Effect of Sulfur Variation on the Vulcanizate Structure of Silica-Filled Styrene-Butadiene Rubber Compounds with a Sulfide–Silane Coupling Agent
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
2.3. Preparation of Vulcanizates
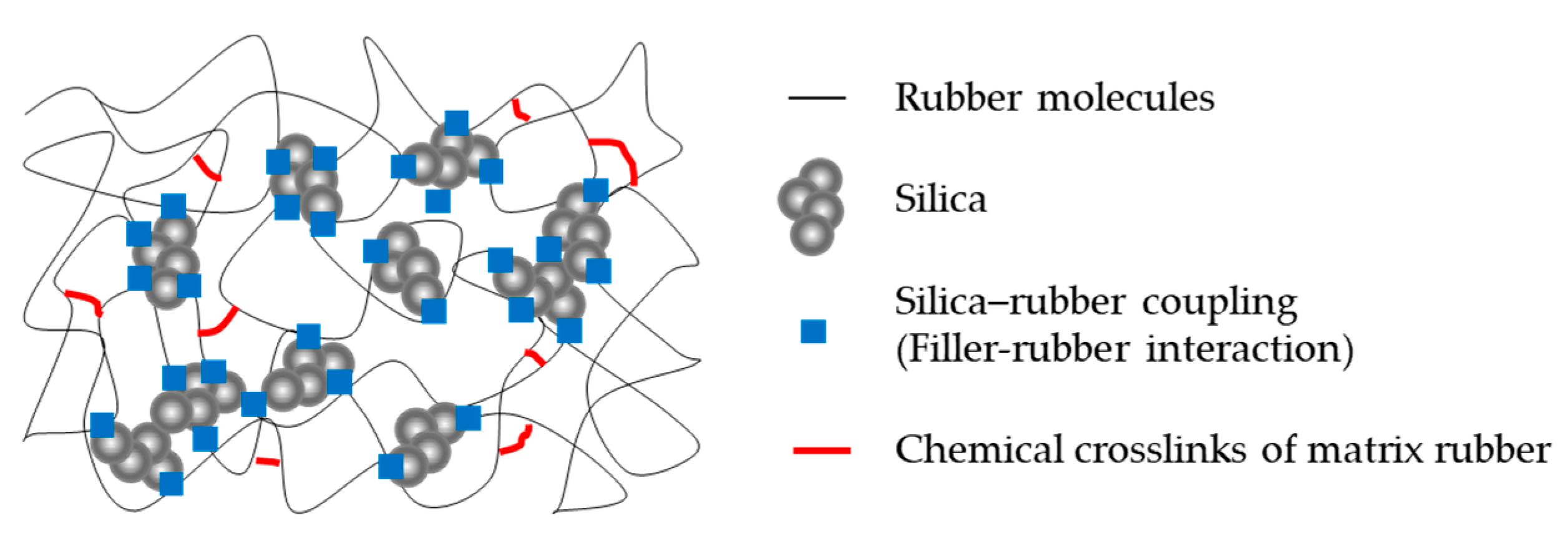
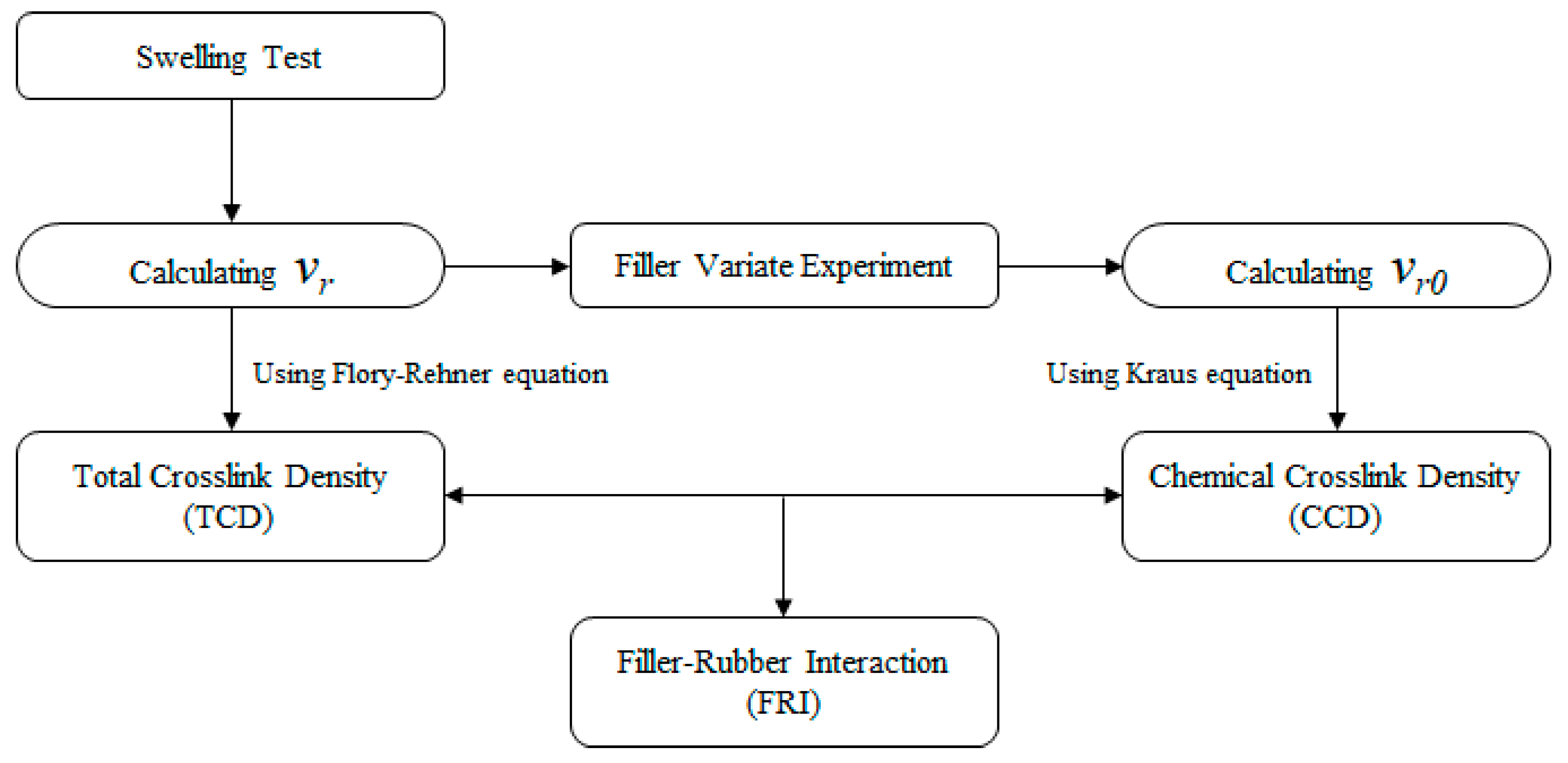
2.4. Analysis of Vulcanizate Structure
3. Results and Discussion
3.1. Vulcanization Characteristics
3.2. Analysis of Vulcanizate Structure
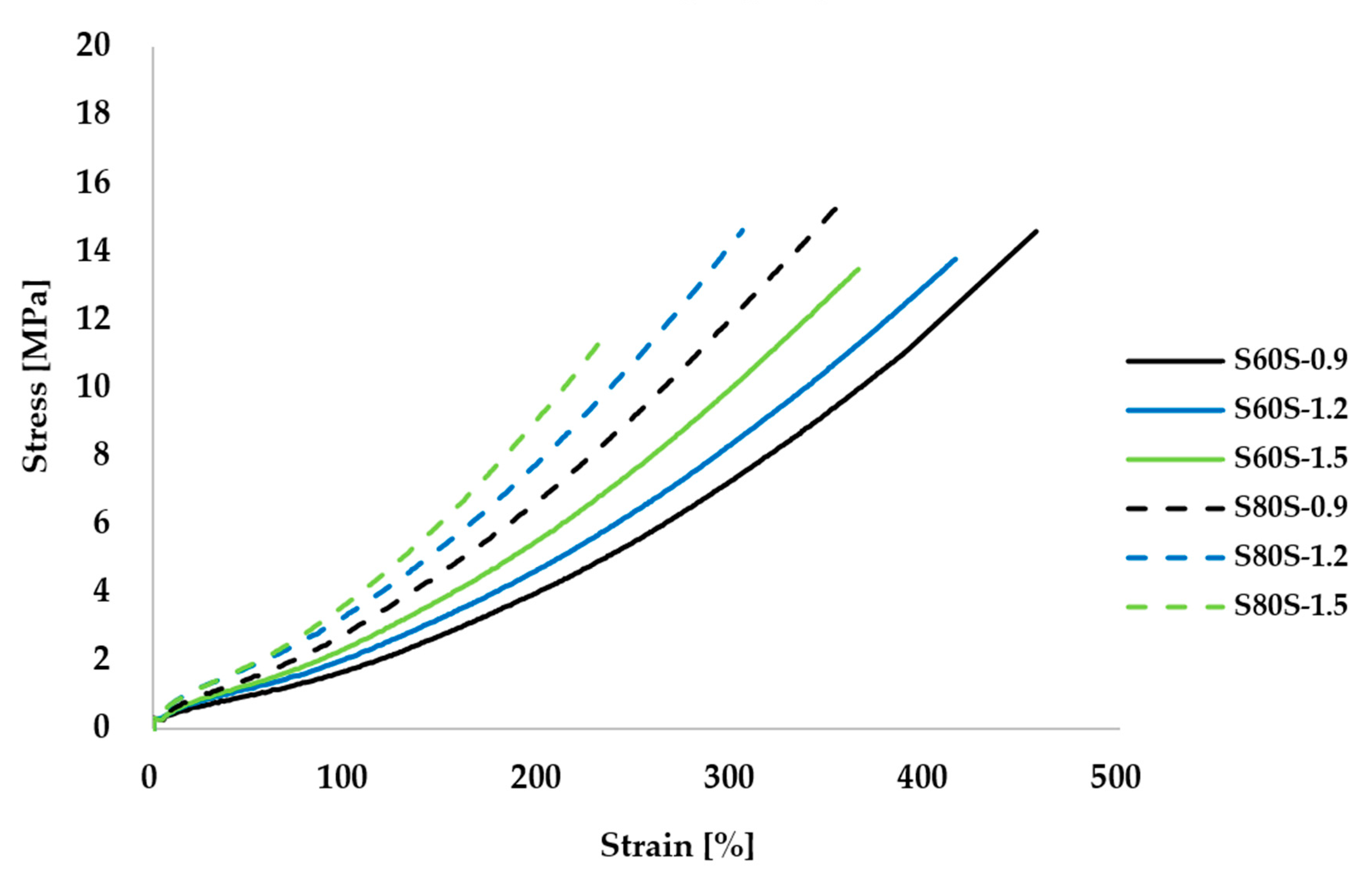
3.3. Effect of Sulfur Variation on Mechanical Properties
3.4. Filler Dispersity of Vulcanizates using FE-SEM-EDX Analysis
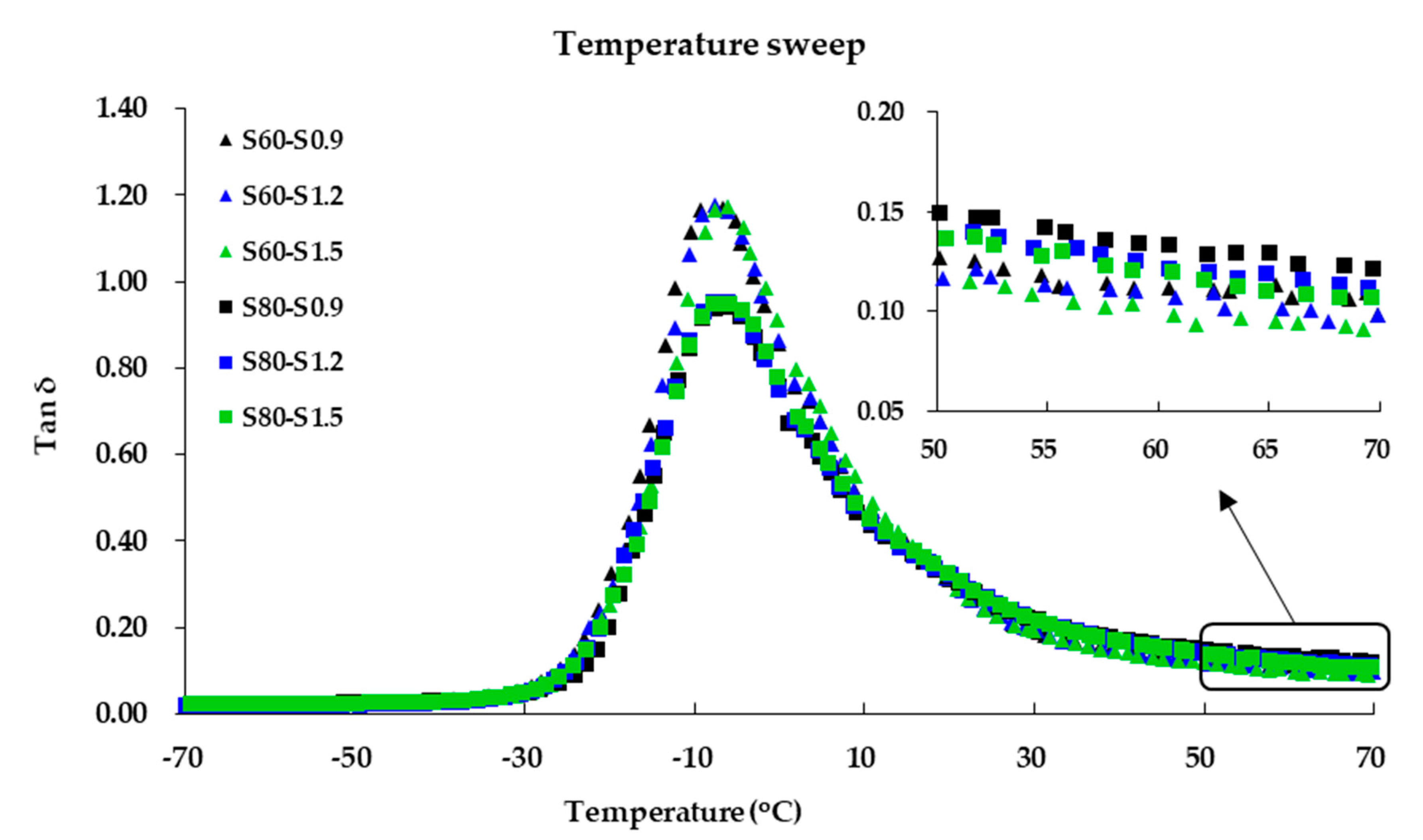
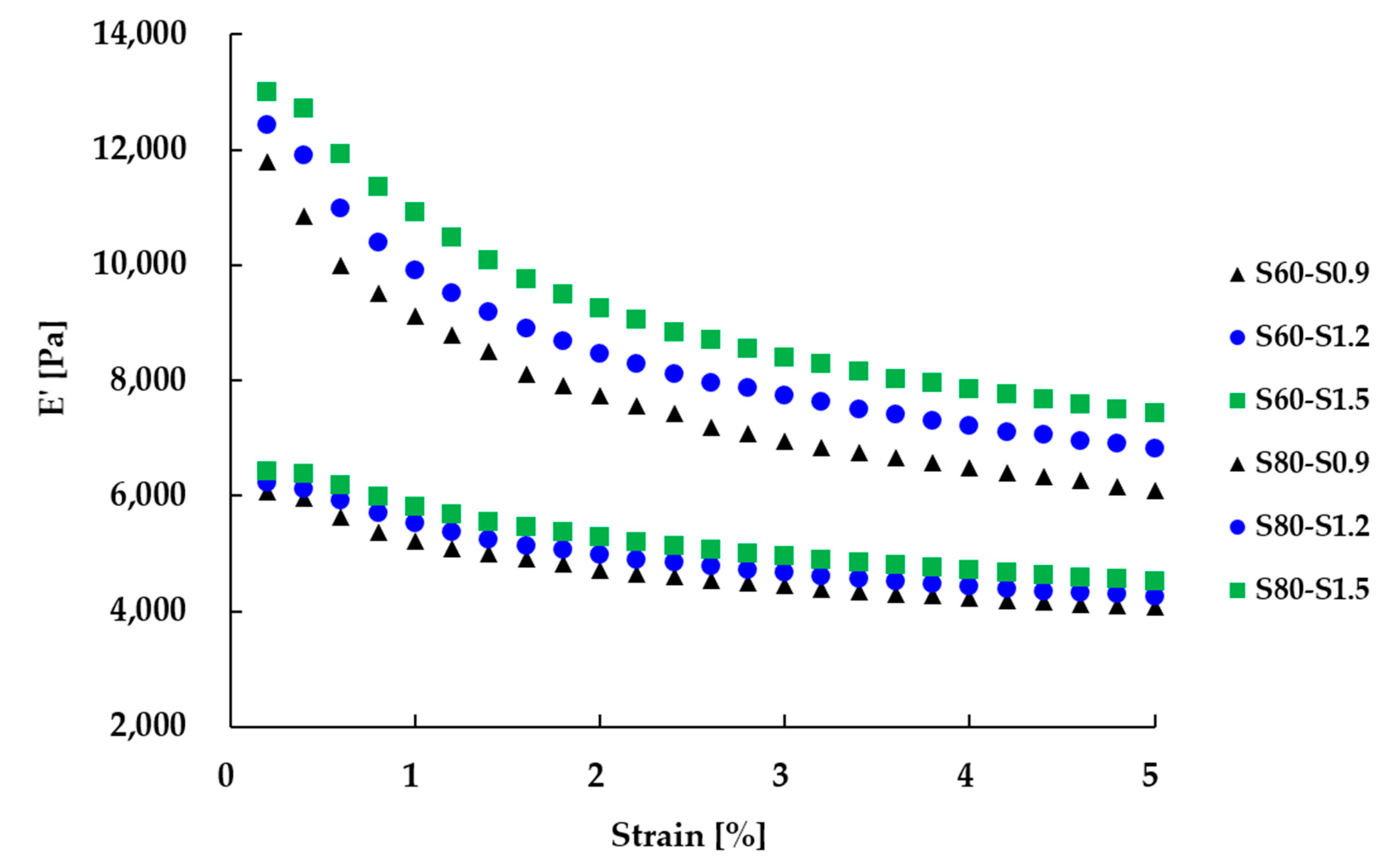
3.5. Viscoelastic Properties
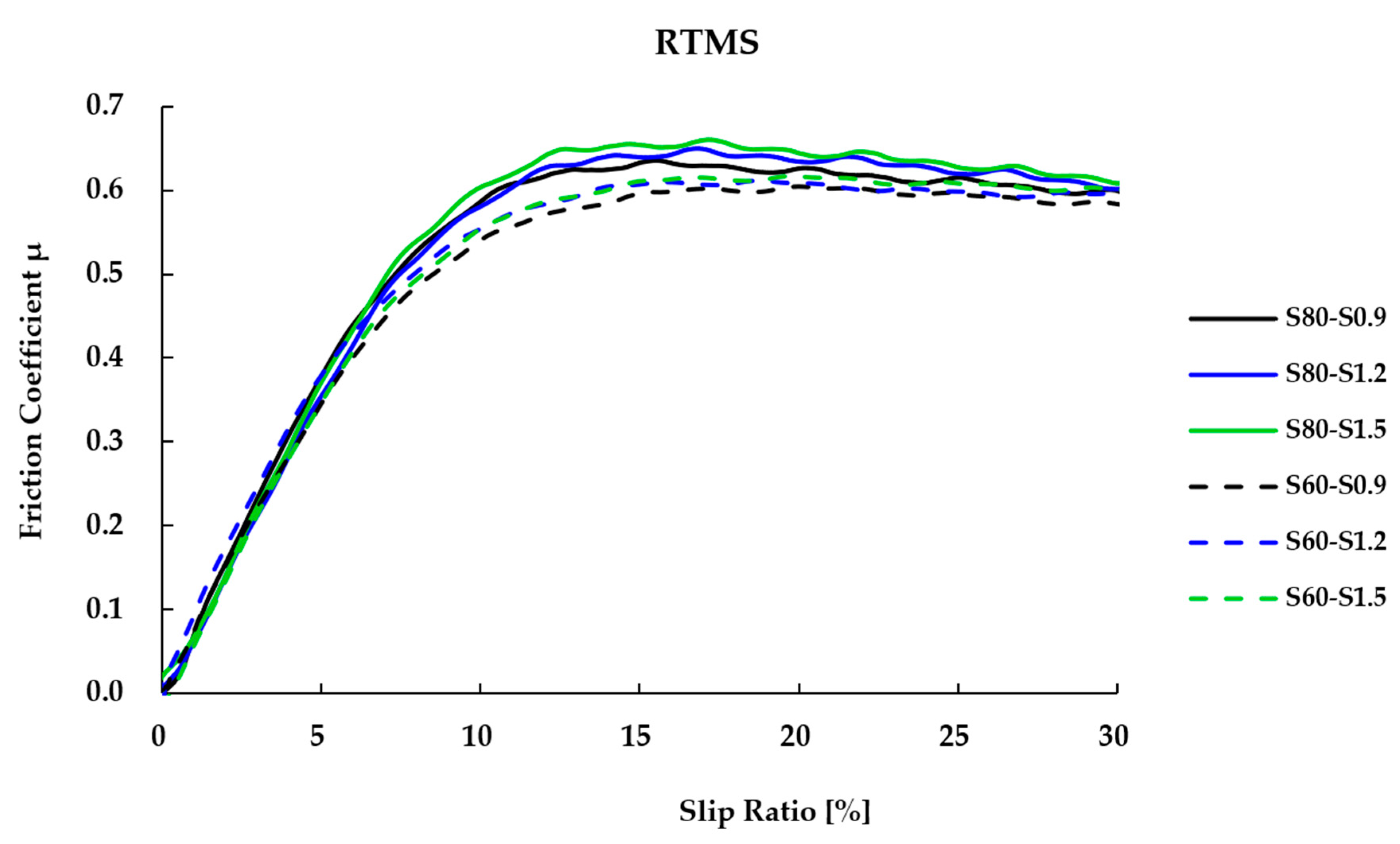
3.6. Wet Friction Coefficient of Vulcanizates Using a Rotational Traction Measuring System (RTMS)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maghami, S. Silica-Filled Tire Tread Compounds: An Investigation into the Viscoelastic Properties of the Rubber Compounds and Their Relation to Tire Performance. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2016. [Google Scholar]
- Gu, J.W.; Zhang, Q.Y.; Li, H.C.; Tang, Y.S.; Kong, J.; Dang, J. Study on preparation of SiO2/epoxy resin hybrid materials by means of sol-gel. Polym. Plast. Technol. Eng. 2007, 46, 1129–1134. [Google Scholar] [CrossRef]
