramaniyan, S.; Xin, H.; Kim, F.S.; Shoaee, S.; Durrant, J.R.; Jenekhe, S.A. Effects of side chains on thiazolothiazole-based copolymer semiconductors for high performance solar cells. Adv. Energy Mater. 2011, 1, 854–860. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, H.; Zhu, Y.; Zheng, R.; Li, T.; Chen, C.; Huang, T.; Zhao, Y.; Wang, R.; Meng, D.; et al. Transparent Hole-Transporting Frameworks: A Unique Strategy to Design High-Performance Semitransparent Organic Photovoltaics. Adv. Mater. 2020, 32, 2003891. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, J.; Zhan, X.; Yang, Y. Constructing High-Performance Organic Photovoltaics via Emerging Non-Fullerene Acceptors and Tandem-Junction Structure. Adv. Energy Mater. 2020, 10, 2000746. [Google Scholar] [CrossRef]
- Kim, D.H.; Seok, W.C.; Leem, J.T.; Han, Y.W.; Kang, J.H.; Song, H.J. Ordered orientation and compact molecule packing due to coplanar backbone structure of interlayer: Improvement in fill factor for photovoltaic device. Eur. Polym. J. 2019, 116, 330–335. [Google Scholar] [CrossRef]
- Manceau, M.; Angmo, D.; Jørgensen, M.; Krebs, F.C. ITO-free flexible polymer solar cells: From small model devices to roll-to-roll processed large modules. Org. Electron. 2011, 12, 566–574. [Google Scholar] [CrossRef]
- Song, H.J.; Kim, D.H.; Lee, E.J.; Moon, D.K. Conjugated polymers consisting of quinacridone and quinoxaline as donor materials for organic photovoltaics: Orientation and charge transfer properties of polymers formed by phenyl structures with a quinoxaline derivative. J. Mater. Chem. A 2013, 1, 6010–6020. [Google Scholar] [CrossRef]
- Song, H.-J.; Kim, D.-H.; Lee, E.-J.; Heo, S.-W.; Lee, J.-Y.; Moon, D.-K. Conjugated Polymer Consisting of Quinacridone and Benzothiadiazole as Donor Materials for Organic Photovoltaics: Coplanar Property of Polymer Backbone. Macromolecules 2012, 45, 7815–7822. [Google Scholar] [CrossRef]
- Zhu, Y.; Champion, R.D.; Jenekhe, S.A. Conjugated donor-acceptor copolymer semiconductors with large intramolecular charge transfer: Synthesis, optical properties, electrochemistry, and field effect carrier mobility of thienopyrazine-based copolymers. Macromolecules 2006, 39, 8712–8719. [Google Scholar] [CrossRef]
- Kuwabara, J.; Nohara, Y.; Choi, S.J.; Fujinami, Y.; Lu, W.; Yoshimura, K.; Oguma, J.; Suenobu, K.; Kanbara, T. Direct arylation polycondensation for the synthesis of bithiophene-based alternating copolymers. Polym. Chem. 2013, 4, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Song, H.J.; Kim, D.H.; Lee, E.J.; Haw, J.R.; Moon, D.K. Correlation of intramolecular charge transfer and orientation properties among quinacridone and acceptor units. Sol. Energy Mater. Sol. Cells 2014, 123, 112–121. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, H.; Zheng, R.; Zhu, Y.; Dai, S.; Li, Z.; Chen, C.; Zhao, Y.; Wang, R.; Meng, D.; et al. Enabling High-Performance Tandem Organic Photovoltaic Cells by Balancing the Front and Rear Subcells. Adv. Mater. 2020, 32, 2002315. [Google Scholar] [CrossRef]
- Osaka, I.; Akita, M.; Koganezawa, T.; Takimiya, K. Quinacridone-based semiconducting polymers: Implication of electronic structure and orientational order for charge transport property. Chem. Mater. 2012, 24, 1235–1243. [Google Scholar] [CrossRef]
- Lin, C.J.; Lee, W.Y.; Lu, C.; Lin, H.W.; Chen, W.C. Biaxially extended thiophene-fused thiophene conjugated copolymers for high performance field effect transistors. Macromolecules 2011, 44, 9565–9573. [Google Scholar] [CrossRef]
