Insights on Potential Formation Damage Mechanisms Associated with the Use of Gel Breakers in Hydraulic Fracturing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fluid Preparation
2.3. Experimental Equipment
2.3.1. HP/HT Rheometer
2.3.2. HP/HT Aging Cell
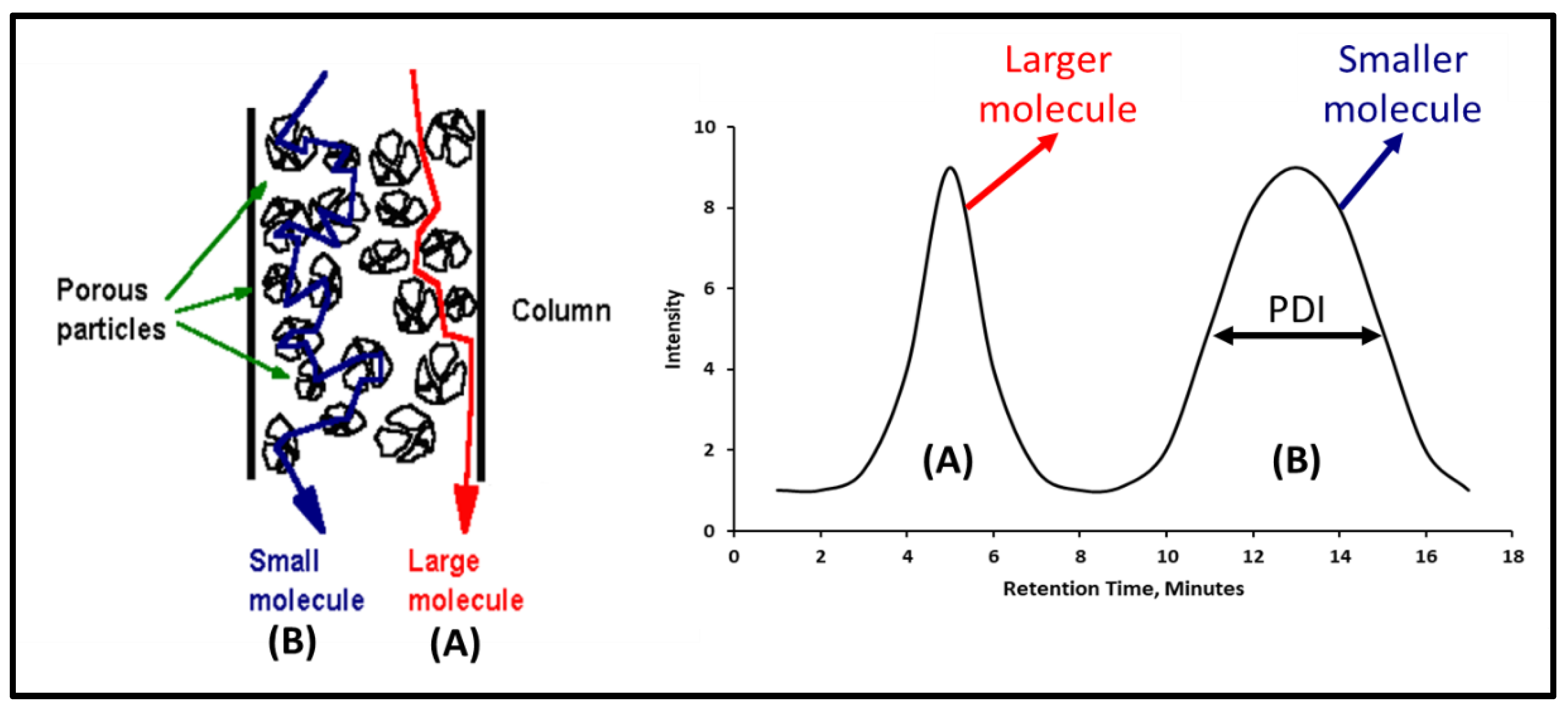
2.3.3. Gel Permeation Chromatography
2.3.4. Zeta Potential