- Hu, J.; Liu, L.; Xie, Y.; Wu, L. Facile synthesis of thermal-responsive P (NIPAM-S)/SiO2 hybrid hollow spheres and their controllable release properties for fragrance. Polym. Chem. 2013, 4, 3293–3299. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Ahn, B.; Kim, W.; Moon, H.; Paik, H.; Kim, W. The effect of accelerator contents on the vulcanizate structures of SSBR/silica vulcanizates. Compos. Interfaces 2017, 24, 563–577. [Google Scholar] [CrossRef]
- Ahn, B.; Park, N.; Kim, D.-H.; Kim, W. Influence of end-functionalized solution styrene-butadiene rubber on silica-filled vulcanizates with various silica-silane systems. Rubber Chem. Technol. 2019, 92, 364–377. [Google Scholar] [CrossRef]
- Ahn, B.; Kim, D.; Kim, K.; Kim, I.J.; Kim, H.J.; Kang, C.H. Effect of the functional group of silanes on the modification of silica surface and the physical properties of solution styrene-butadiene rubber/silica composites. Compos. Interfaces 2019, 26, 585–596. [Google Scholar] [CrossRef]
- Mun, H.; Hwang, K.; Kim, W. Synthesis of emulsion styrene butadiene rubber by reversible addition-fragmentation chain transfer polymerization and its properties. J. Appl. Polym. Sci. 2019, 136, 47069. [Google Scholar] [CrossRef]
- Park, N.; Ahn, B.; Lee, J.-Y.; Kim, W.; Moon, H.; Kim, W. Effect of organosilane agents on the vulcanizate structure and physical properties of silica-filled solution styrene butadiene rubber compounds. Compos. Interfaces 2017, 25, 1–15. [Google Scholar] [CrossRef]
- Han, S.; Kim, W.-S.; Mun, D.-Y.; Ahn, B.; Kim, W. Effect of coupling agents on the vulcanizate structure of carbon black filled natural rubber. Compos. Interfaces 2020, 27, 355–370. [Google Scholar] [CrossRef]