- Jiang, J.M.; Yang, P.A.; Hsieh, T.H.; Wei, K.H. Crystalline low-band gap polymers comprising thiophene and 2,1,3-benzooxadiazole units for bulk heterojunction solar cells. Macromolecules 2011, 44, 9155–9163. [Google Scholar] [CrossRef]
- Song, K.W.; Song, H.J.; Lee, T.H.; Heo, S.W.; Moon, D.K. An effect on the side chain position of D-π-A-type conjugated polymers with sp2-hybridized orbitals for organic photovoltaics. Polym. Chem. 2013, 4, 3225–3235. [Google Scholar] [CrossRef]
- Liu, J.; Choi, H.; Kim, J.Y.; Bailey, C.; Durstock, M.; Dai, L. Highly crystalline and low bandgap donor polymers for efficient polymer solar cells. Adv. Mater. 2012, 24, 538–542. [Google Scholar] [CrossRef]
- Lee, E.J.; Heo, S.W.; Han, Y.W.; Moon, D.K. An organic–inorganic hybrid interlayer for improved electron extraction in inverted polymer solar cells. J. Mater. Chem. C 2016, 4, 2463–2469. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.-G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef]
- Yu, R.; Yao, H.; Cui, Y.; Hong, L.; He, C.; Hou, J. Improved Charge Transport and Reduced Nonradiative Energy Loss Enable over 16% Efficiency in Ternary Polymer Solar Cells. Adv. Mater. 2019, 31, 1902302. [Google Scholar] [CrossRef]
- Chang, Y.; Lau, T.K.; Pan, M.A.; Lu, X.; Yan, H.; Zhan, C. The synergy of host-guest nonfullerene acceptors enables 16%-efficiency polymer solar cells with increased open-circuit voltage and fill-factor. Mater. Horizons 2019, 6, 2094–2102. [Google Scholar] [CrossRef]
- Coropceanu, V.; Chen, X.K.; Wang, T.; Zheng, Z.; Brédas, J.L. Charge-transfer electronic states in organic solar cells. Nat. Rev. Mater. 2019, 4, 689–707. [Google Scholar] [CrossRef]



| Polymer | Mn (kg/mol) | Mw (kg/mol) | PDI | Td (°C) | Absorption, λmax (nm) | Eoxonset (V) | EHOMO (eV) a | ELUMO (eV) b | Eopt (eV) e | |
|---|---|---|---|---|---|---|---|---|---|---|
| Solution a | Film b | |||||||||
| PAFDQ | 13,200 | 36,400 | 2.74 | 385 | 256, 284, 362, 516 | 259, 286, 365, 529 | 0.99 | −5.37 | −3.42 | 1.95 |
| Active Layer | Weight Ratio (P:A, w/w) | VOC (V) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| Polymer: Acceptor | |||||
| PAFDQ: PC71BM | 1:2 | 0.86 | 4.97 | 51.63 | 2.21 |
| average | 0.85 ± 0.02 | 4.83 ± 0.09 | 51.66 ± 0.64 | 2.11 ± 0.06 | |
| PAFDQ: ITIC | 1:1 | 0.96 | 3.94 | 31.56 | 1.20 |
| average | 0.93 ± 0.02 | 3.77 ± 0.12 | 32.10 ± 1.63 | 1.12 ± 0.06 | |
| PAFDQ: Y6-BO-4Cl | 1:1 | 0.76 | 9.86 | 44.23 | 3.32 |
| average | 0.75 ± 0.01 | 9.63 ± 0.34 | 44.20 ± 1.52 | 3.18 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.J.; Song, H.J. Donor-Acceptor Polymer Based on Planar Structure of Alkylidene-Fluorene Derivative: Correlation of Power Conversion Efficiency among Polymer and Various Acceptor Units. Polymers 2020, 12, 2859. https://doi.org/10.3390/polym12122859
Lee EJ, Song HJ. Donor-Acceptor Polymer Based on Planar Structure of Alkylidene-Fluorene Derivative: Correlation of Power Conversion Efficiency among Polymer and Various Acceptor Units. Polymers. 2020; 12(12):2859. https://doi.org/10.3390/polym12122859
Chicago/Turabian StyleLee, Eui Jin, and Ho Jun Song. 2020. "Donor-Acceptor Polymer Based on Planar Structure of Alkylidene-Fluorene Derivative: Correlation of Power Conversion Efficiency among Polymer and Various Acceptor Units" Polymers 12, no. 12: 2859. https://doi.org/10.3390/polym12122859
APA StyleLee, E. J., & Song, H. J. (2020). Donor-Acceptor Polymer Based on Planar Structure of Alkylidene-Fluorene Derivative: Correlation of Power Conversion Efficiency among Polymer and Various Acceptor Units. Polymers, 12(12), 2859. https://doi.org/10.3390/polym12122859





