Defluorination of Polytetrafluoroethylene Surface by Hydrogen Plasma
Abstract
1. Introduction
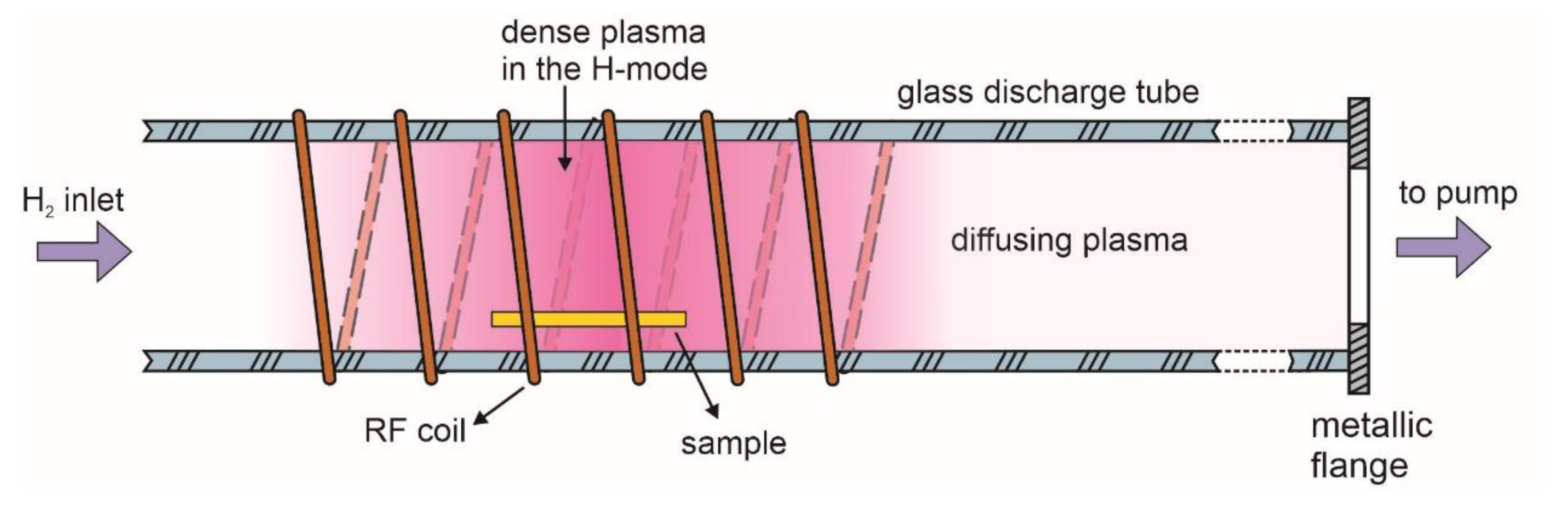
2. Materials and Methods
3. Results and Discussion
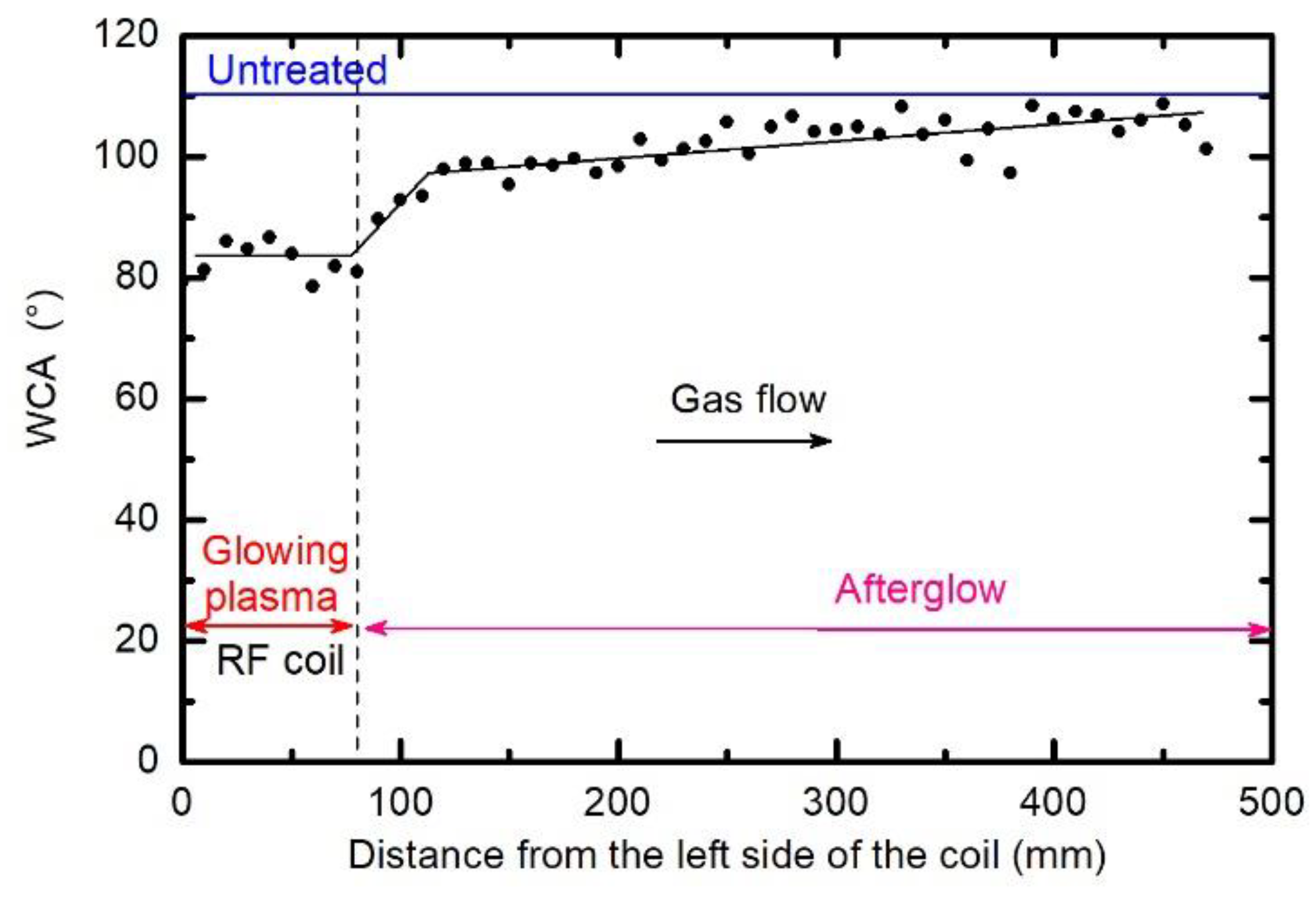
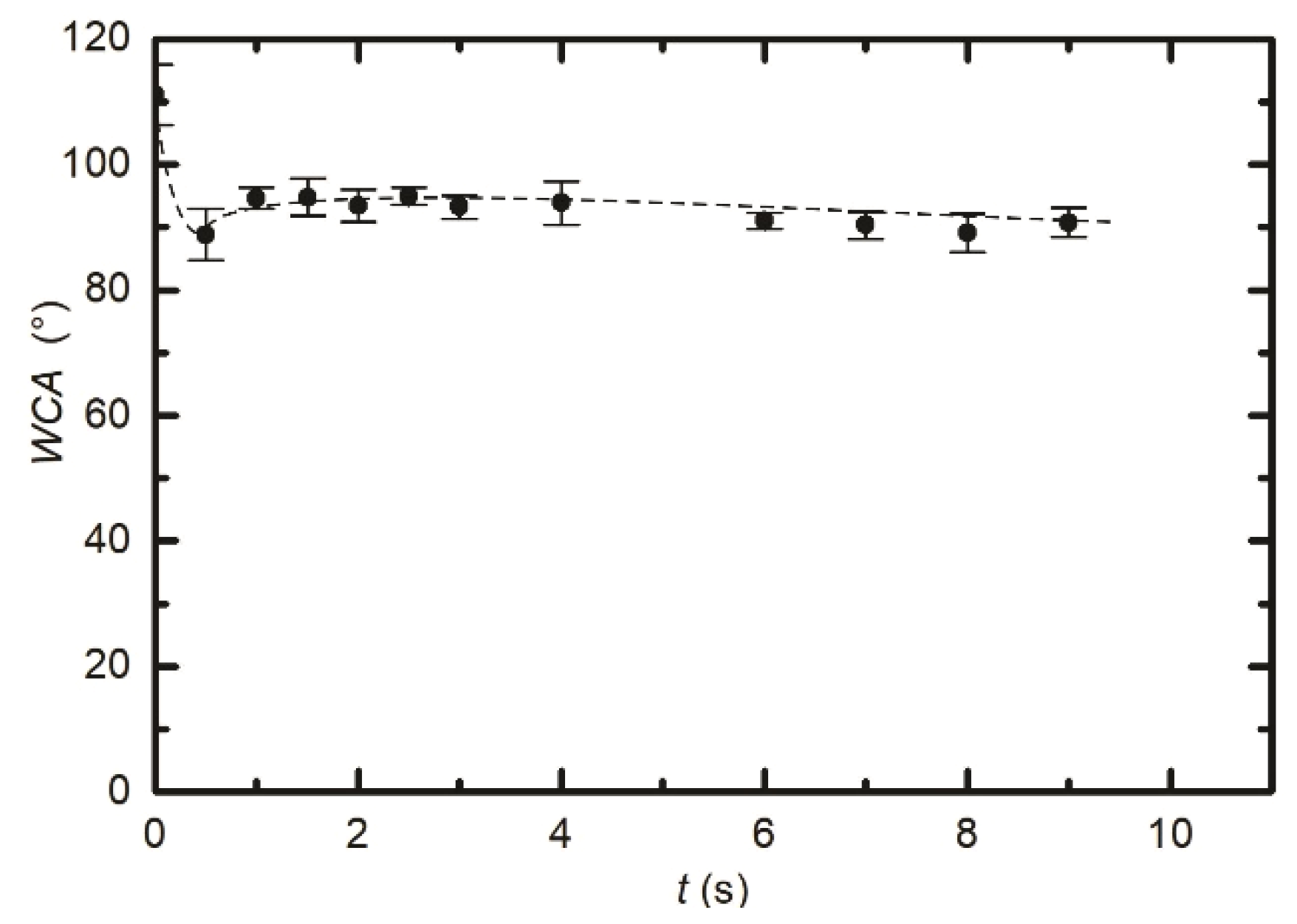
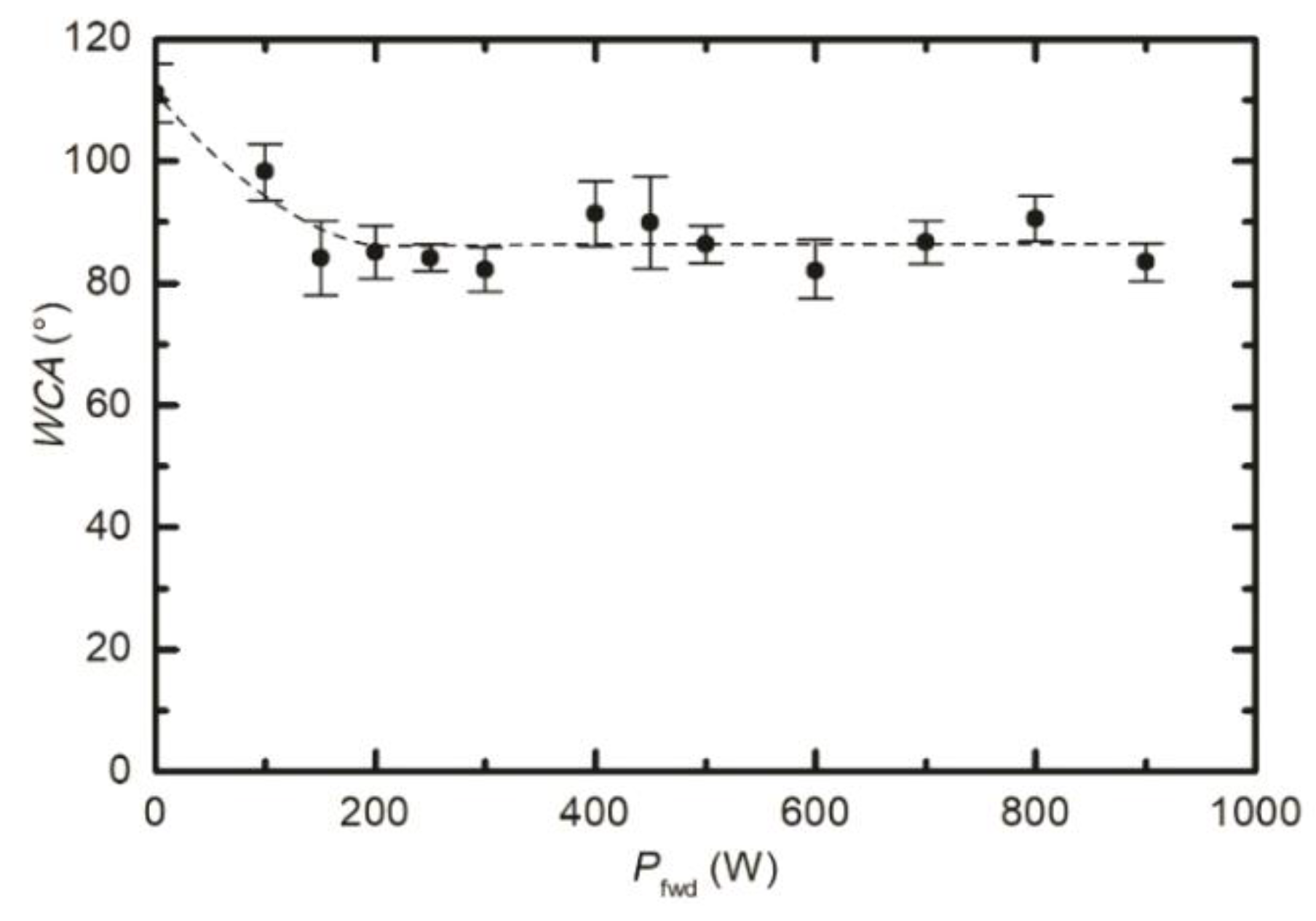
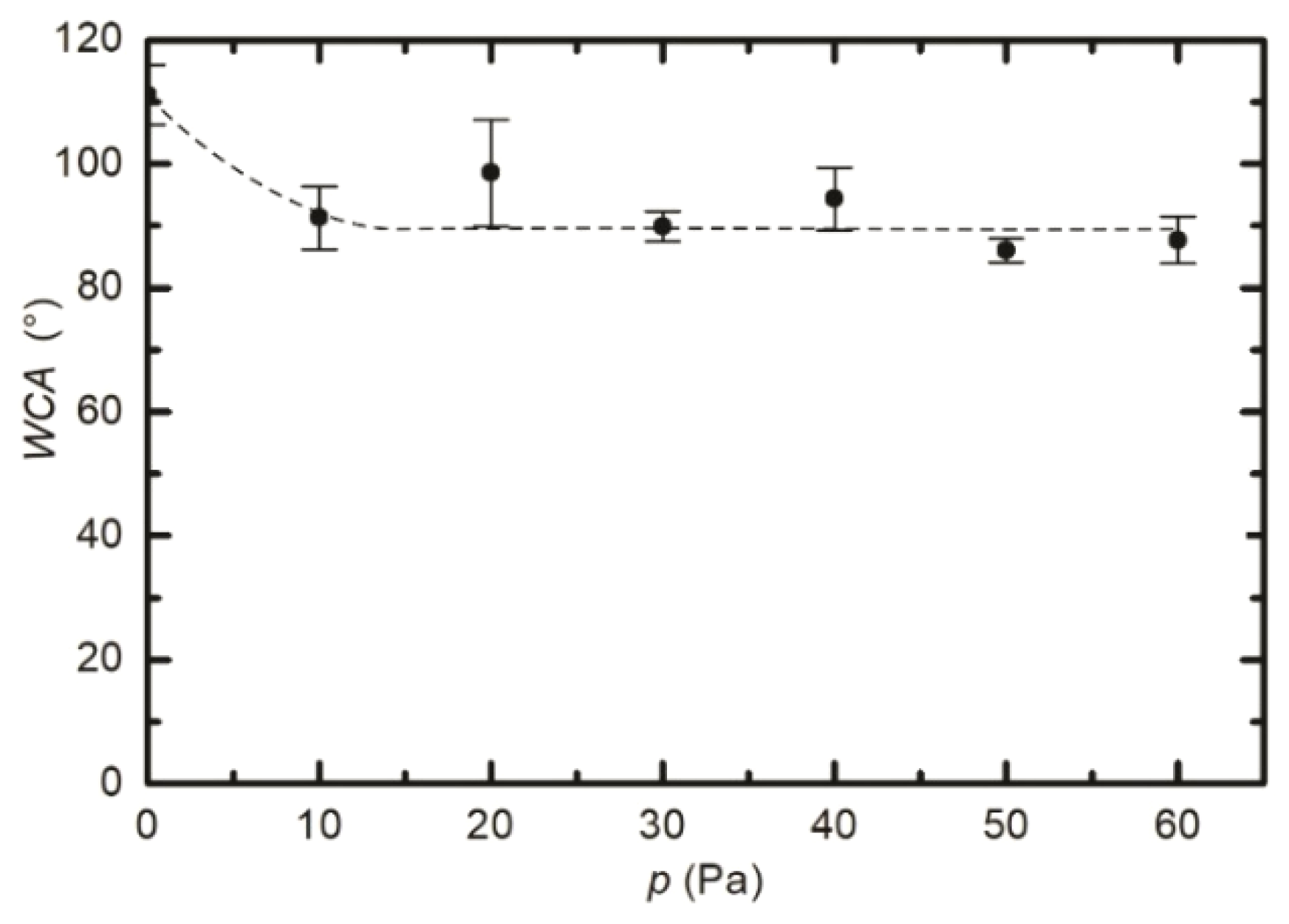
3.1. Modification of the Surface Wettability
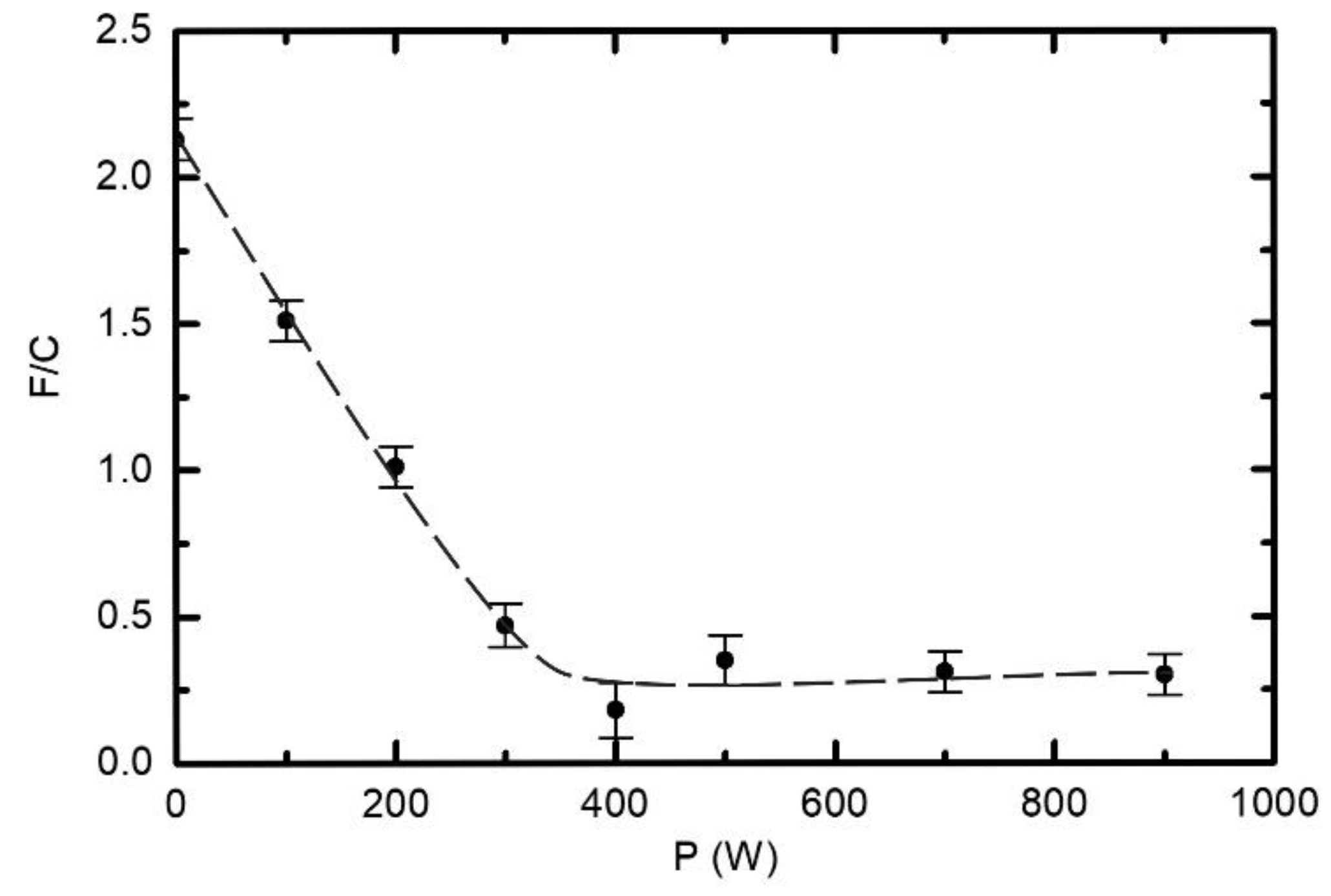
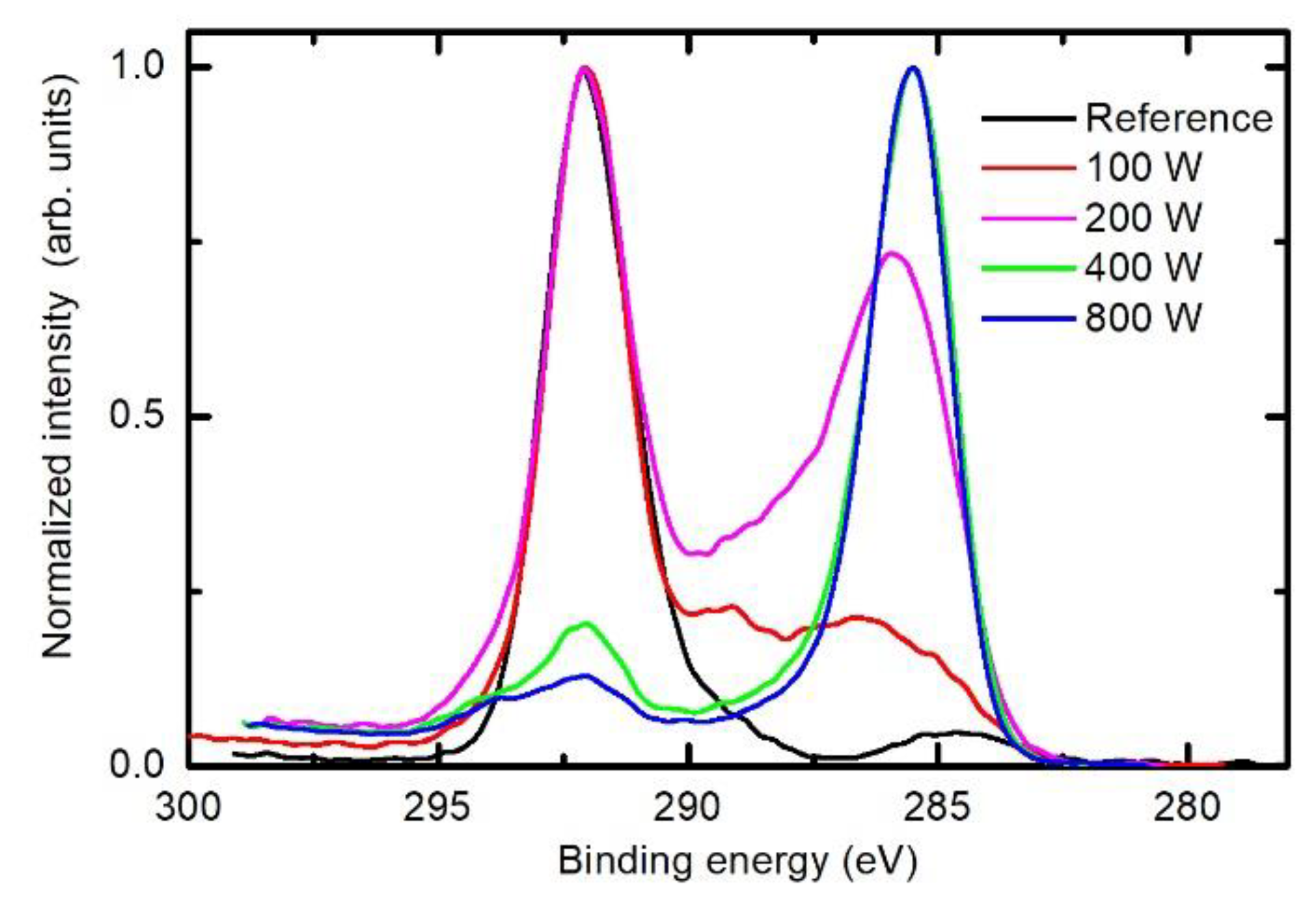
3.2. Chemical Modifications as Determined by X-ray Photoelectron Spectroscopy (XPS)
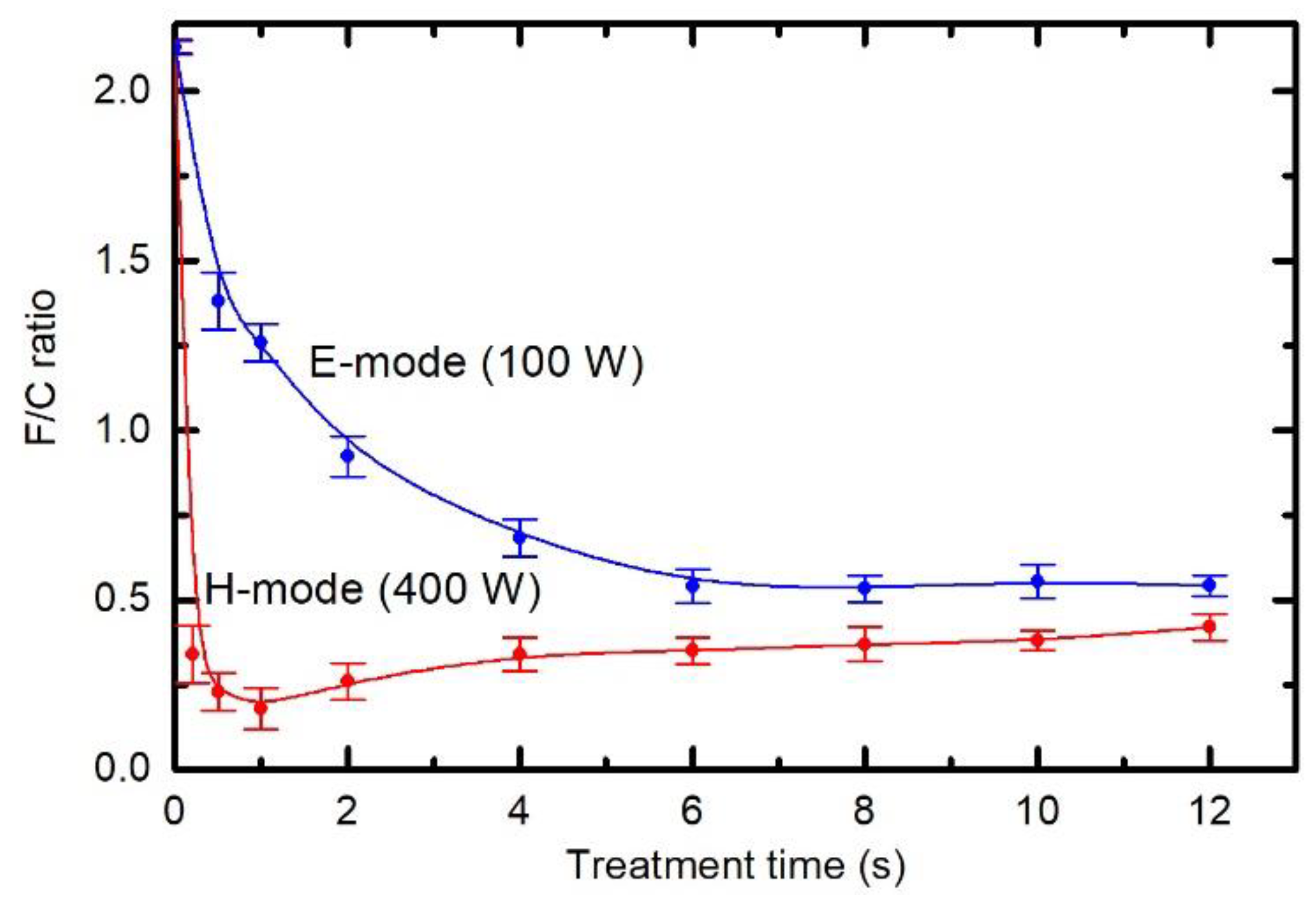
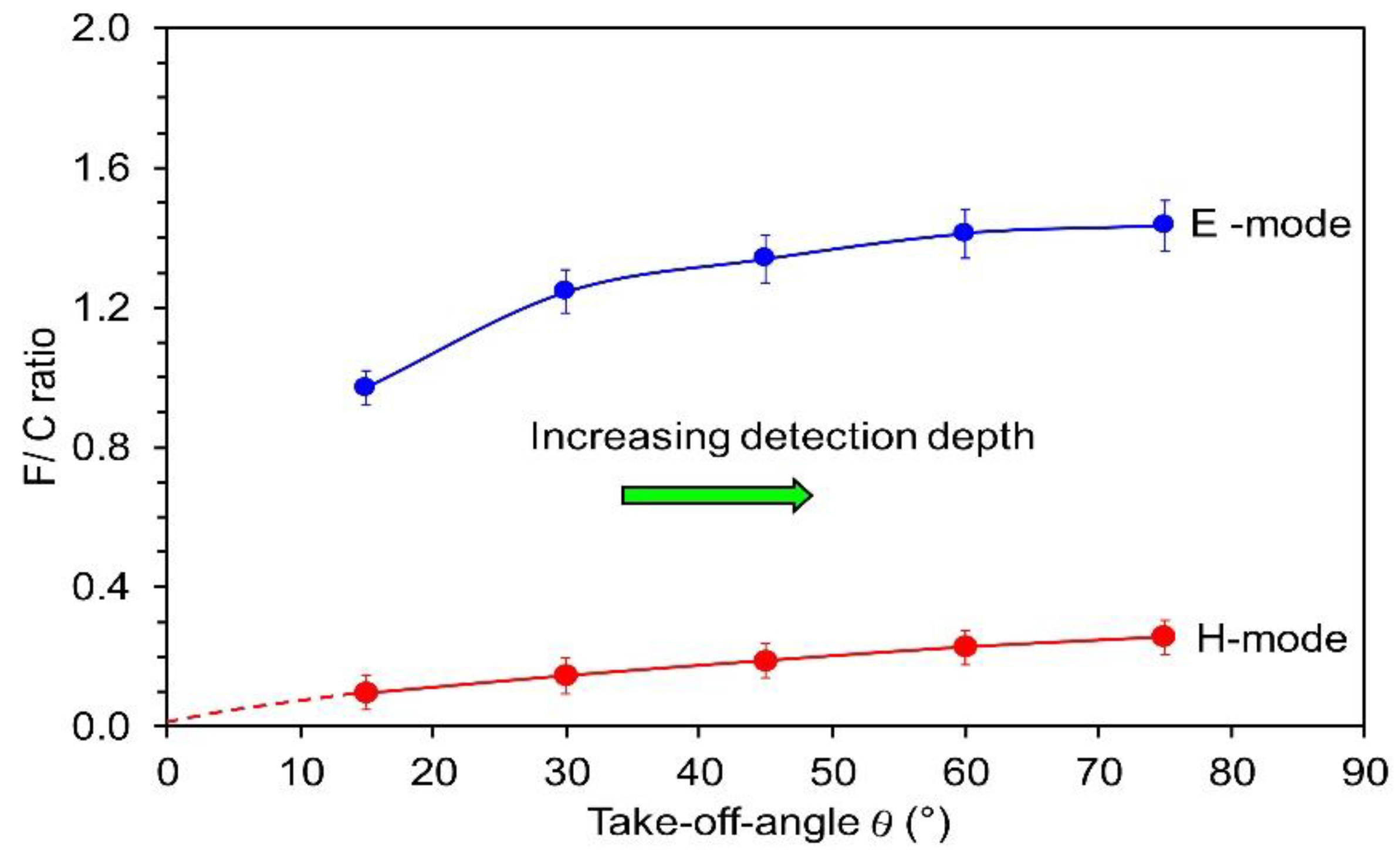
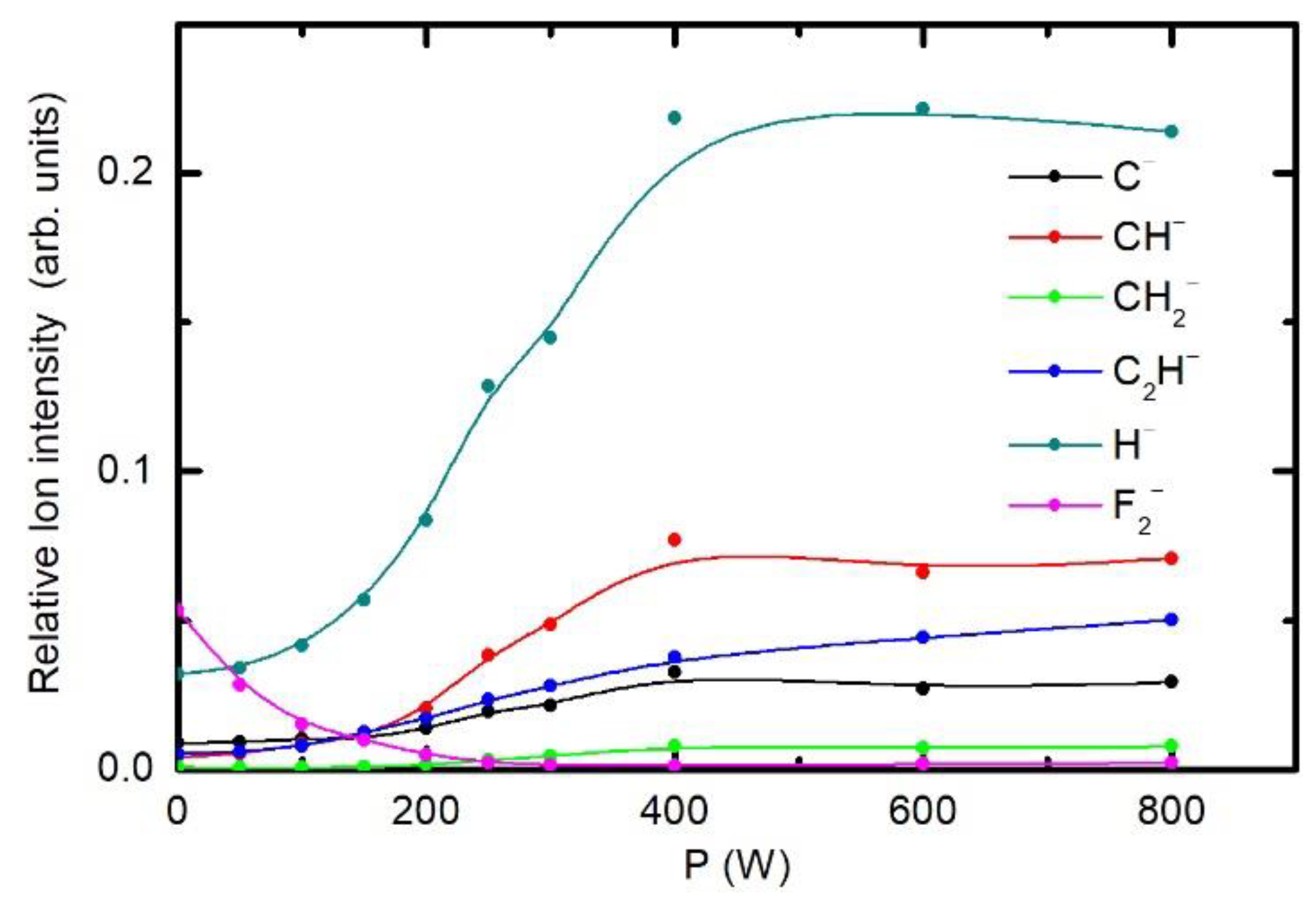
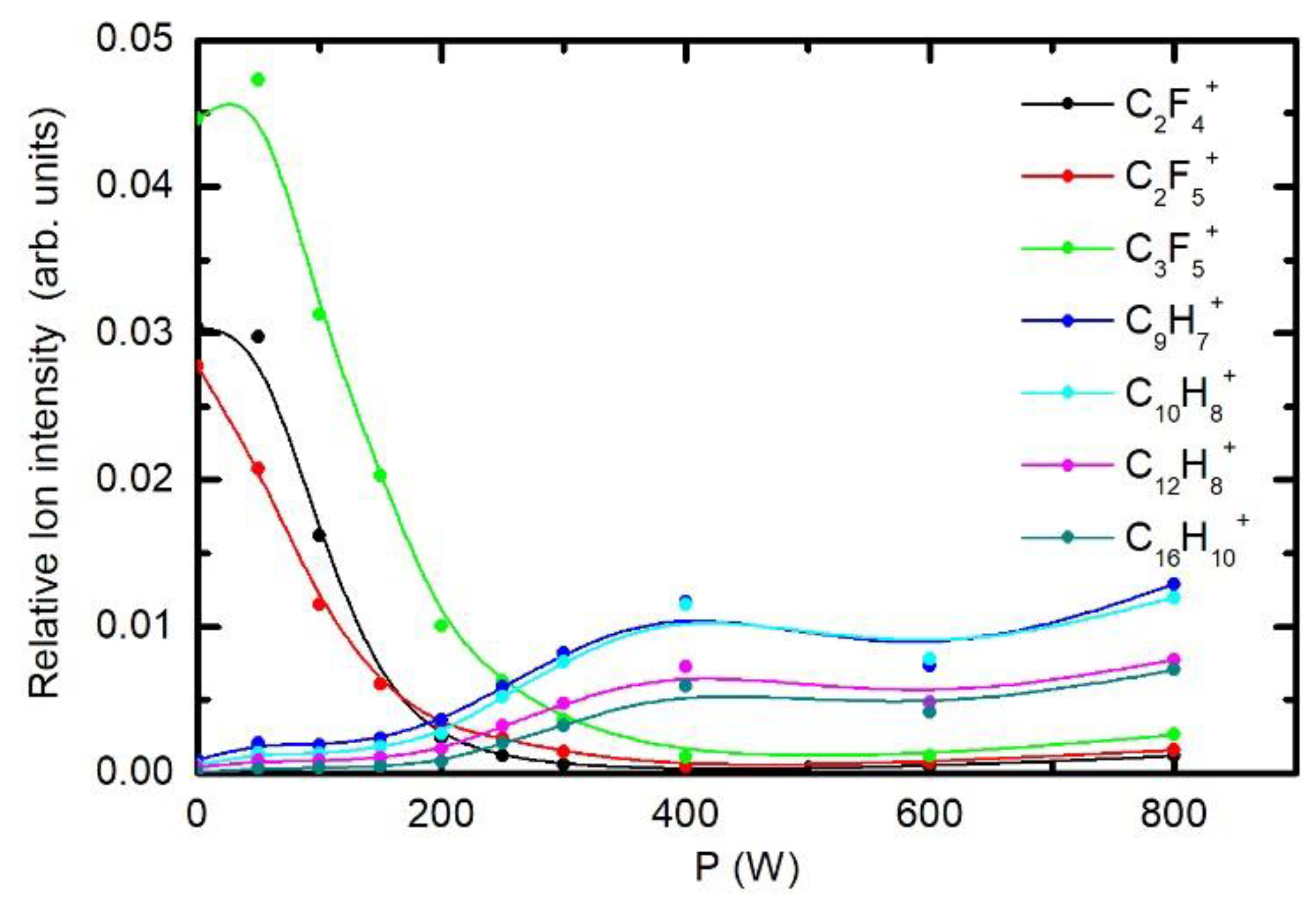
3.3. Chemical Modifications as Determined by Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Améduri, B. The promising future of fluoropolymers. Macromol. Chem. Phys. 2020, 221, 1900573. [Google Scholar] [CrossRef]
