Fabrication and Characterization of Activated Carbon Fibers from Oil Palm Trunk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. ACFs Preparation
2.3. Characterization
2.4. Chromium Removal Experiments
3. Results
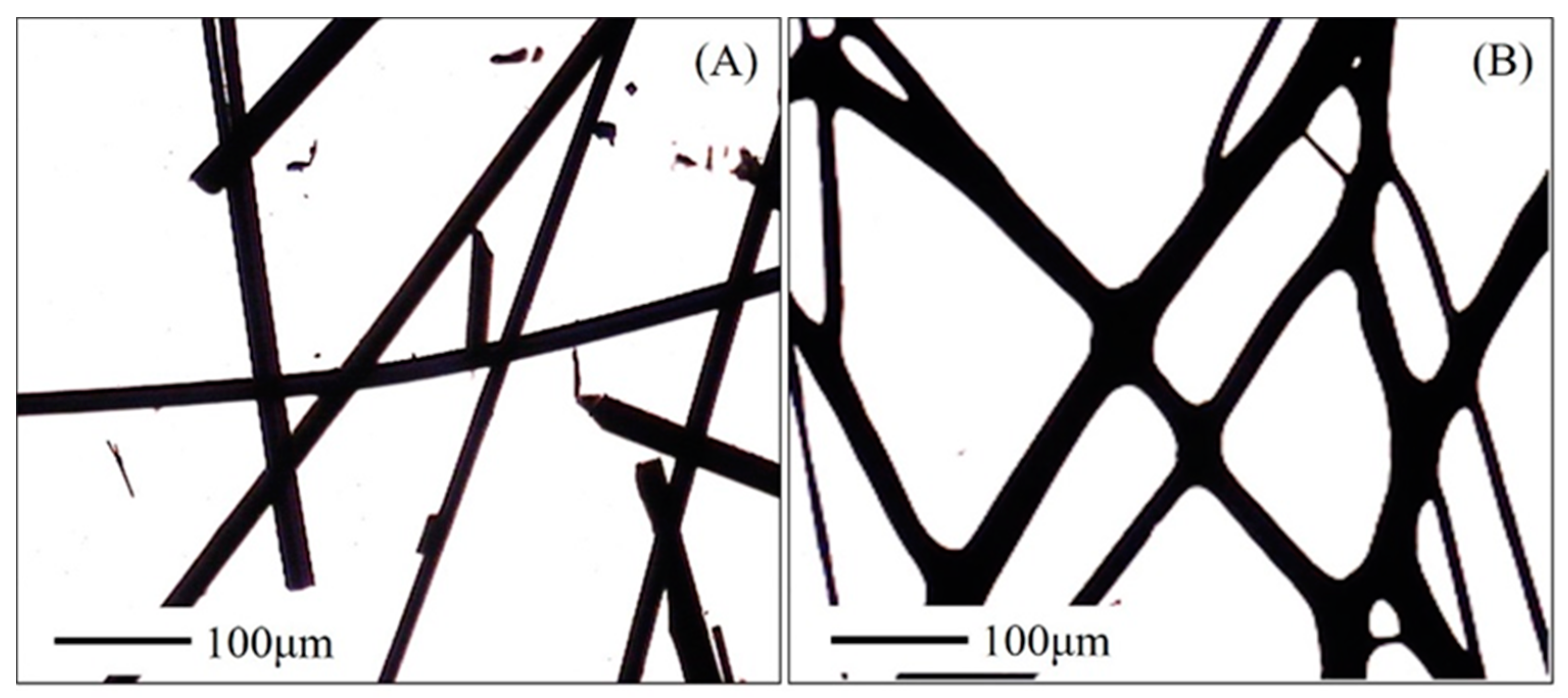
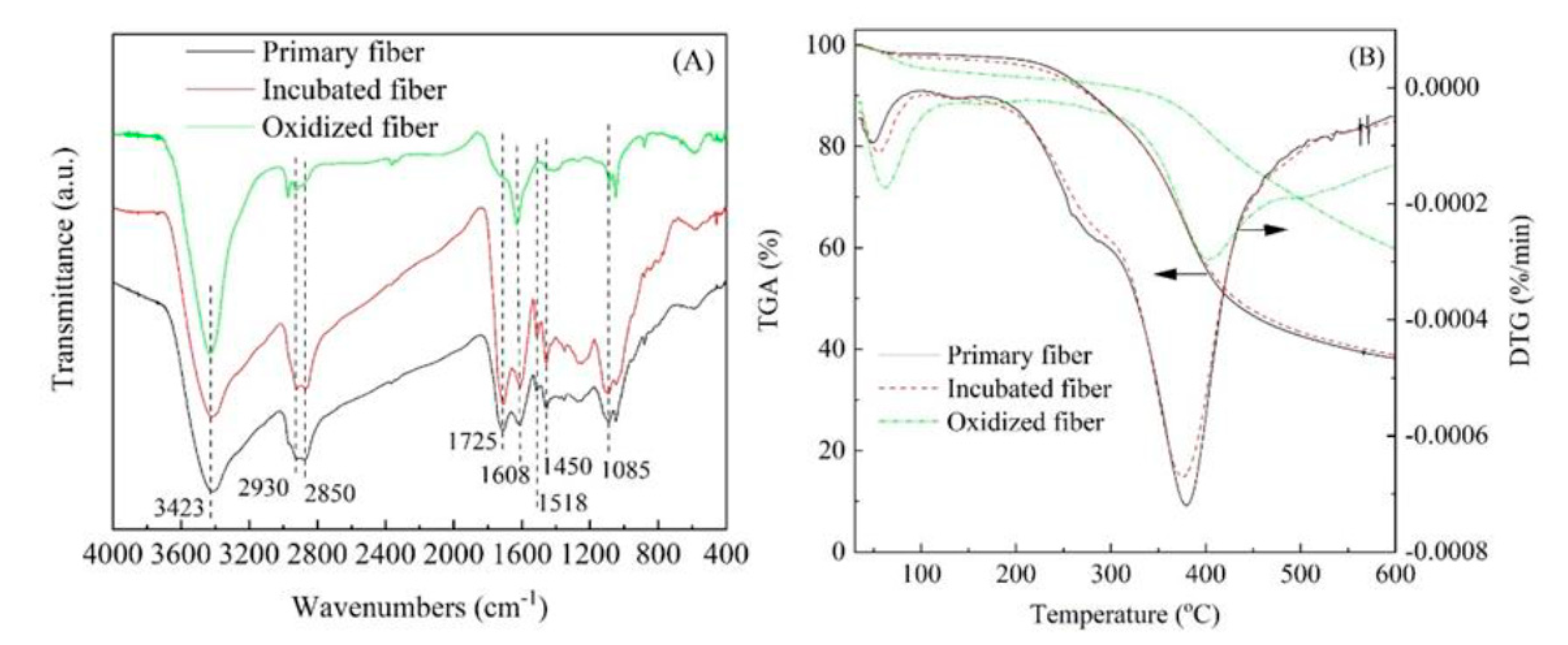
3.1. Infusible Precursor of ACFs
3.2. Porosity Characterization of ACFs
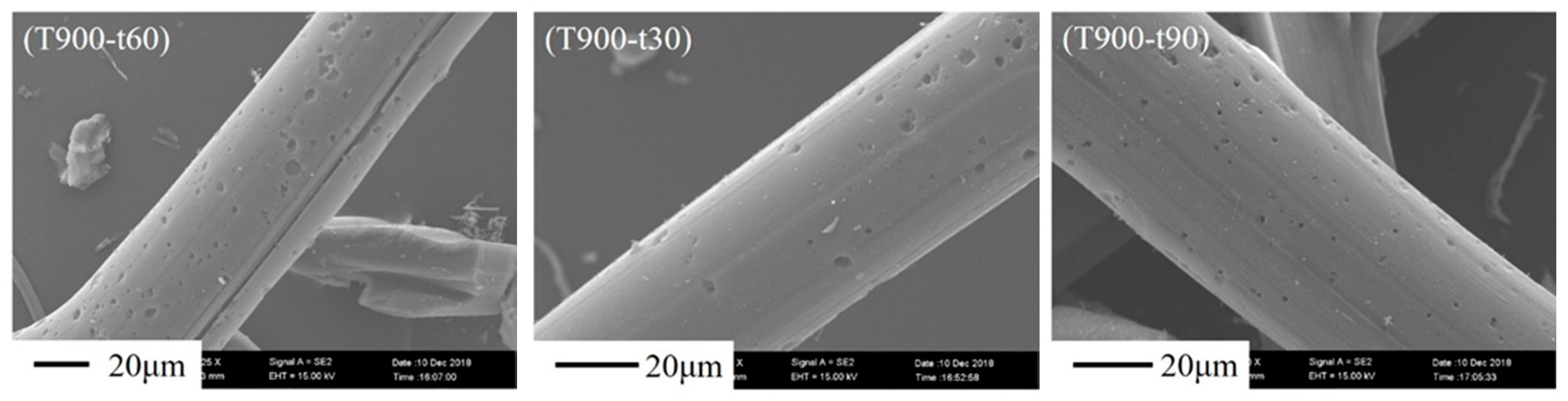
3.3. Morphology
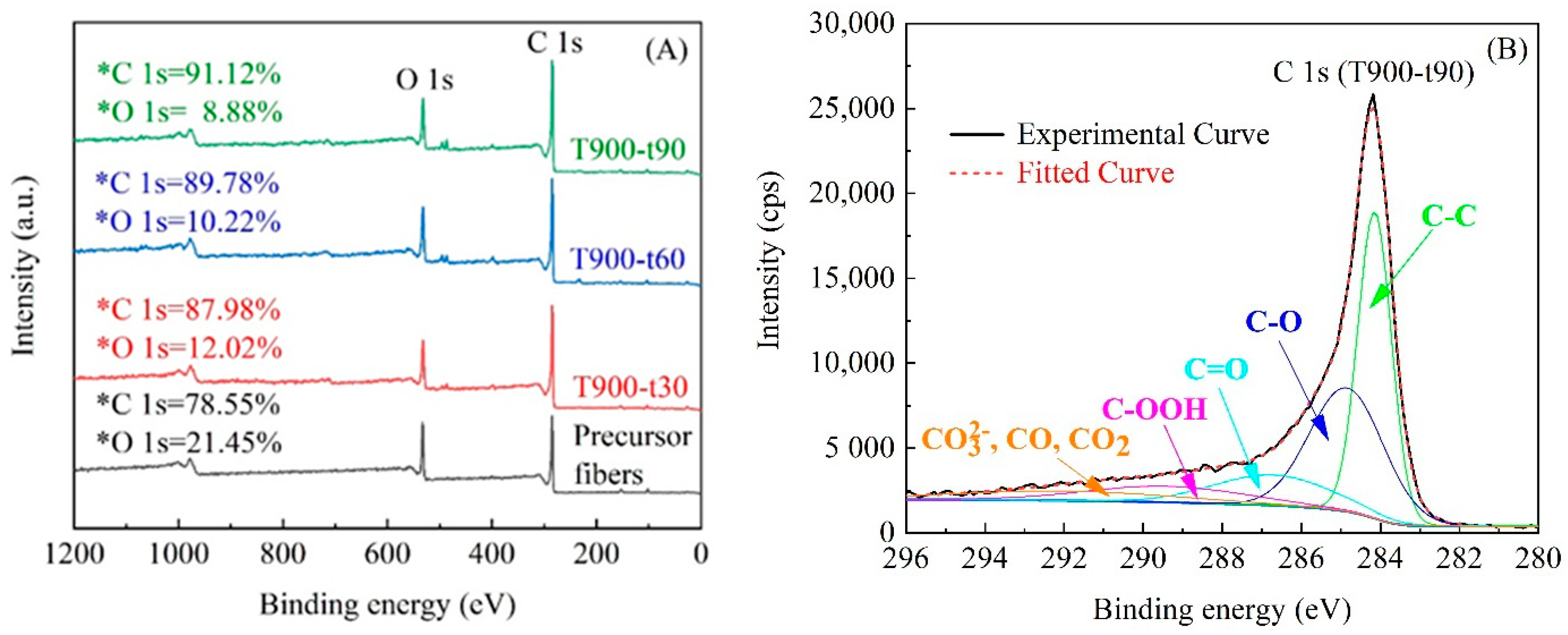
3.4. Surface Chemistry
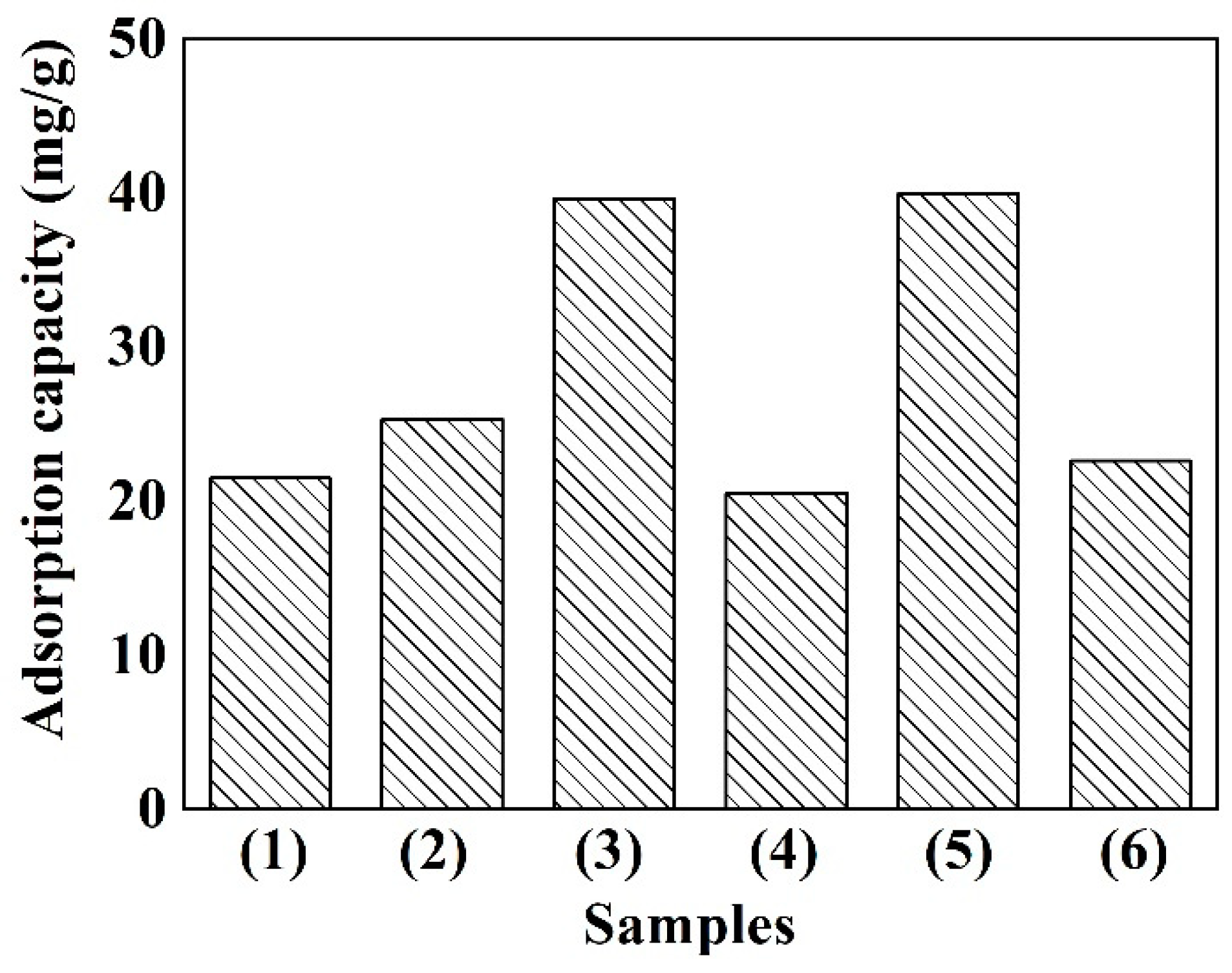
3.5. Cr(VI) Removal Capacities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Zeng, F.L.; Pan, D. The structural transitions of rayon under the promotion of a phosphate in the preparation of ACF. Cellulose 2008, 15, 91–99. [Google Scholar] [CrossRef]
- Ryu, S.K.; Kim, S.Y.; Gallego, N.; Edie, D.D. Physical properties of silver-containing pitch-based activated carbon fibers. Carbon 1999, 37, 1619–1625. [Google Scholar] [CrossRef]
- Mangun, C.L.; Benak, K.R.; Economy, J.; Foster, K.L. Surface chemistry, pore sizes and adsorption properties of activated carbon fibers and precursors treated with ammonia. Carbon 2001, 39, 1809–1820. [Google Scholar] [CrossRef]
- Sun, J.F.; He, C.J.; Zhu, S.J.; Wang, Q.R. Effects of oxidation time on the structure and properties of polyacrylonitrile-based activated carbon hollow fiber. J. Appl. Polym. Sci. 2007, 106, 470–474. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, Q.H.; Zhao, G.J. Influence of activation time on the microstructure and antibacterial activity of nanosilver-containing activated carbon fibers prepared from liquefied wood. Fibers Polym. 2015, 16, 522–528. [Google Scholar] [CrossRef]