- Kim, I.J.; Ahn, B.; Kim, D.; Lee, H.J.; Kim, H.J.; Kim, W. Vulcanizate structures and mechanical properties of rubber compounds with silica and carbon black binary filler systems. Rubber Chem. Technol. 2020, 93. [Google Scholar] [CrossRef]
- Hasse, A.; Klockmann, O.; Wehmeier, A.; Luginsland, H.-D. Influence of the amount of di- and polysulfane silanes on the crosslinking density of silica-filled rubber compounds. Kautsch. Und Gummi Kunstst. 2002, 55, 236–243. [Google Scholar]
- Qu, L.; Yu, G.; Xie, X.; Wang, L.; Li, J.; Zhao, Q. Effect of silane coupling agent on filler and rubber interaction of silica reinforced solution styrene butadiene rubber. Polym. Compos. 2013, 34, 1575–1582. [Google Scholar] [CrossRef]
- Wang, M.-J. Effect of polymer-filler and filler-filler interactions on dynamic properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Gorl, U.; Munzenberg, J.; Luginsland, D.; Muller, A. Investigations on the reaction silica/organosilane and organosilane/polymer-Part 4: Studies on the chemistry of the silane sulfur chain. Kautsch. Und Gummi Kunstst. 1999, 52, 588–597. [Google Scholar]
- Mihara, S.; Datta, R.N.; Noordermeer, J.W.M. Flocculation in silica reinforced rubber compounds. Rubber Chem. Technol. 2009, 82, 524–540. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Optimization of mixing conditions for silica-reinforced natural rubber tire tread compounds. Rubber Chem. Technol. 2012, 85, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Cooperative effects of epoxide functional groups on natural rubber and silane coupling agents on reinforcing efficiency of silica. Rubber Chem. Technol. 2014, 87, 291–310. [Google Scholar] [CrossRef]