- McKeen, L.W. Chapter 11—Fluoropolymers. In Fatigue and Tribological Properties of Plastics and Elastomers, 2nd ed.; McKeen, L.W., Ed.; William Andrew Publishing: Oxford, UK, 2010; pp. 249–264. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Shibahara, M.; Nagatani, A.; Honda, K.; Endo, K.; Yamamura, K. Comparison between adhesion properties of adhesive bonding and adhesive-free adhesion for heat-assisted plasma-treated polytetrafluoroethylene (PTFE). J. Adhes. 2020, 96, 776–796. [Google Scholar] [CrossRef]
- Badey, J.P.; Espuche, E.; Jugnet, Y.; Chabert, B.; Duct, T.M. Influence of chemical and plasma treatments on the adhesive properties of PTFE with an epoxy resin. Int. J. Adhes. Adhes. 1996, 16, 173–178. [Google Scholar] [CrossRef]
- Lee, S.W.; Hong, J.W.; Wye, M.Y.; Kim, J.H.; Kang, H.J.; Lee, Y.S. Surface modification and adhesion improvement of PTFE film by ion beam irradiation. Nucl. Instrum. Methods Phys. Res. B 2004, 219, 963–967. [Google Scholar] [CrossRef]
- Baumgärtner, K.M.; Schneider, J.; Schulz, A.; Feichtinger, J.; Walker, M. Short-time plasma pre-treatment of polytetrafluoroethylene for improved adhesion. Surf. Coat. Technol. 2001, 142, 501–506. [Google Scholar] [CrossRef]