- Song, M.; Zhang, W.; Chen, Y.S.; Luo, J.M.; Crittenden, J.C. The preparation and performance of lignin-based activated carbon fiber adsorbents for treating gaseous streams. Front. Chem. Sci. Eng. 2017, 11, 328–337. [Google Scholar] [CrossRef]
- Hu, S.X.; Zhang, S.L.; Pan, N.; Hsieh, Y.L. High energy density supercapacitors from lignin derived submicron activated carbon fibers in aqueous electrolytes. J. Power Sources 2014, 270, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Espinoza-Acosta, J.L.; Torres-Chavez, P.I.; Olmedo-Martinez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in storage and renewable energy application: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Dong, X.Z.; Lu, C.X.; Zhou, P.C.; Zhang, S.C.; Wang, L.Y.; Li, D.H. Polyacrylonitrile/lignin sulfonated blend fiber for low-cost carbon fiber. RSC Adv. 2015, 5, 42259–42265. [Google Scholar] [CrossRef]
- Komariah, R.N.; Miyamato, T.; Tanaka, S.; Prasetiyo, K.W.; Syamani, F.A.; Subyakto; Umezawa, T.; Kanayama, K.; Umemura, K. High-performance binderless particle board from the inner part of oil palm trunk by addition of ammonium dihydrogen phosphate. Ind. Crop Prod. 2019, 141, 111761. [Google Scholar] [CrossRef]