- Debnath, S.C.; Datta, R.N.; Noordermeer, J.W.M. Understanding the chemistry of the rubber/silane reaction for silica reinforcement, using model olefins. Rubber Chem. Technol. 2003, 76, 1311–1328. [Google Scholar] [CrossRef]
- Luginsland, H.D. Reactivity of the sulfur chains of the tetrasulfane silane Si 69 and the disulfane silane TESPD. Kautsch. Und Gummi Kunstst. 2000, 53, 10–19. [Google Scholar]
- Jin, J. Influence of Compounding and Mixing on Filler Dispersion and Curing Behavior of Silica Compounds. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2020. [Google Scholar]
- Yang, J.-K.; Park, W.; Ryu, C.; Kim, S.J. Roles of sulfur and accelerators in the vulcanization of SBR compounds deduced through simulation. Rubber Chem. Technol. 2018, 91, 595–608. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Reuvekamp, L.A.E.M.; Dierkes, W.K.; Blume, A.; Noordermeer, J.W.M.; Sahakaro, K. Enhancing the silanization reaction of the silica-Silane system by different amines in model and practical silica-filled natural rubber compounds. Polymers 2018, 10, 584. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Kraus, G. Degree of cure in filler-reinforced vulcanizates by the swelling method. Rubber Chem. Technol. 1957, 30, 928–951. [Google Scholar] [CrossRef]
- Ramesan, M.T. The effects of filler content on cure and mechanical properties of dichlorocarbene modified styrene butadiene rubber/carbon black composites. J. Polym. Res. 2005, 11, 333–340. [Google Scholar] [CrossRef]