- Koh, S.-K.; Park, S.-C.; Kim, S.-R.; Choi, W.-K.; Jung, H.-J.; Pae, K.D. Surface modification of polytetrafluoroethylene by Ar+ irradiation for improved adhesion to other materials. J. Appl. Polym. Sci. 1997, 64, 1913–1921. [Google Scholar] [CrossRef]
- Hopp, B.; Kresz, N.; Kokavecz, J.; Smausz, T.; Schieferdecker, H.; Döring, A.; Marti, O.; Bor, Z. Adhesive and morphological characteristics of surface chemically modified polytetrafluoroethylene films. Appl. Surf. Sci. 2004, 221, 437–443. [Google Scholar] [CrossRef]
- Ohkubo, Y.; Ishihara, K.; Shibahara, M.; Nagatani, A.; Honda, K.; Endo, K.; Yamamura, K. Drastic improvement in adhesion property of polytetrafluoroethylene (PTFE) via heat-assisted plasma treatment using a heater. Sci. Rep. 2017, 7, 9476. [Google Scholar] [CrossRef]
- Gilman, A.; Piskarev, M.; Yablokov, M.; Kechek’Yan, A.; Kuznetsov, A.A. Adhesive properties of PTFE modified by DC discharge. J. Phys. Conf. Ser. 2014, 516, 012012. [Google Scholar] [CrossRef]
- Marchesi, J.T.; Ha, K.; Garton, A.; Swei, G.S.; Kristal, K.W. Adhesion to sodium naphthalenide treated fluoropolymers. Part II. Effects of treatment conditions and fluoropolymer structure. J. Adhes. 1991, 36, 55–69. [Google Scholar] [CrossRef]
- Kavan, L.; Dousek, F.P.; Janda, P.; Weber, J. Carbonization of highly oriented poly(tetrafluoroethylene). Chem. Mater. 1999, 11, 329–335. [Google Scholar] [CrossRef]
- Ha, K.; McClain, S.; Suib, S.L.; Garton, A. Adhesion to sodium naphthalenide treated fluoropolymers Part I-Analytical methodology. J. Adhes. 1991, 33, 169–184. [Google Scholar] [CrossRef]
- Clark, D.T.; Hutton, D.R. Surface modification by plasma techniques. 1. The interactions of a hydrogen plasma with fluoropolymer surfaces. J. Polym. Sci. Part A Polym. Chem. 1987, 25, 2643–2664. [Google Scholar] [CrossRef]
- Wang, L.; Angert, N.; Trautmann, C.; Vetter, J. Effect of ion irradiation and heat treatment on adhesion in the Cu / Teflon system. J. Adhes. Sci. Technol. 1995, 9, 1523–1529. [Google Scholar] [CrossRef]
- Takata, R.; Iwao, T.; Yumoto, M. Surface modification of PTFE using low-energy nitrogen ion irradiation: Improvement in adhesive strength on modification of deep modifying layer. Electron. Commun. Jpn. 2016, 99, 93–99. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M. New developments in surface functionalization of polymers using controlled plasma treatments. J. Phys. D Appl. Phys. 2017, 50, 293001. [Google Scholar] [CrossRef]
- Primc, G. Recent advances in surface activation of polytetrafluoroethylene (PTFE) by gaseous plasma treatments. Polymers 2020, 12, 2295. [Google Scholar] [CrossRef]
- Badey, J.P.; Espuche, E.; Sage, D.; Chabert, B.; Jugnet, Y.; Batier, C.; Duc, T.M. A comparative study of the effects of ammonia and hydrogen plasma downstream treatment on the surface modification of polytetrafluoroethylene. Polymer 1996, 37, 1377–1386. [Google Scholar] [CrossRef]
- Yamada, Y.; Yamada, T.; Tasaka, S.; Inagaki, N. Surface modification of poly(tetrafluoroethylene) by remote hydrogen plasma. Macromolecules 1996, 29, 4331–4339. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Umehara, T. Effects of surface modification by remote hydrogen plasma on adhesion in poly(tetrafluoroethylene)/copper composites. J. Appl. Polym. Sci. 1999, 71, 2191–2200. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Narushima, K.; Teranishi, K. Surface modification of poly(tetrafluoroethylene) with pulsed hydrogen plasma. J. Appl. Polym. Sci. 2002, 83, 340–348. [Google Scholar] [CrossRef]
- König, U.; Nitschke, M.; Pilz, M.; Simon, F.; Arnhold, C.; Werner, C. Stability and ageing of plasma treated poly(tetrafluoroethylene) surfaces. Colloid. Surf. B 2002, 25, 313–324. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, K.; Kogoma, M. Defluorination of polytetrafluoroethylene by combination of atmospheric pressure glow plasma treatment and chemical transport method. In Proceedings of the 20th International Symposium on Plasma Chemistry, Philadelphia, PA, USA, 24–29 July 2011. [Google Scholar]
- Hunke, H.; Soin, N.; Shah, T.; Kramer, E.; Pascual, A.; Karuna, M.; Siores, E. Low-pressure H2, NH3 microwave plasma treatment of polytetrafluoroethylene (PTFE) powders: Chemical, thermal and wettability analysis. Materials 2015, 8, 2258–2275. [Google Scholar] [CrossRef]