- Prawitwong, P.; Kosugi, A.; Arai, T.; Deng, L.; Lee, K.C.; Ibrahim, D.; Murata, Y.; Sulaiman, O.; Hashim, R.; Sudesh, K.; et al. Efficient ethanol production from separated parenchyma and vascular bundle of oil palm trunk. Bioresour. Technol. 2012, 125, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Lamaming, J.; Hashim, R.; Sulaiman, O.; Leh, C.P.; Sugimoto, T.; Nordin, N.A. Cellulose nanocrystals isolated from oil palm trunk. Carbohyd. Polym. 2015, 127, 202–208. [Google Scholar] [CrossRef]
- Lamaming, J.; Hashim, R.; Leh, C.P.; Sulaiman, O.; Sugimoto, T.; Nasir, M. Isolation and characterization of cellulose nanocrystals from parenchyma and vascular bundle of oil palm trunk (Elaeis guineensis). Carbohyd. Polym. 2015, 134, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Oramahi, H.A.; Wahdina; Diba, F.; Nurhaida; Yoshimura, T. Optimization of production of Lignocellulosic biomass bio-oil from oil palm trunk. Procedia Environ. Sci. 2015, 28, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Ashaari, Z.; Ang, A.F.; Halip, J.A.; Lum, W.C. Effects of two-step post heat-treatment in palm oil on the properties of oil palm trunk particleboard. Ind. Crop Prod. 2018, 116, 249–258. [Google Scholar] [CrossRef]
- Aizat, A.G.; Zaidon, A.; Nabil, F.L.; Bakar, E.S.; Rasmina, H. Effects of diffusion process and compression on polymer loading of laminated compreg oil palm (Elaeis guineensis) wood and its relation to properties. J. Biobased Mater. Bio. 2014, 8, 519–525. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Nur Firdaus, M.Y.; Jawaid, M.; Anis, M.; Ridzuan, R.; Mohamed, A.R. Development and material properties of new hybrid plywood from oil palm biomass. Mater. Des. 2010, 31, 417–424. [Google Scholar] [CrossRef]