- Waddell, W.H.; Evans, L.R. Use of nonblack fillers in tire compounds. Rubber Chem. Technol. 1996, 69, 377–423. [Google Scholar] [CrossRef]
- Suchiva, K.; Sirisinha, C.; Sae-oui, P.; Thapthong, P. Development of tyre tread compounds for good wet-grip: Effects of rubber type. IOP Conference Series. Mater. Sci. Eng. 2019, 526, 012035. [Google Scholar]
- Tomita, Y.; Azuma, K.; Naito, M. Computational evaluation of strain-rate-dependent deformation behavior of rubber and carbon-black-filled rubber under monotonic and cyclic straining. Int. J. Mech. Sci. 2008, 50, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Kummer, H.W. Unified Theory of Rubber and Tire Friction. Engineering Research Bulletin B-94; Pennsylvania State University: State College, PA, USA, 1966; pp. 100–101. [Google Scholar]
- Woodward, D.; Millar, P.; Lantieri, C.; Sangiorgi, C.; Vignali, V. The wear of Stone Mastic Asphalt due to slow speed high stress simulated laboratory trafficking. Constr. Build. Mater. 2016, 110, 270–277. [Google Scholar] [CrossRef]
- Dunford, A. Friction and the Texture of Aggregate Particles Used in the Road Surface Course; Dissertation, University of Nottingham: Nottingham, UK, 2013. [Google Scholar]
- Little, D.N.; Bhasin, A. Using Surface Energy Measurements to Select Materials for Asphalt Pavement. No. NCHRP Project; Transportation Research Board: Washington, DC, USA, 2006; pp. 9–37. [Google Scholar]
- Berg, J. Interfaces and Colloids; World Scientific: Hackensack, NJ, USA, 2010; pp. 250–268. [Google Scholar]


| Step | Materials | S60S-0.9 | S60S-1.2 | S60S-1.5 | S80S-0.9 | S80S-1.2 | S80S-1.5 |
|---|---|---|---|---|---|---|---|
| Stage 1 | S-SBR | 137.5 | 137.5 | 137.5 | 137.5 | 137.5 | 137.5 |
| Silica | 60 | 60 | 60 | 80 | 80 | 80 | |
| TESPT | 4.8 | 4.8 | 4.8 | 6.4 | 6.4 | 6.4 | |
| Zinc oxide | 2 | 2 | 2 | 2 | 2 | 2 | |
| Stearic acid | 3 | 3 | 3 | 3 | 3 | 3 | |
| Stage 2 | Sulfur | 0.9 | 1.2 | 1.5 | 0.9 | 1.2 | 1.5 |
| CBS | 1 | 1 | 1 | 1 | 1 | 1 | |
| DPG | 2 | 2 | 2 | 2 | 2 | 2 | |
| ZBEC | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Step | Time (min:seconds) | Revolutions per minute (RPM) | Action |
|---|---|---|---|
| Stage 1 | 0:00–0:30 | 15 | Rubber |
| 0:30–1:30 | 30 | Silica and chemicals | |
| 1:30–1:40 | 30 | Sweep | |
| 1:40–5:30 | Variable | Mixing and silanization (during 3 min at 145 ℃) | |
| 5:30 | Variable | Dump at 145℃ after silanization reaction | |
| Sheeting | - | Two roll mill | |
| Stage 2 | 0:00–0:20 | 15 | Compounds of stage 1 |
| 0:20–1:00 | 30 | Add sulfur, cure accelerator | |
| 1:00–2:00 | 30 | Extra mix and dump (under 100 ℃) | |
| Sheeting | - | Two roll mill |
| Compound | S60S-0.9 | S60S-1.2 | S60S-1.5 | S80S-0.9 | S80S-1.2 | S80S-1.5 | |
|---|---|---|---|---|---|---|---|
| Cure time 10% vulcanization (t10) | min | 3.7 | 3.6 | 3.4 | 3.5 | 3.3 | 3.2 |
| Cure time 90% vulcanization (t90) | min | 14.7 | 14.9 | 15.1 | 17.2 | 17.9 | 18.2 |
| Torque [△T], Tmax − Tmin | N·m | 2.079 | 2.192 | 2.429 | 2.248 | 2.429 | 2.633 |
| Crosslink Density (10−4 mol/g) | S60S-0.9 | S60S-1.2 | S60S-1.5 | S80S-0.9 | S80S-1.2 | S80S-1.5 |
|---|---|---|---|---|---|---|
| TCD a | 1.14 | 1.26 | 1.39 | 1.44 | 1.64 | 1.75 |
| CCD b | 0.55 | 0.63 | 0.70 | 0.55 | 0.63 | 0.70 |
| FRI c (= a − b) | 0.59 | 0.63 | 0.69 | 0.89 | 1.02 | 1.05 |
| Compound | S60S-0.9 | S60S-1.2 | S60S-1.5 | S80S-0.9 | S80S-1.2 | S80S-1.5 |
|---|---|---|---|---|---|---|
| Hardness (Shore A) | 57 | 60 | 61 | 67 | 68 | 70 |
| M100 (MPa) | 1.7 | 2.1 | 2.4 | 2.8 | 3.4 | 3.7 |
| M300 (MPa) | 7.3 | 8.4 | 10.1 | 12.1 | 14.3 | - |
| Tensile strength (MPa) | 14.6 | 13.8 | 13.5 | 15.3 | 14.6 | 11.3 |
| Elongation at break (%) | 460 | 420 | 370 | 350 | 310 | 230 |
| Abrasion (weight loss, %) | 10.7 | 7.4 | 6.1 | 0.9 | 0.6 | 0.5 |
| Range (µm) | S60S-0.9 | S60S-1.5 | |
|---|---|---|---|
| Class 1 | 0.5–1.0 | 18.73 | 34.53 |
| Class 2 | 1.0–1.5 | 56.64 | 46.39 |
| Class 3 | 1.5–2.0 | 13.21 | 9.28 |
| Class 4 | 2.0–2.5 | 4.12 | 4.06 |
| Class 5 | 2.5–3.0 | 1.99 | 1.95 |
| Class 6 | 3.0–5.0 | 1.13 | 0.74 |
| Class 7 | 5.0–10.0 | 2.06 | 2.00 |
| Class 8 | 10.0–20.0 | 1.73 | 1.05 |
| Class 9 | 20.0–50.0 | 0.33 | - |
| Class 10 | 50.0–100.0 | 0.07 | - |
| Condition | Property | S60S-0.9 | S60S-1.2 | S60S-1.5 | S80S-0.9 | S80S-1.2 | S80S-1.5 |
|---|---|---|---|---|---|---|---|
| Temperature sweep (−80–70 ℃ under 0.2% strain) | E” at 0 ℃ [MPa] | 25 | 25 | 31 | 45 | 49 | 50 |
| tan δ at 0 ℃ | 0.847 | 0.853 | 0.898 | 0.744 | 0.745 | 0.767 | |
| tan δ at 60 ℃ | 0.112 | 0.108 | 0.101 | 0.134 | 0.123 | 0.120 | |
| Strain sweep (0.2–5% at 60 ℃) | E’ at 0.2% [Pa] | 6079 | 6222 | 6423 | 11,788 | 12,439 | 13,000 |
| E’ at 5% [Pa] | 4070 | 4255 | 4518 | 6096 | 6823 | 7423 | |
| ΔE’ (0.2–5%) [Pa] | 2009 | 1967 | 1906 | 5691 | 5616 | 5577 |
| Compound | S60S-0.9 | S60S-1.2 | S60S-1.5 | S80S-0.9 | S80S-1.2 | S80S-1.5 |
|---|---|---|---|---|---|---|
| μ peak average (higher is better) | 0.604 | 0.610 | 0.612 | 0.636 | 0.643 | 0.660 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Gu, B.; Kim, S.; Kim, S.; Mun, D.; Morita, K.; Kim, D.; Kim, W. Effect of Sulfur Variation on the Vulcanizate Structure of Silica-Filled Styrene-Butadiene Rubber Compounds with a Sulfide–Silane Coupling Agent. Polymers 2020, 12, 2815. https://doi.org/10.3390/polym12122815
Han S, Gu B, Kim S, Kim S, Mun D, Morita K, Kim D, Kim W. Effect of Sulfur Variation on the Vulcanizate Structure of Silica-Filled Styrene-Butadiene Rubber Compounds with a Sulfide–Silane Coupling Agent. Polymers. 2020; 12(12):2815. https://doi.org/10.3390/polym12122815
Chicago/Turabian StyleHan, Sangwook, Bonyoung Gu, Sungwoo Kim, Seongrae Kim, Dalyong Mun, Koichi Morita, Donghyuk Kim, and Wonho Kim. 2020. "Effect of Sulfur Variation on the Vulcanizate Structure of Silica-Filled Styrene-Butadiene Rubber Compounds with a Sulfide–Silane Coupling Agent" Polymers 12, no. 12: 2815. https://doi.org/10.3390/polym12122815
APA StyleHan, S., Gu, B., Kim, S., Kim, S., Mun, D., Morita, K., Kim, D., & Kim, W. (2020). Effect of Sulfur Variation on the Vulcanizate Structure of Silica-Filled Styrene-Butadiene Rubber Compounds with a Sulfide–Silane Coupling Agent. Polymers, 12(12), 2815. https://doi.org/10.3390/polym12122815