- Zanini, S.; Barni, R.; Pergola, R.D.; Riccardi, C. Modification of the PTFE wettability by oxygen plasma treatments: Influence of the operating parameters and investigation of the ageing behaviour. J. Phys. D Appl. Phys. 2014, 47, 325202. [Google Scholar] [CrossRef]
- Abourayana, H.M.; Dowling, D.P. Plasma Processing for Tailoring the Surface Properties of Polymers. In Surface Energy; Aliofkhazraei, M., Ed.; Intech Open: Rijeka, Croatia, 2015. [Google Scholar]
- Wilken, R.; Holländer, A.; Behnisch, J. Quantitative comparison between vacuum-ultraviolet irradiation and remote hydrogen plasma treatment of hydrocarbon polymers. Plasmas Polym. 1998, 3, 165–175. [Google Scholar] [CrossRef]
- Fantz, U.; Briefi, S.; Rauner, D.; Wünderlich, D. Quantification of the VUV radiation in low pressure hydrogen and nitrogen plasmas. Plasma Sources Sci. Technol. 2016, 25, 045006. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Lopes da Silva, J.D.; Botelho do Rego, A.M. XPS studies of directly fluorinated HDPE: Problems and solutions. Polymer 2003, 44, 7241–7249. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesel, A.; Lojen, D.; Zaplotnik, R.; Primc, G.; Mozetič, M.; Ekar, J.; Kovač, J.; Gorjanc, M.; Kurečič, M.; Stana-Kleinschek, K. Defluorination of Polytetrafluoroethylene Surface by Hydrogen Plasma. Polymers 2020, 12, 2855. https://doi.org/10.3390/polym12122855
Vesel A, Lojen D, Zaplotnik R, Primc G, Mozetič M, Ekar J, Kovač J, Gorjanc M, Kurečič M, Stana-Kleinschek K. Defluorination of Polytetrafluoroethylene Surface by Hydrogen Plasma. Polymers. 2020; 12(12):2855. https://doi.org/10.3390/polym12122855
Chicago/Turabian StyleVesel, Alenka, Dane Lojen, Rok Zaplotnik, Gregor Primc, Miran Mozetič, Jernej Ekar, Janez Kovač, Marija Gorjanc, Manja Kurečič, and Karin Stana-Kleinschek. 2020. "Defluorination of Polytetrafluoroethylene Surface by Hydrogen Plasma" Polymers 12, no. 12: 2855. https://doi.org/10.3390/polym12122855
APA StyleVesel, A., Lojen, D., Zaplotnik, R., Primc, G., Mozetič, M., Ekar, J., Kovač, J., Gorjanc, M., Kurečič, M., & Stana-Kleinschek, K. (2020). Defluorination of Polytetrafluoroethylene Surface by Hydrogen Plasma. Polymers, 12(12), 2855. https://doi.org/10.3390/polym12122855