- Choowang, R.; Lin, J.; Zhao, G.J. Efficiency of blended polyhydric alcohols solution on liquefaction of oil palm trunk (Elaeis guineensis Jacq.). Bioresources 2019, 14, 2759–2772. [Google Scholar]
- Okada, K.; Yamamoto, N.; Kameshima, Y.; Yasumori, A. Porous properties of activated carbons from waste newspaper prepared by chemical and physical activation. J. Colloid Interface Sci. 2003, 262, 179–193. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Micropor. Mesopor. Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Shimada, M.; Iida, T.; Kawarada, K.; Chiba, Y.; Mamoto, T.; Okayama, T. Porous structure of activated carbon prepared from waste newspaper. J. Mater. Cycles Waste Manag. 2000, 2, 100–108. [Google Scholar]
- RodriguezReinoso, F.; LopezGonzalez, J.D.; Berenguer, C. Activated carbon from almost shells-II: Characterization of the pore structure. Carbon 1984, 22, 13–18. [Google Scholar] [CrossRef]
- Lastoskie, C.; Gubbins, K.E.; Quirke, N. Pore size distribution analysis of microporous carbon: A density functional theory approach. J. Phys. Chem. 1993, 97, 4786–4796. [Google Scholar] [CrossRef]
- Troppová, I.; Matějová, L.; Kuboňová, L.; Strašák, T.; Študentová, S.; Kustrowski, P.; Obalová, L. Molecular dimensions and porous structure of activated carbons for sorption of xylene and isoocatane. Chem. Eng. Technol. 2017, 40, 6–17. [Google Scholar] [CrossRef]