2.3.5. Sour Environment Compatibility Tests
2.3.6. Environmental Scanning Electron Microscope
3. Results and Discussion
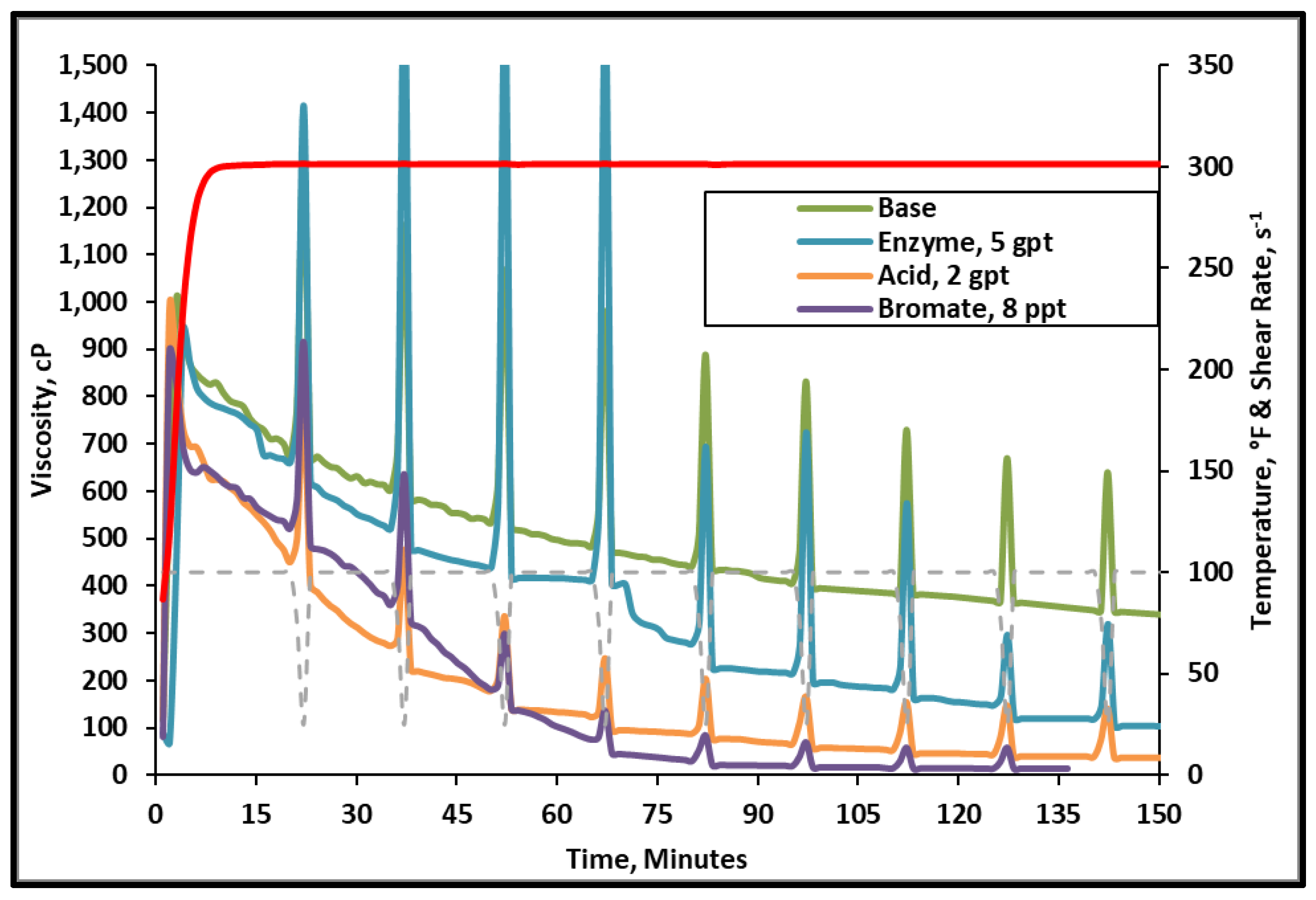
3.1. Fracturing Fluid Viscosity Tests
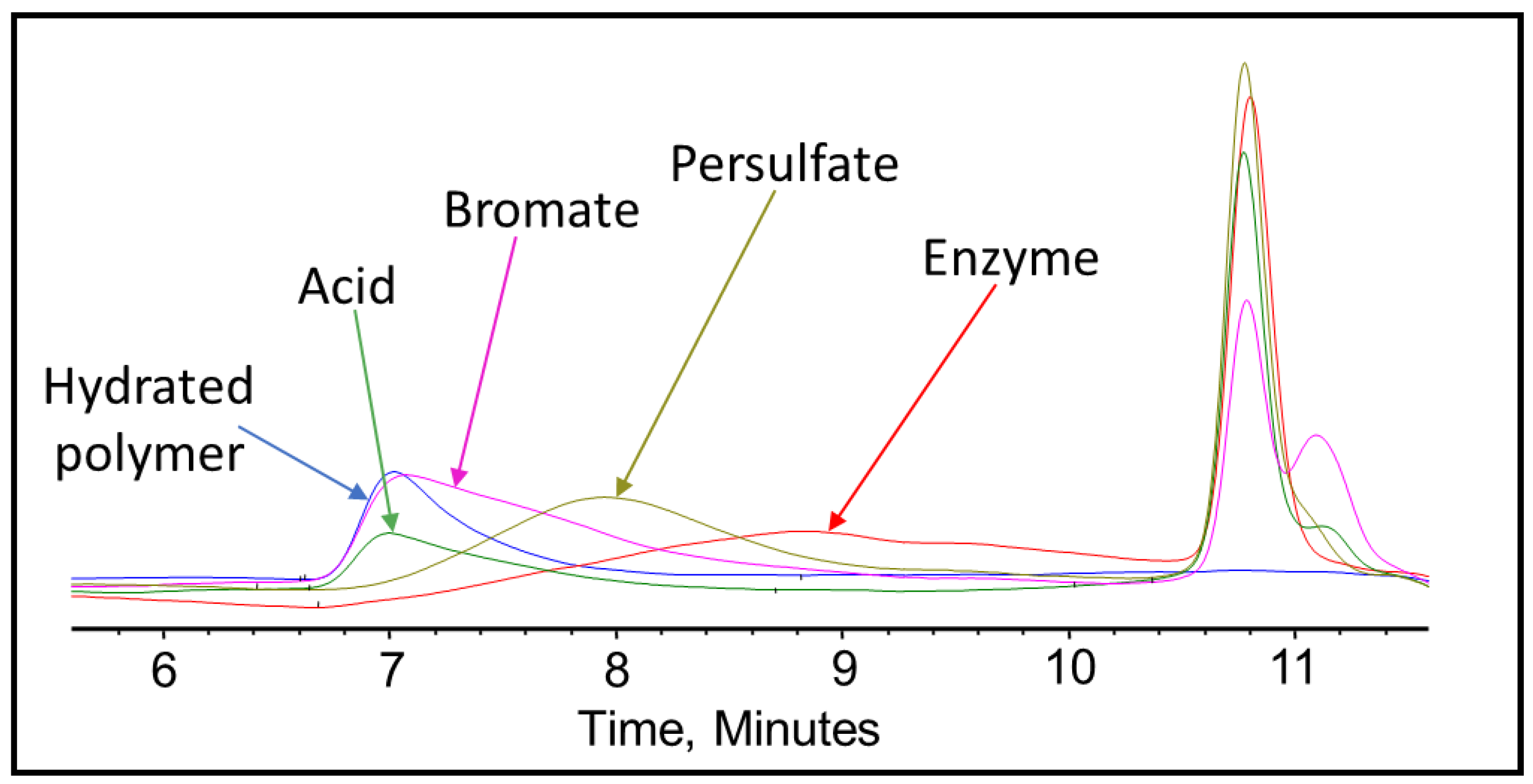
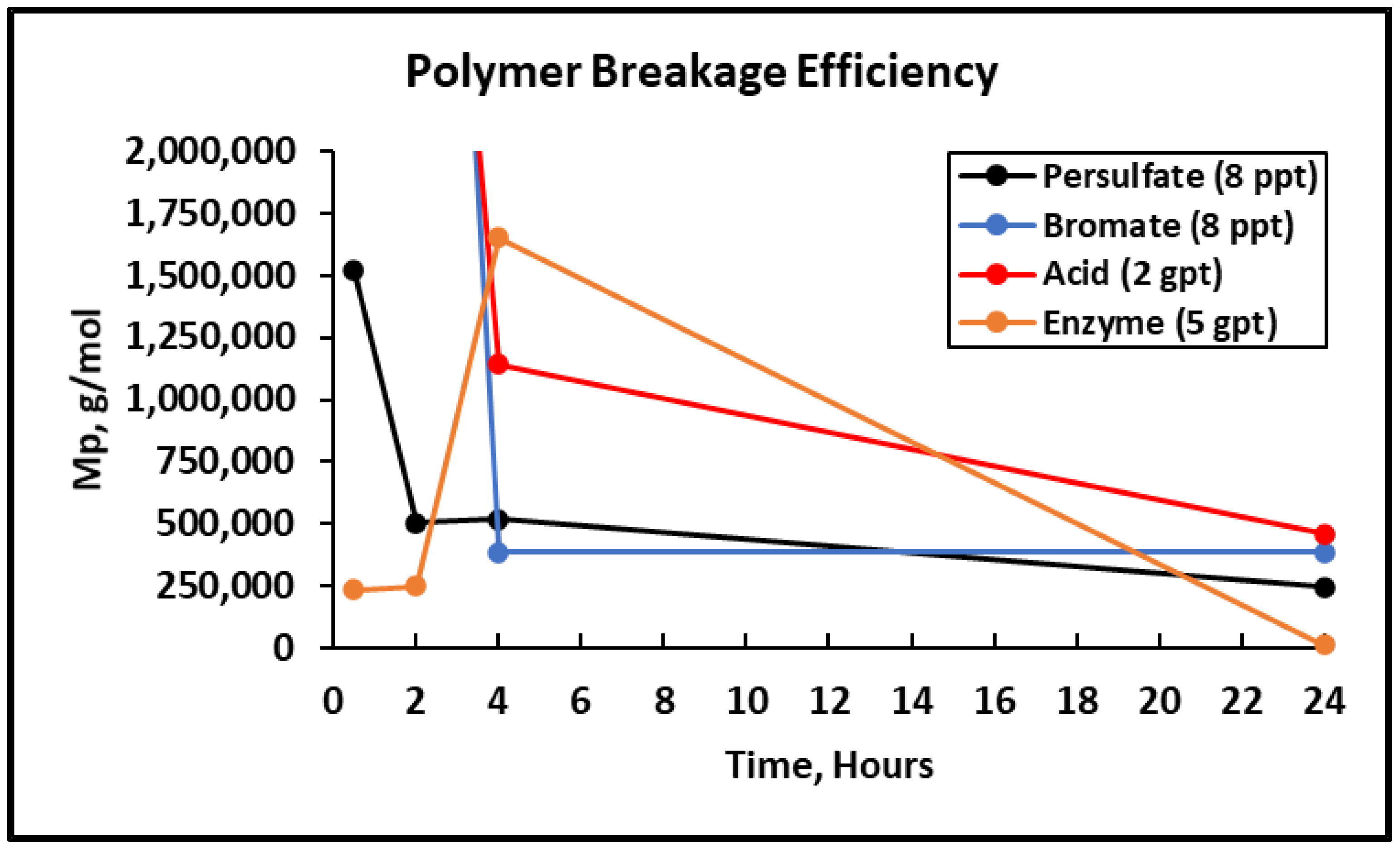
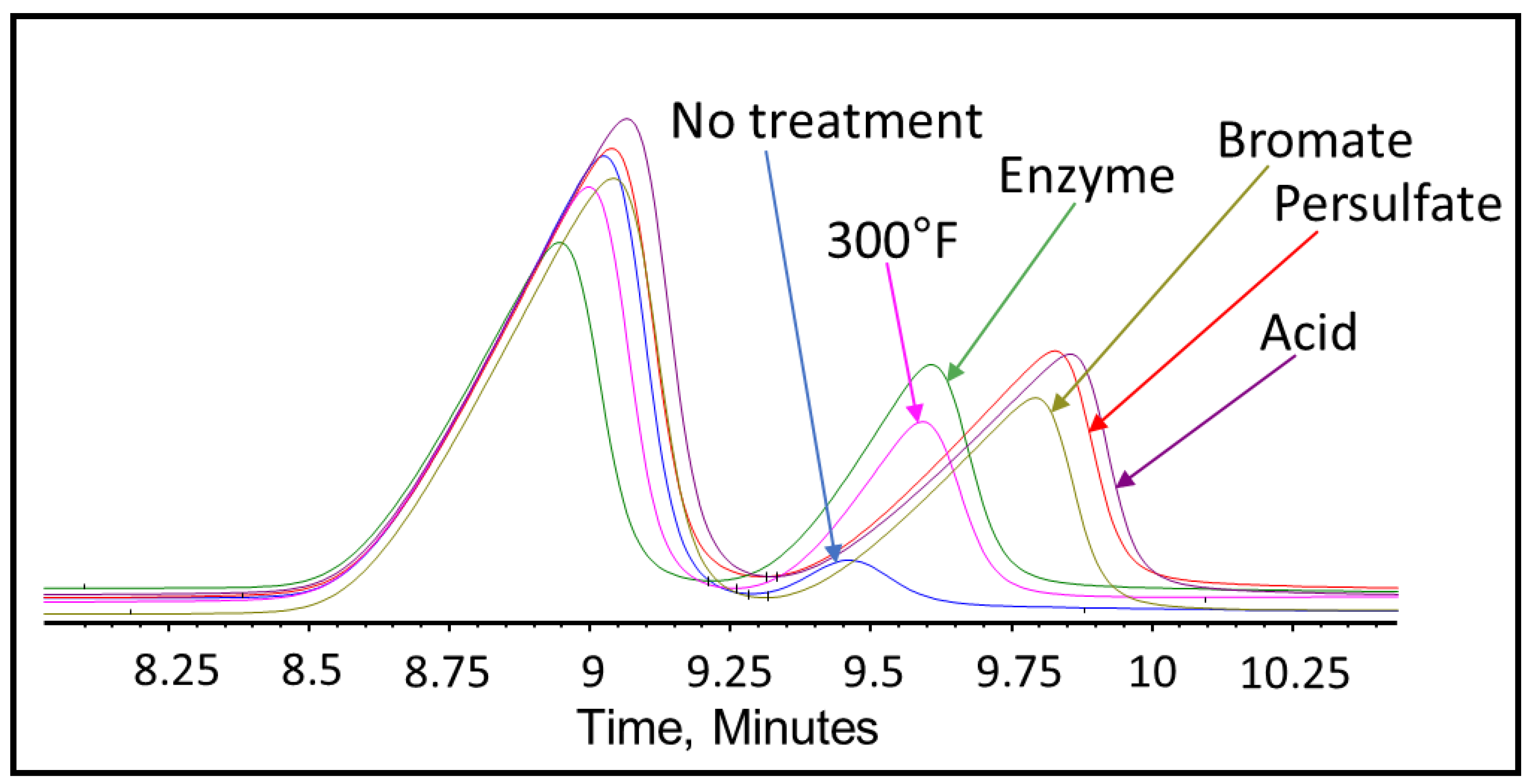
3.2. Fracturing Fluid Polymer-Gel Breaker GPC Analysis
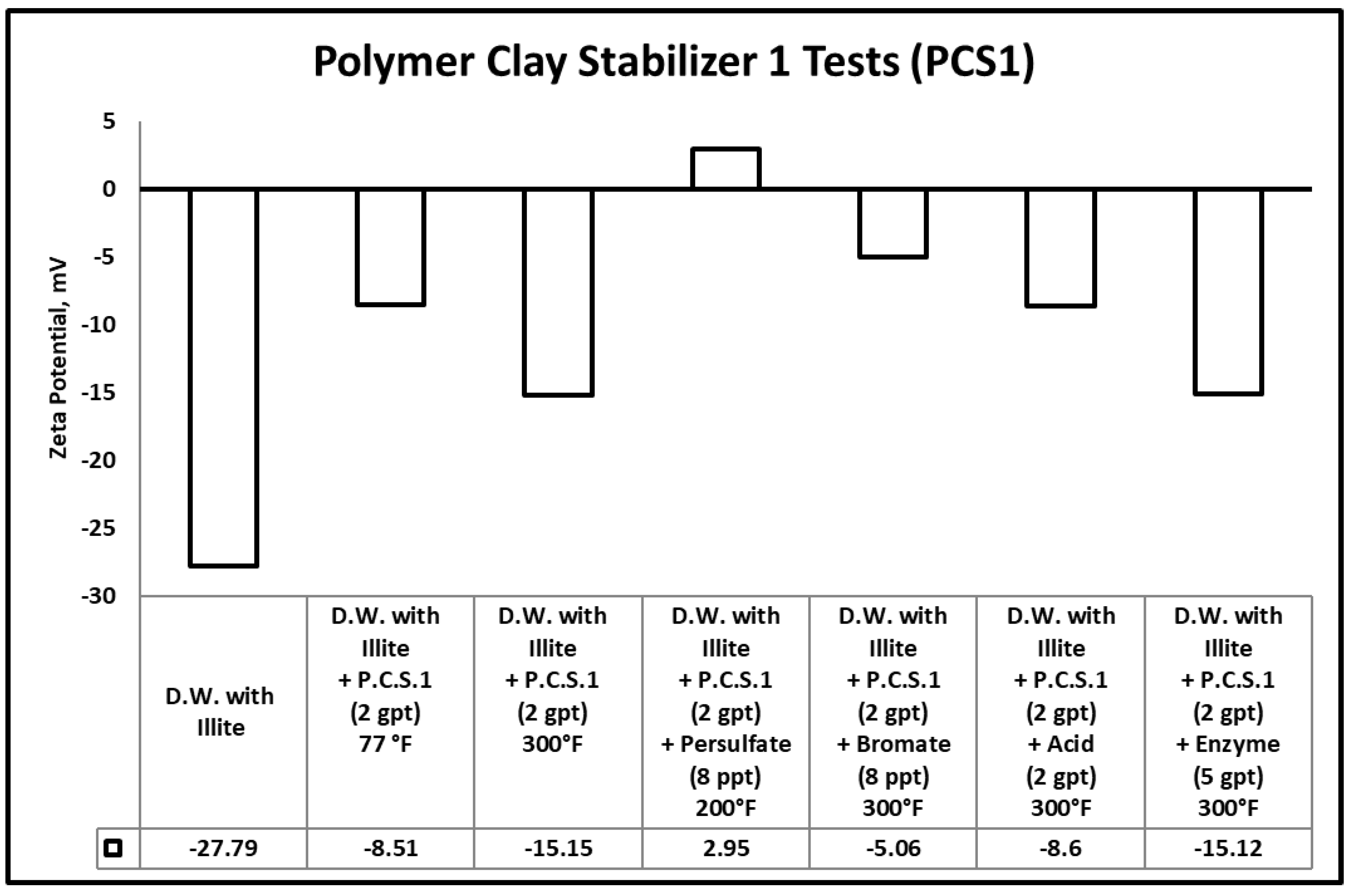
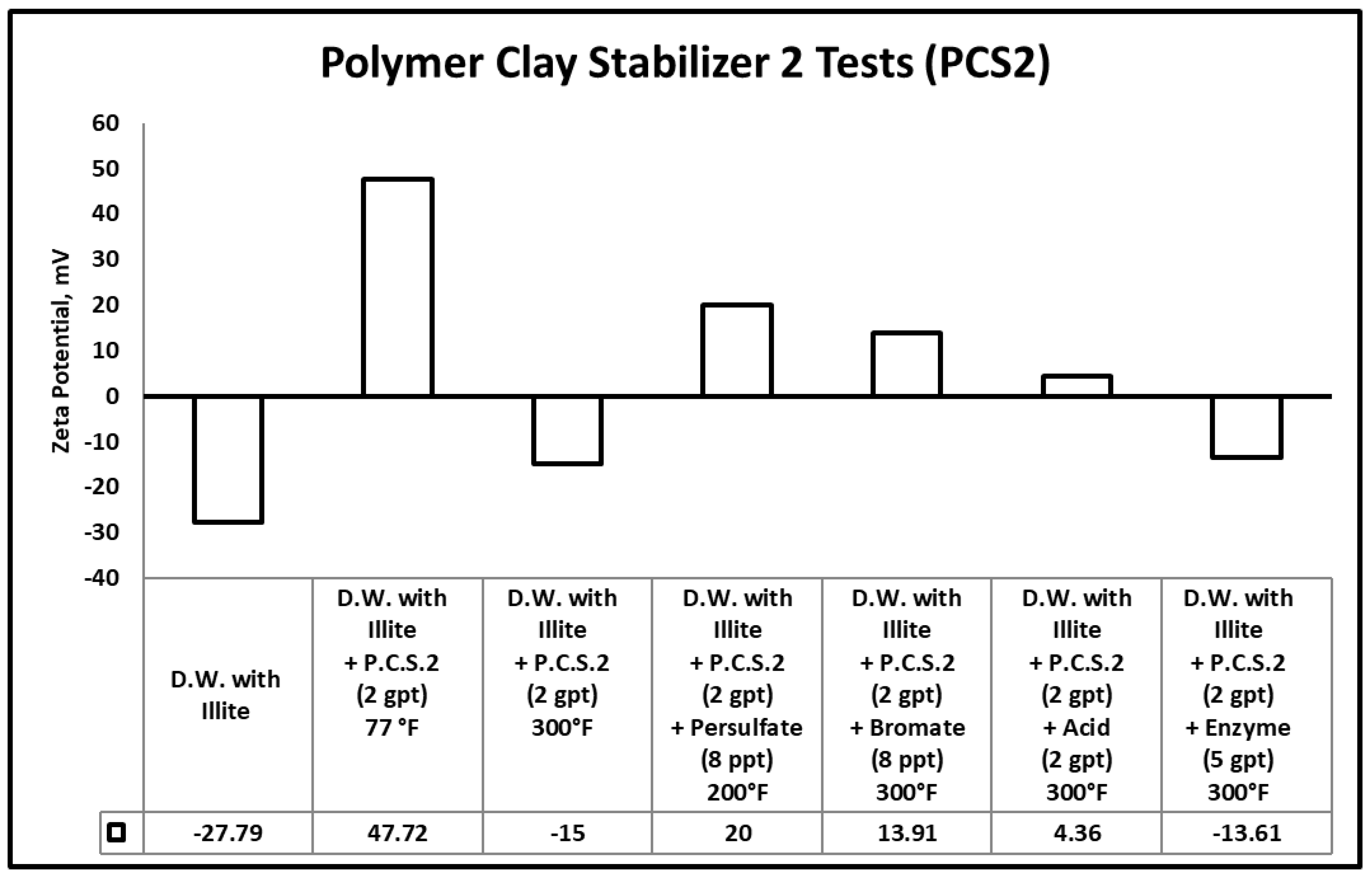
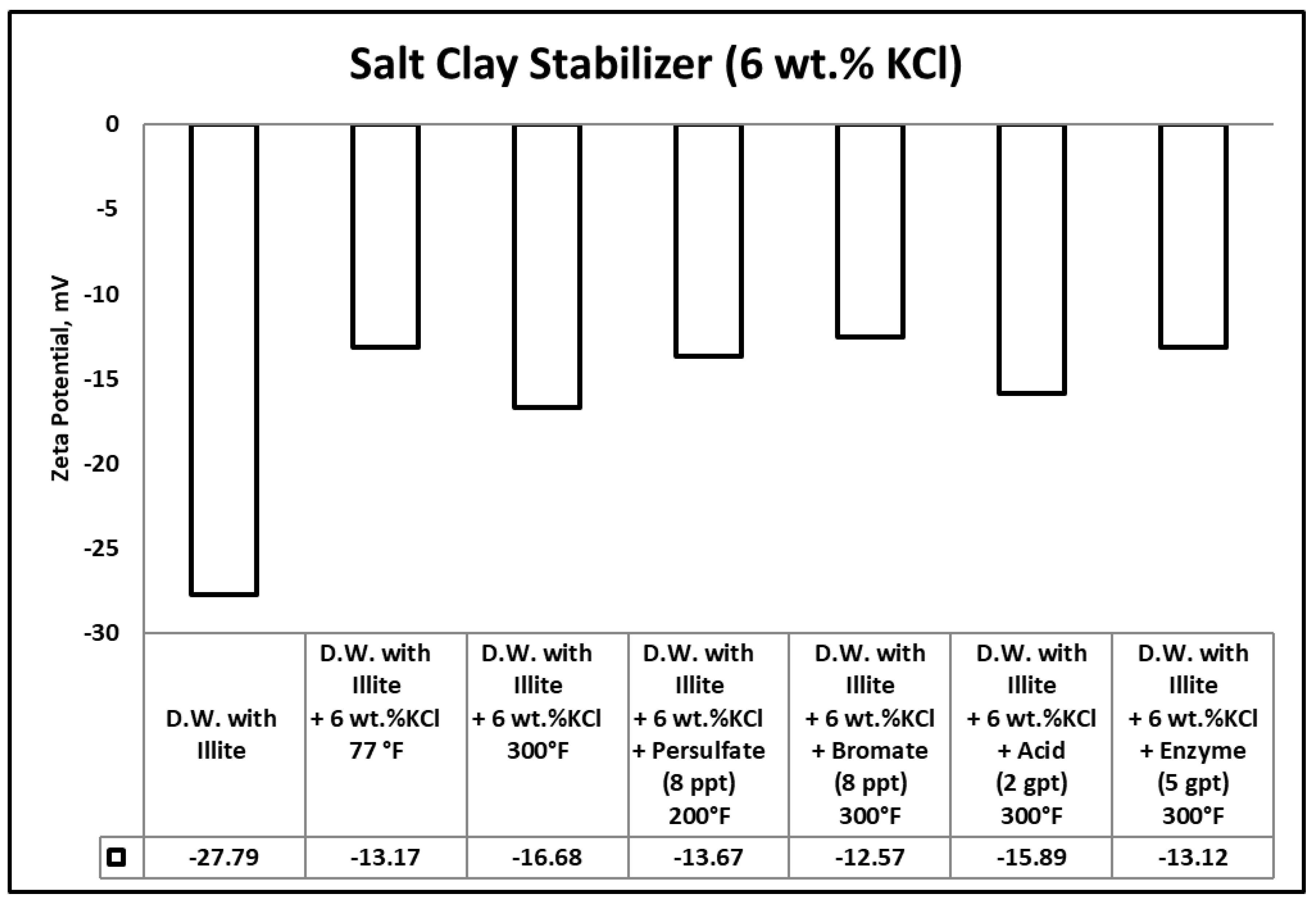
3.3. Zeta Potential
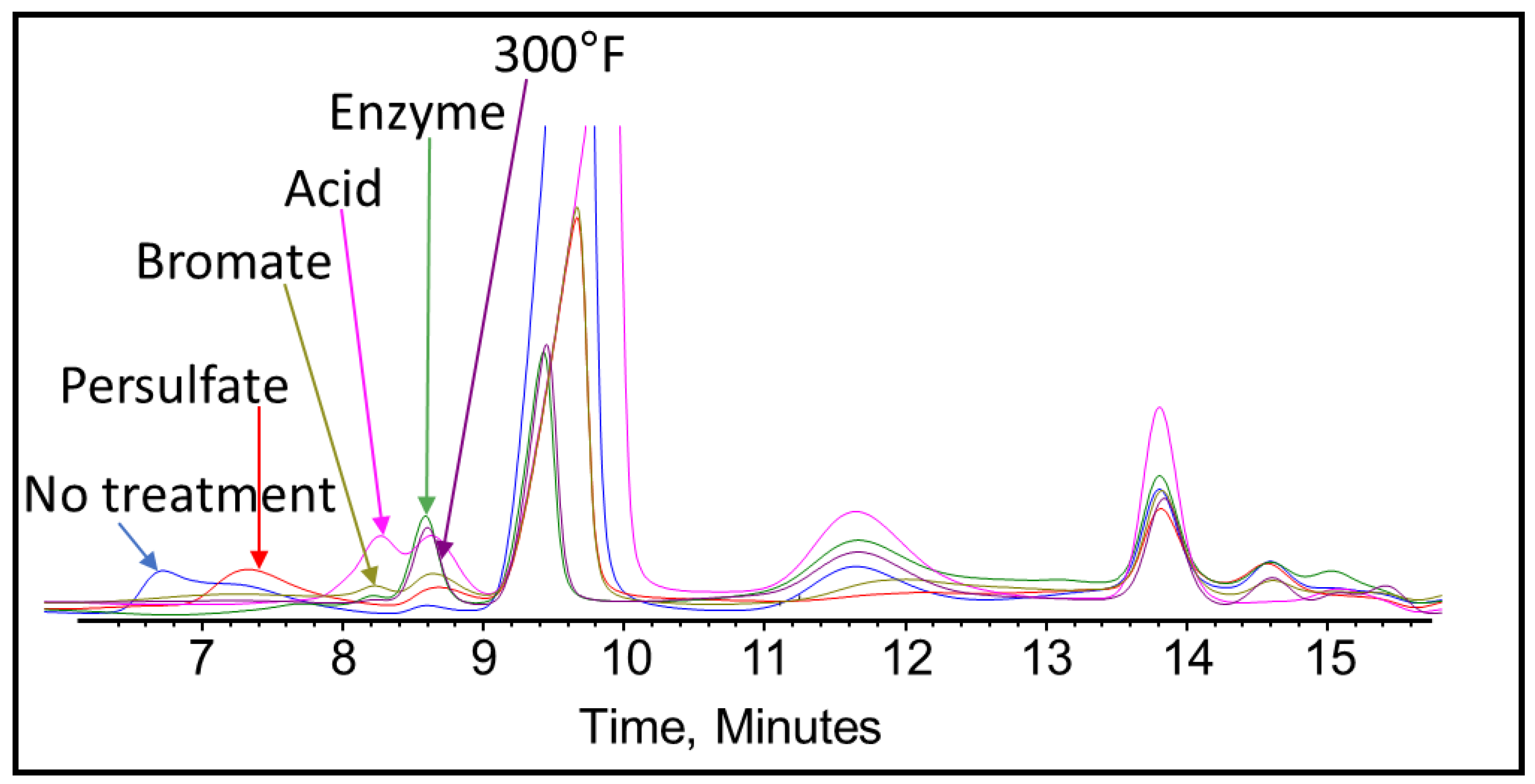
3.4. Polymeric Clay Stabilizer-Gel Breaker GPC Analysis
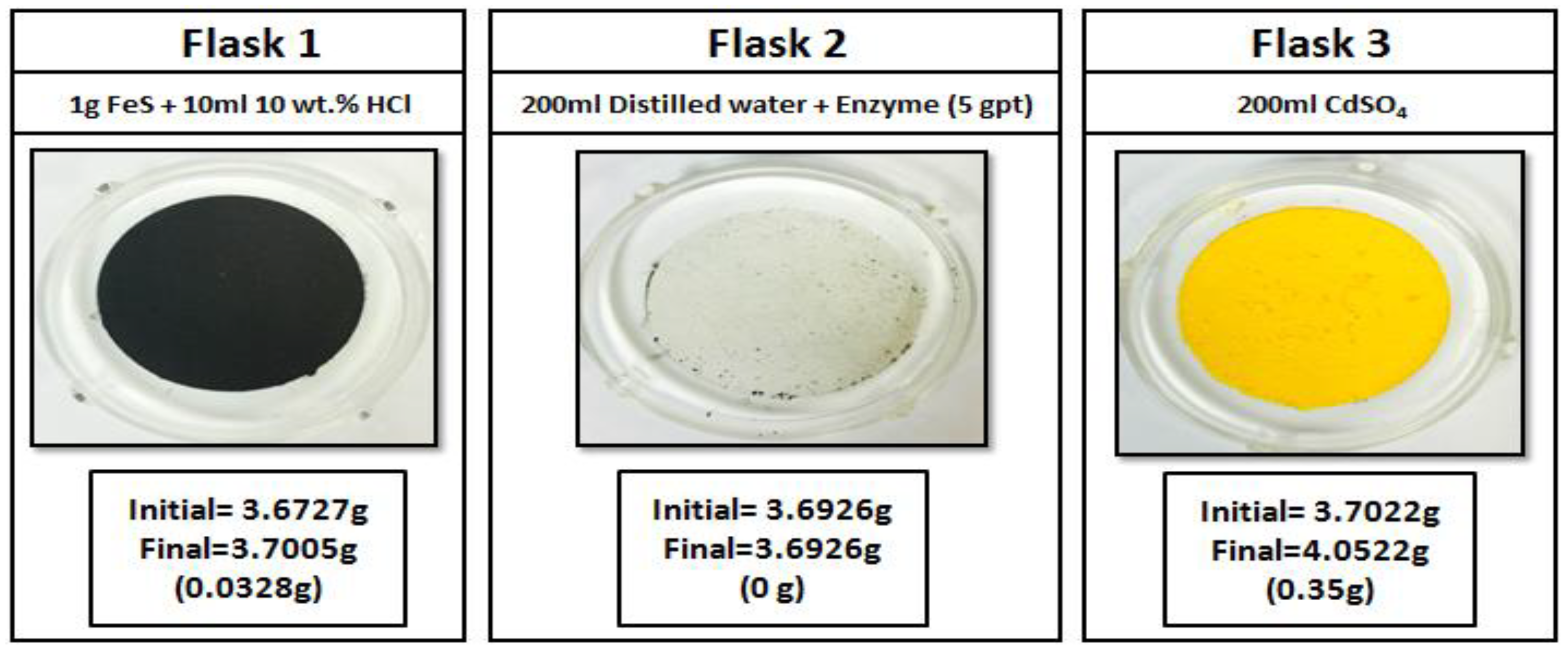
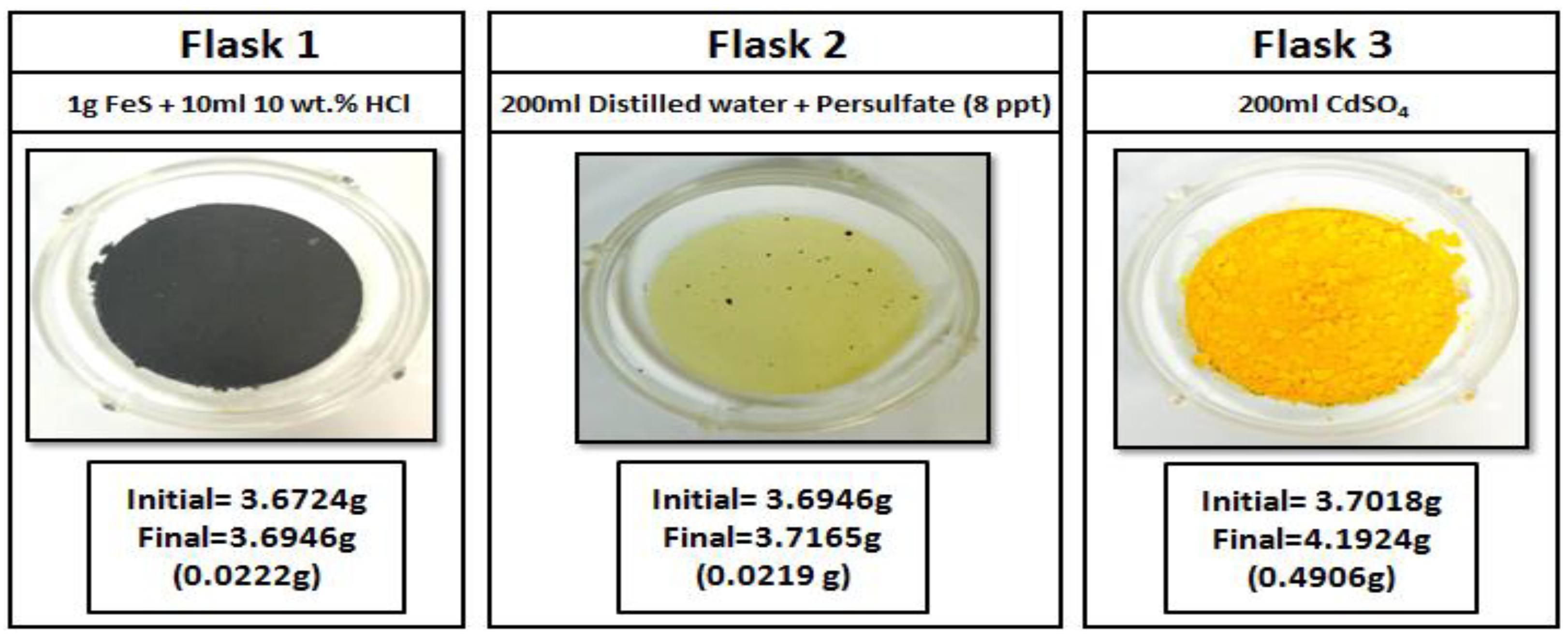
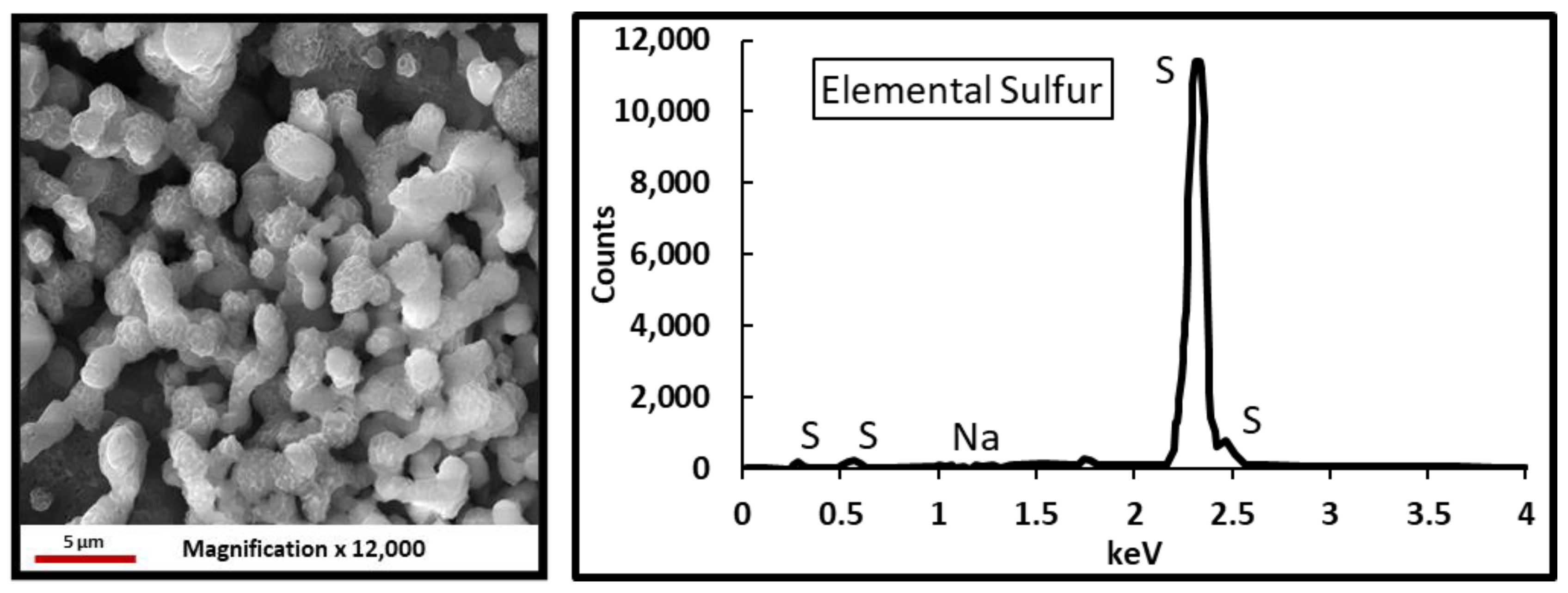
3.5. H2S-Gel Breaker Interactions
4. Conclusions
- The tested gel breakers were all effective in lowering the viscosity of the 45 lb/1000 gal crosslinked fracturing fluid at 300 °F.
- The amount of visual polymer residue generated from the use of oxidizer and acid gel breakers is significant and may cause damage to the fracture conductivity.
- The bromate gel breaker’s intended reactions stopped after 4 h at 300 °F as the broken fracturing fluid polymer size remained constant.
- Enzyme gel breakers took a longer duration to operate fully; however, they generated the smallest broken polymer fragments and the least residue in comparison to oxidizers and acid gel breakers after 24 h at 300 °F.
- Heat (300 °F) and gel breakers (acid, bromate) contributed to the break of polymeric clays stabilizers used in this work, the reduction in the size of polymeric clay stabilizers has negatively influenced its performance, which was evidenced by zeta potential measurements.
- Elemental sulfur precipitation was observed when oxidizers were exposed to H2S.
5. Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| µm | Micrometer. |
| Å | Ampere. |
| CdSO4 | Cadmium sulfate. |
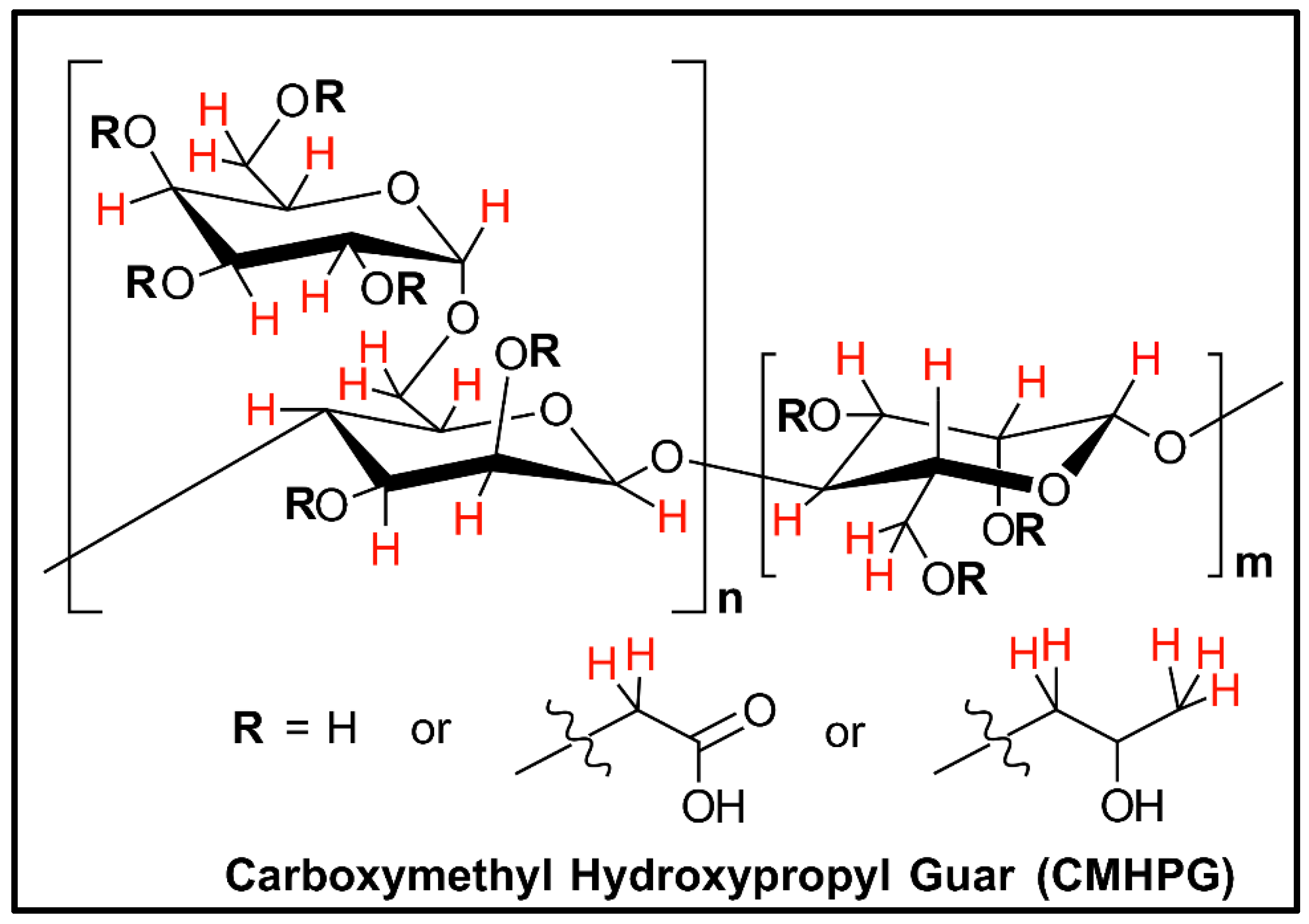
| CMHPG | Carboxymethyl hydroxypropyl guar. |
| DI | Distilled water. |
| EDS | Energy dispersive x-ray spectroscopy. |
| ESEM | