Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. List of Symbols and Acronyms
- LLDP—liquid–liquid displacement porometry;
- NIPS—non–solvent induced phase separation;
- NMP—N–methyl–2–pyrrolidone;
- PAA—polyamic acid;
- PI—polyimide;
- PSD—pore size distribution;
- SEM—scanning electron microscopy;
- d—total thickness of the polymer layer, µm;
- t—time, s;
- v—deposition rate in limited layer, μm/s;
- D—diameter, nm;
- γ—interfacial tension, 10−3 N/m;
- θ—contact angle, °;
- J1—flux of the displacing liquid in the presence of the wetting liquid, L/m2 h;
- J2—flux the displacing liquid only, L/m2 h;
- Δp—differential pressure, bar;
- η—viscosity, mPa∙s;
- P—permeance, kg/m2 h bar;
- C—concentration, wt.%.
2.2. Materials
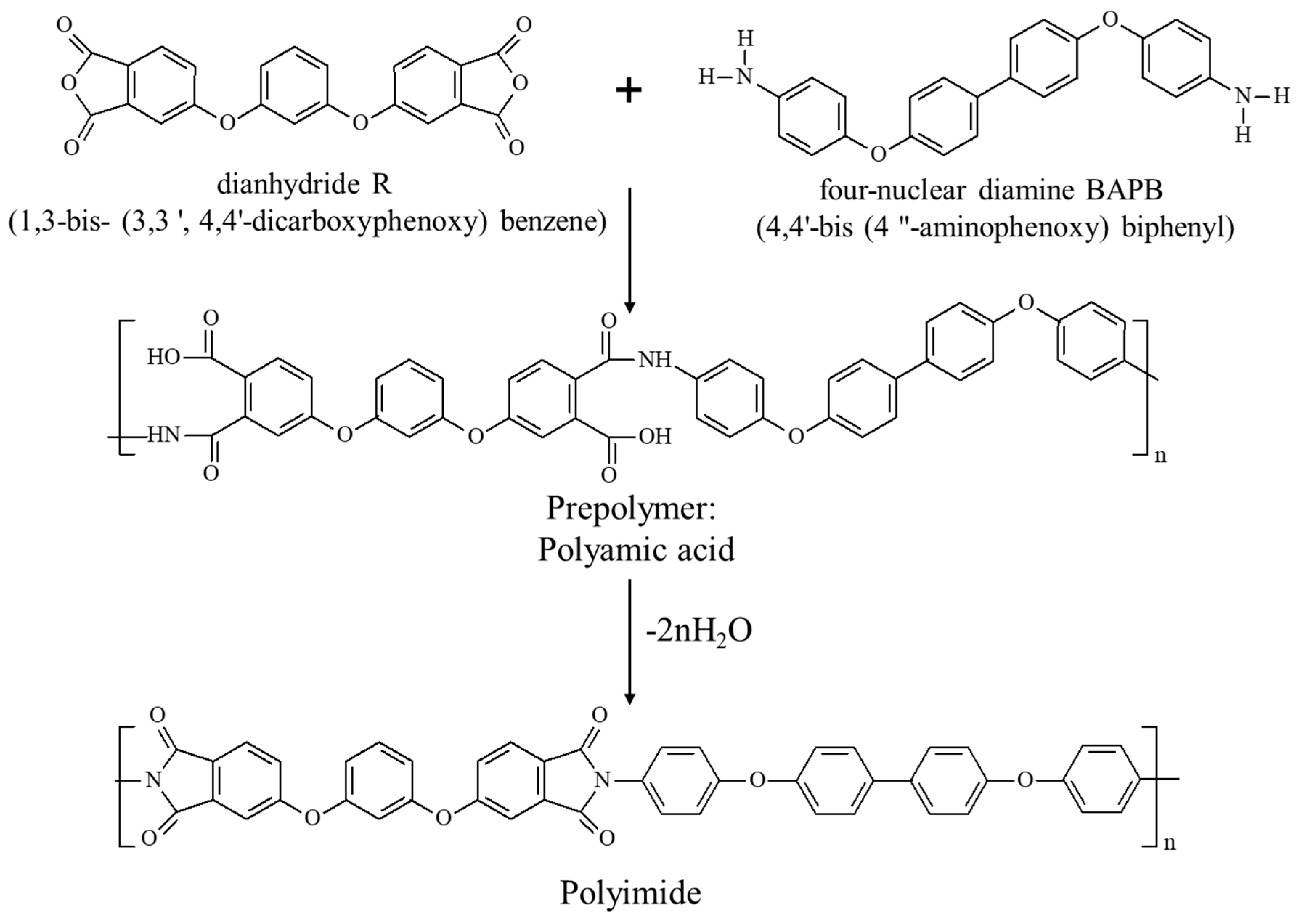
2.3. PAA Synthesis
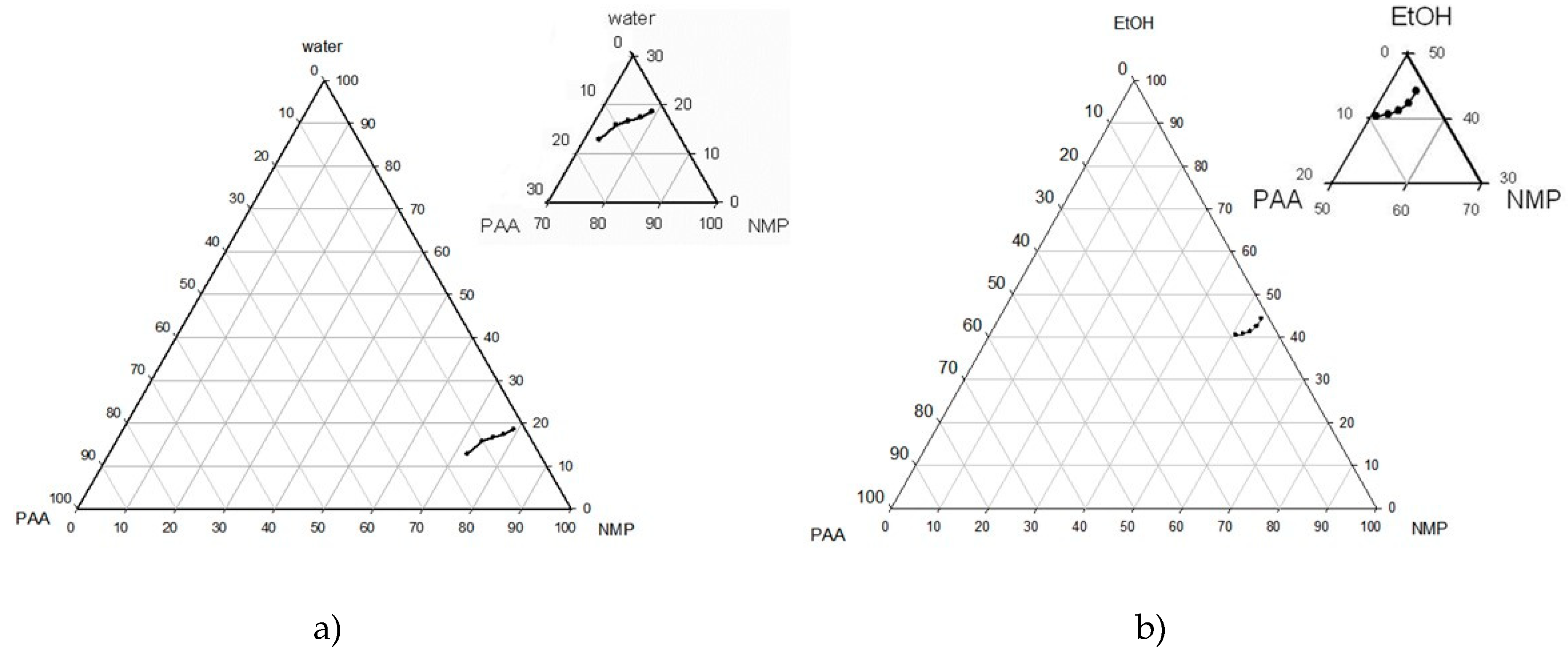
2.4. Ternary Phase Diagrams
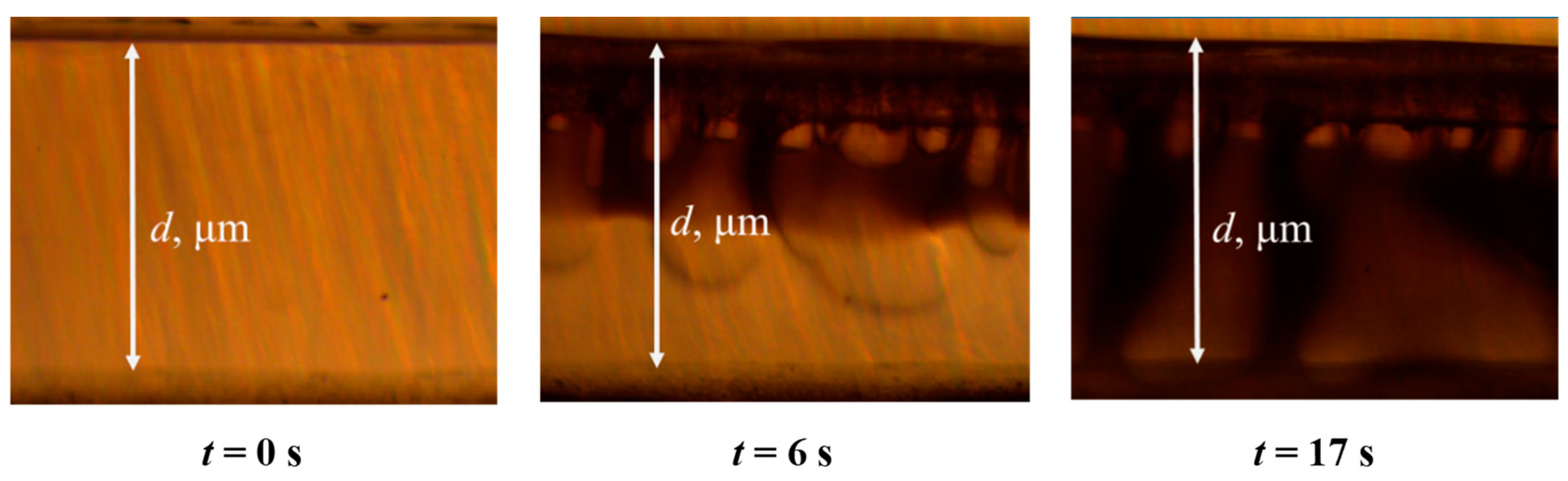
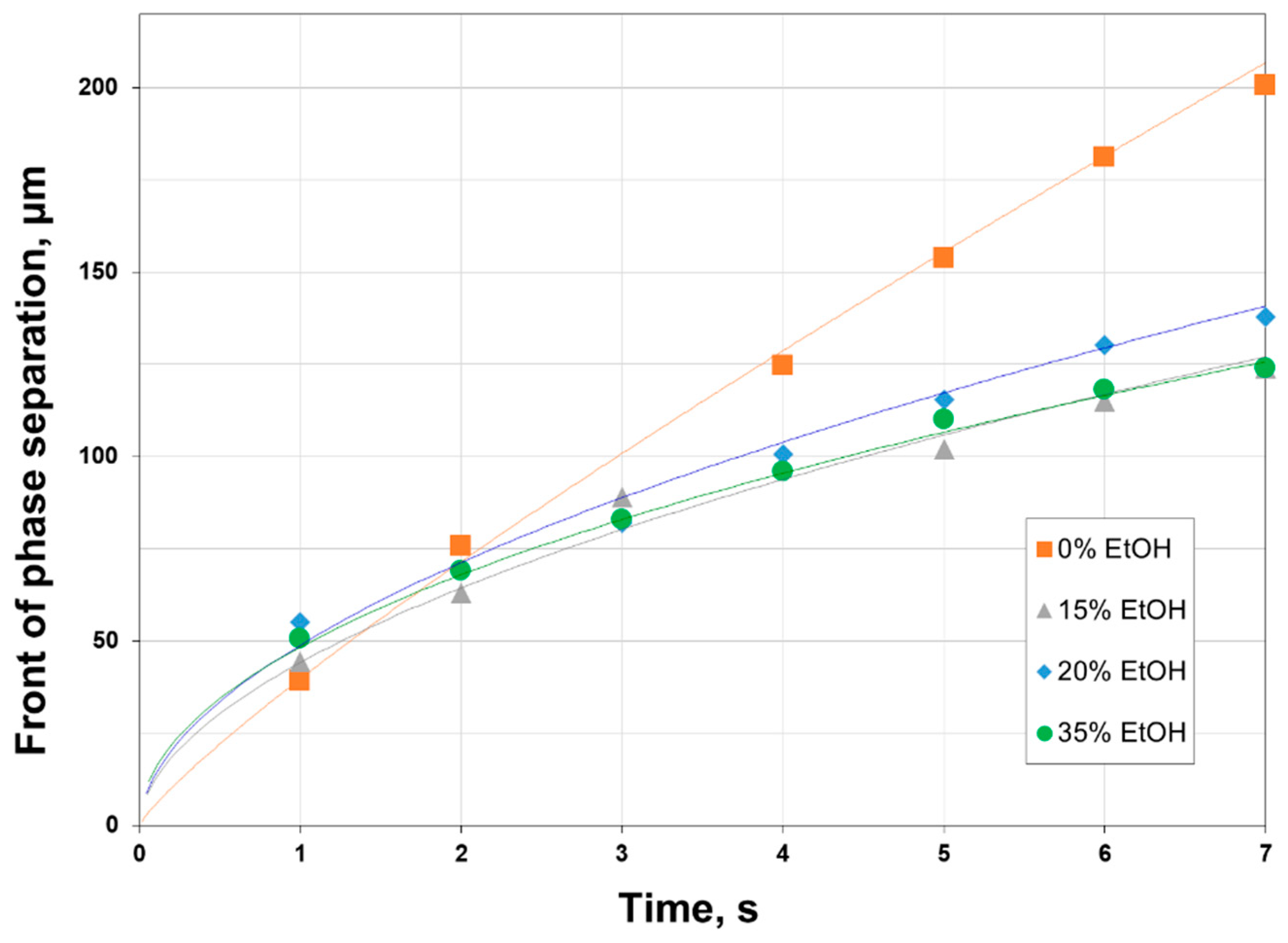
2.5. Determination of Precipitation Rate
2.6. Preparation of Flat Asymmetric Membranes Based on PAA
2.7. Scanning Electron Microscopy
2.8. Study of Pore Size and Membrane Permeance
3. Results
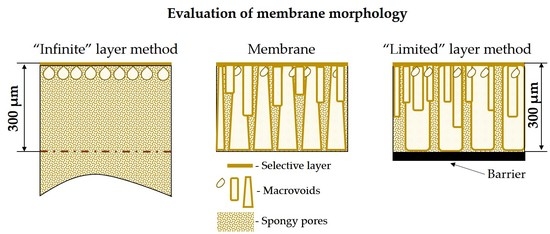
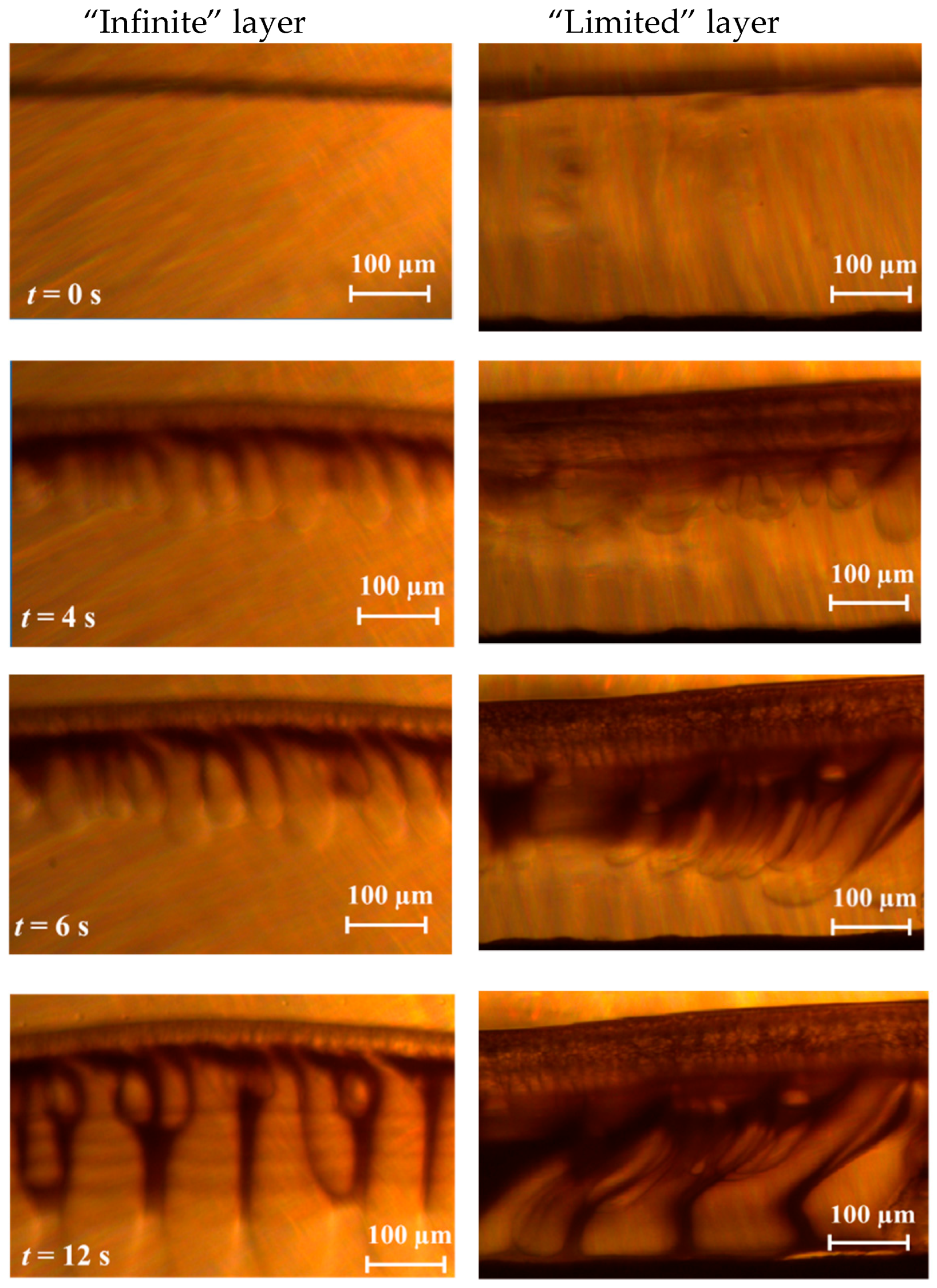
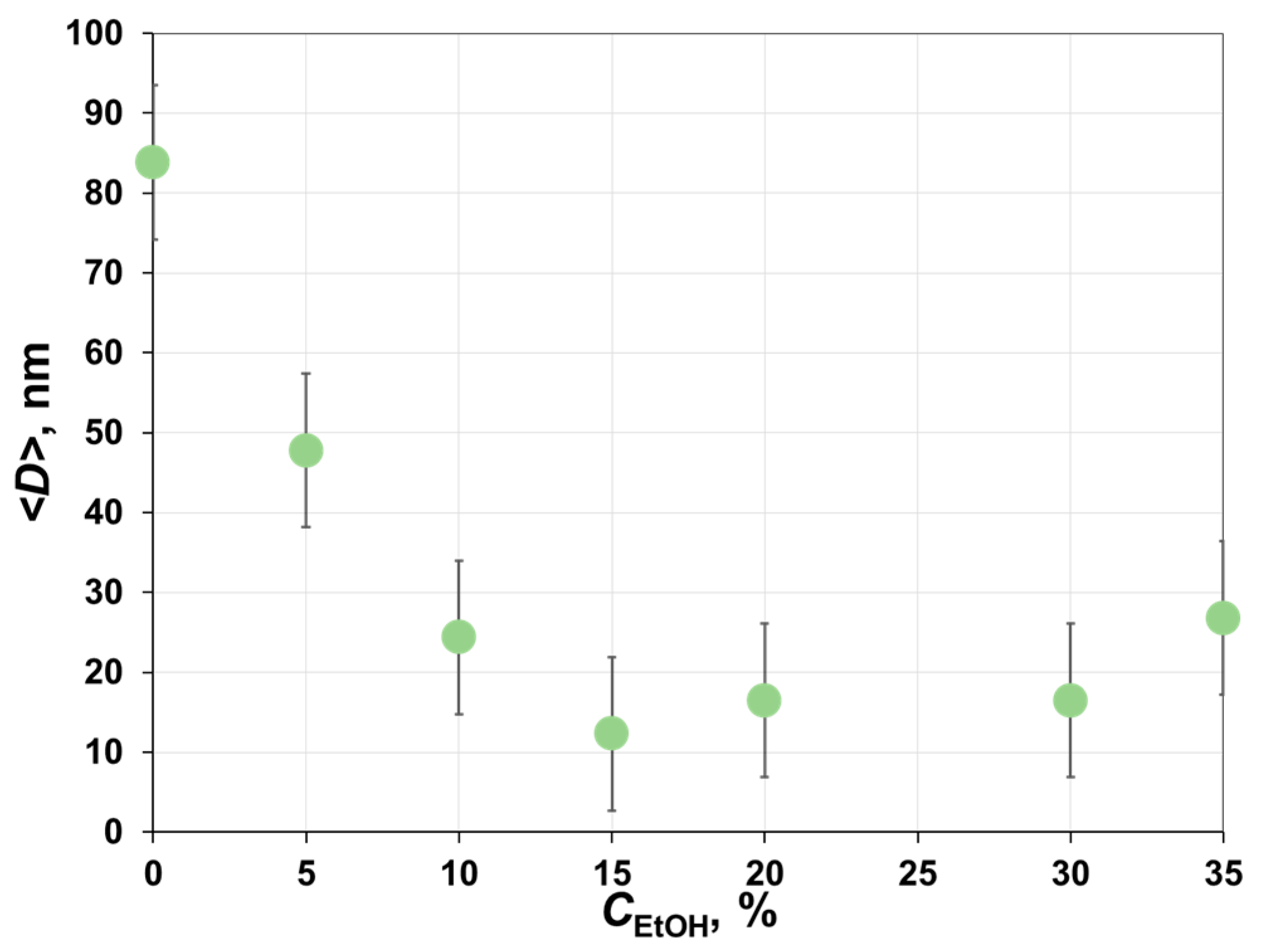
3.1. Phase Inversion of Polymer/Solvent Solution
3.2. Phase Inversion of Polymer/Solvent/Non-solvent Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vanherck, K.; Koeckelberghs, G.; Vankelecom, I.F.J. Crosslinking polyimides for membrane applications: A review. Prog. Polym. Sci. 2013, 38, 874–896. [Google Scholar] [CrossRef]
- Sroog, C.E. Polyimides. Prog. Polym. Sci. 1991, 16, 561–694. [Google Scholar] [CrossRef]
- Abadie, M.J.M.; Rusanov, A.L. Practical Guide to Polyimides Shawbury; Rapra: Shrewsbury, UK, 2007; 84p. [Google Scholar]
- Pulyalina, A.; Tataurov, M.; Faykov, I.; Rostovtseva, V.; Polotskaya, G. Polyimide Asymmetric Membrane vs. Dense Film for Purification of MTBE Oxygenate by Pervaporation. Symmetry 2020, 12, 436. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M.; Gubanova, G.N.; Didenco, A.L.; Suchanova, T.E.; Kudryavtsev, V.V. Semicrystalline polyimide matrices for composites: Crystallization and properties. J. Appl. Polym. Sci. 2002, 83, 2873–2882. [Google Scholar] [CrossRef]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Synthesis, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Schumann, W.; Strathmann, H. Polyimide Membrane and Process for Making Same. U.S. Patent 3,925,211, 30 January 1975. [Google Scholar]
- Li, Y.; Cao, B.; Li, P. Fabrication of PMDA-ODA hollow fibers with regular cross-section morphologies and study on the formation mechanism. J. Membr. Sci. 2017, 544, 1–11. [Google Scholar] [CrossRef]
- Guillen, G.R.; Ramon, G.Z.; Kavehpour, H.P.; Kaner, R.B.; Hoek, E.M.V. Direct microscopic observation of membrane formation by nonsolvent induced phase separation. J. Membr. Sci. 2013, 431, 212–220. [Google Scholar] [CrossRef]
- Huang, T.; Song, J.; He, H.; Zhang, Y.-B.; Li, X.-M.; He, T. Impact of SPEEK on PEEK membranes: Demixing, morphology and performance enhancement in lithium membrane extraction. J. Membr. Sci. 2020, 615, 118448. [Google Scholar] [CrossRef]
- Yudin, V.E.; Svetlichnyi, V.M.; Gubanova, G.N.; Didenco, A.L.; Popova, E.N.; Sukhanova, T.E.; Grigoriev, A.I.; Kostereva, T.A.; Arbel, I.; Marom, G. Influence of crystallinity of R-BAPB-type polyimide matrix on thermal and mechanical properties of carbon-fiber-reinforced composites. In Polyimides and Other High Temperature Polymers: Synthesis, Characterization and Applications; Mittal, K.L., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 299–316. [Google Scholar]
- Smirnova, V.E.; Gofman, I.V.; Ivankova, E.M.; Didenko, A.L.; Svetlichnyi, V.M.; Krestinin, A.V.; Zvereva, G.I.; Yudin, V.E. Effect of single-walled carbon nanotubes and carbon nanofibers on the structure and mechanical properties of thermoplastic polyimide matrix films. Polym. Sci. Ser. A 2013, 55, 268–278. [Google Scholar] [CrossRef]
- Vaganov, G.V.; Didenko, A.L.; Ivan’kova, E.M.; Popova, E.N.; Elokhovskii, V.Y.; Yudin, V.E.; Volkov, A.V. Preparation and properties of a thermoplastic partially crystalline polyimide in the oriented state. Russ. J. Appl. Chem. 2020, 93, 72–79. [Google Scholar] [CrossRef]
- Volgin, I.V.; Andreeva, M.V.; Larin, S.V.; Didenko, A.L.; Vaganov, G.V.; Borisov, I.L.; Volkov, A.V.; Klushin, L.I.; Lyulin, S.V. Transport Properties of Thermoplastic R-BAPB Polyimide: Molecular Dynamics Simulations and Experiment. Polymers 2019, 11, 1775. [Google Scholar] [CrossRef]
- Silinskaya, I.G.; Svetlichnyi, V.M.; Kalinina, N.A.; Didenko, A.L.; Filippov, A.P.; Kudryavtsev, V.V. Molecular characteristics and solution behavior of prepolymers of several polyimides: Effect of synthesis conditions. Polym. Sci. Ser. A 2006, 48, 787–792. [Google Scholar] [CrossRef]
- Praneeth, K.; Bhargava Suresh, K.; Tardio, J.; Sridhar, S. Design of novel ultrafiltration systems based on robust polyphenylsulfone hollow fiber membranes for treatment of contaminated surface water. Chem. Eng. J. 2014, 248, 297–306. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Karslyan, Y.A.; Ovcharova, A.A.; Volkov, V.V. Development of high flux ultrafiltration polyphenylsulfone membranes applying the systems with upper and lower critical solution temperatures: Effect of polyethylene glycol molecular weight and coagulation bath temperature. J. Membr. Sci. 2018, 565, 266–280. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Hliavitskay, T.A.; Penkova, A.V.; Ermakov, S.S.; Ulbricht, M. Modification of Polysulfone Ultrafiltration Membranes via Addition of Anionic Polyelectrolyte Based on Acrylamide and Sodium Acrylate to the Coagulation Bath to Improve Antifouling Performance in Water Treatment. Membranes 2020, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Zeman, L.J.; Zydney, A.L. Microfiltration and Ultrafiltration: Principles and Applications; Marcel Dekker: New York, NY, USA, 1996; pp. 197–201. [Google Scholar] [CrossRef]
- Antón, E.; Calvo, J.I.; Álvarez, J.R.; Hernández, A.; Luque, S. Fitting approach to liquid–liquid displacement porometry based on the log-normal pore size distribution. J. Membr. Sci. 2014, 470, 219–228. [Google Scholar] [CrossRef]
- Morison, K.R. A comparison of liquid–liquid porosimetry equations for evaluation of pore size distribution. J. Membr. Sci. 2008, 325, 301–310. [Google Scholar] [CrossRef]
- Giglia, S.; Bohonak, D.; Greenhalgh, P.; Leahy, A. Measurement of pore size distribution and prediction of membrane filter virus retention using liquid–liquid porometry. J. Membr. Sci. 2015, 476, 399–409. [Google Scholar] [CrossRef]
- Simanovich, I.E.; Fedorova, G.N.; Mikhailova, N.V.; Bobasheva, A.S.; Gribanov, A.V.; Sazanov Yu, N. Thermal analysis of polyamic acid-furyl alcohol compositions. J. Therm. Anal. 1988, 34, 289–295. [Google Scholar] [CrossRef]
- Lee, H.; Won, J.; Park, H.; HLee, H.; Kang, Y. Effect of poly (amic acid) imidication on solution characteristics and membrane morphology. J. Membr. Sci. 2000, 178, 35–41. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anokhina, T.; Borisov, I.; Yushkin, A.; Vaganov, G.; Didenko, A.; Volkov, A. Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties. Polymers 2020, 12, 2785. https://doi.org/10.3390/polym12122785
Anokhina T, Borisov I, Yushkin A, Vaganov G, Didenko A, Volkov A. Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties. Polymers. 2020; 12(12):2785. https://doi.org/10.3390/polym12122785
Chicago/Turabian StyleAnokhina, Tatiana, Ilya Borisov, Alexey Yushkin, Gleb Vaganov, Andrey Didenko, and Alexey Volkov. 2020. "Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties" Polymers 12, no. 12: 2785. https://doi.org/10.3390/polym12122785
APA StyleAnokhina, T., Borisov, I., Yushkin, A., Vaganov, G., Didenko, A., & Volkov, A. (2020). Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties. Polymers, 12(12), 2785. https://doi.org/10.3390/polym12122785