- Kadla, J.F.; Kubo, S.; Venditti, R.A.; Gilbert, R.D.; Compere, A.L.; Griffith, W. Lignin-based carbon fiber for composite fiber applications. Carbon 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Morita, K.; Miyachi, H.; Hiramatsu, T. Stabilization of acrylic fibers by sulfur atoms mechanism of stabilization. Carbon 1981, 19, 11–18. [Google Scholar] [CrossRef]
- Lin, S.S. Oxidative stabilization in production of pitch based carbon-fiber. Sampe J. 1991, 27, 9–14. [Google Scholar]
- Kim, K.W.; Lee, H.M.; Kim, B.S.; Hwang, S.H.; Kim, B.J. Preparation and thermal properties of polyethylene-based carbonized fibers. Carbon Lett. 2015, 16, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Yoo, M.J.; Ko, H.J.; Lim, Y.S.; Kim, M.S. Modification of isotropic coal-tar pitch by acid treatments for carbon fiber melt-spinning. Carbon Lett. 2014, 15, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Rasrendra, C.B.; Windt, M.; Wang, Y.; Adisasmito, S.; Makertihartha, I.G.B.N.; Van Eck, E.R.H.; Meier, D.; Heeres, H.J. Experimental studies on the pyrolysis of humins from the acid-catalysed dehydration of C6-sugars. J. Anal. Appl. Pyrolysis 2013, 104, 299–307. [Google Scholar] [CrossRef]
- Qin, W.; Kadla, J.F. Carbon fibers based on pyrolytic lignin. J. Appl. Polym. Sci. 2011, 126, E203–E212. [Google Scholar] [CrossRef]
- Duan, X.H.; Srinivasakannan, C.; Yang, K.B.; Yang, K.B.; Peng, J.H.; Zhang, L.B. Effects of heating method and activating agent on the porous structure of activated carbons from coconut shells. Waste Biomass Valor. 2012, 3, 131–139. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y. Preparation and characterization of Mn/N co-doped TiO2 loaded on wood-based activated carbon fiber and its visible light photodegradation. Polymers 2015, 7, 1660–1673. [Google Scholar] [CrossRef] [Green Version]
- Hoang, T.M.C.; Van Eck, E.R.H.; Bula, W.P.; Gardeniers, J.G.E.; Lefferts, L.; Seshan, K. Humin based by-product from biomass processing as a potential carbonaceous source for synthesis gas production. Green Chem. 2015, 17, 959–972. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Lee, C.C.; Lee, H.C. Characterization of microstructure and surface properties of heat-treated PAN-and rayon-based activated carbon fibers. J. Porous Mater. 2007, 14, 227–237. [Google Scholar] [CrossRef]
- Lee, I.; Park, J.A.; Kang, J.K.; Kim, J.H.; Son, J.W.; Yi, I.G.; Kim, S.B. Batch and Flow-Through Column Studies for Cr(VI) Sorption to Activated Carbon Fiber. Environ. Eng. Res. 2014, 19, 157–163. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pathania, D.; Sharma, S.; Singh, P. Preparation of bio-based porous carbon by microwave assisted phosphoric acid activation and its use for adsorption of Cr(VI). J. Colllid Interface Sci. 2013, 401, 125–132. [Google Scholar] [CrossRef]
- Huang, M.L.; Mishra, S.B.; Liu, S.Q. Waste Glass Fiber Fabric as a Support for Facile Synthesis of Microporous Carbon to Adsorb Cr(VI) from Wastewater. ACS Sustain. Chem. Eng. 2017, 5, 8127–8136. [Google Scholar] [CrossRef]


| Samples | Specific Surface Area (m2/g) | Pore Volume (cc/g) | %Vmeso | Average Pore Dimension (nm) | ||||
|---|---|---|---|---|---|---|---|---|
| SBET | Smicro | Smeso | Vtot | Vmicro | Vmeso | |||
| Precursor fibers | 4.3 | 1.9 | 2.9 | 0.001 | 0.000 | 0.001 | 100 | 4.00 |
| T700-t60 | 542 | 438 | 171 | 0.234 | 0.200 | 0.096 | 41.0 | 1.99 |
| T800-t60 | 857 | 665 | 231 | 0.343 | 0.296 | 0.128 | 37.3 | 1.90 |
| T900-t60 | 1018 | 715 | 435 | 0.442 | 0.366 | 0.234 | 52.9 | 2.19 |
| T900-t30 | 1109 | 830 | 280 | 0.413 | 0.364 | 0.148 | 35.8 | 1.87 |
| T900-t90 | 1865 | 1132 | 835 | 0.735 | 0.588 | 0.436 | 59.3 | 2.27 |
| Samples | Peak from C 1s Spectrum Binding Energy (%) | ||||
|---|---|---|---|---|---|
| C-C | C-OH | C=O | COOH | CO32−, CO, CO2 | |
| T900-t30 | 65.9 | 13.6 | 3.7 | 2.9 | 13.9 |
| T900-t60 | 58.1 | 20.2 | 4.7 | 4.3 | 12.7 |
| T900-t90 | 43.5 | 28.8 | 9.2 | 7.9 | 10.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Choowang, R.; Zhao, G. Fabrication and Characterization of Activated Carbon Fibers from Oil Palm Trunk. Polymers 2020, 12, 2775. https://doi.org/10.3390/polym12122775
Lin J, Choowang R, Zhao G. Fabrication and Characterization of Activated Carbon Fibers from Oil Palm Trunk. Polymers. 2020; 12(12):2775. https://doi.org/10.3390/polym12122775
Chicago/Turabian StyleLin, Jian, Rattana Choowang, and Guangjie Zhao. 2020. "Fabrication and Characterization of Activated Carbon Fibers from Oil Palm Trunk" Polymers 12, no. 12: 2775. https://doi.org/10.3390/polym12122775
APA StyleLin, J., Choowang, R., & Zhao, G. (2020). Fabrication and Characterization of Activated Carbon Fibers from Oil Palm Trunk. Polymers, 12(12), 2775. https://doi.org/10.3390/polym12122775





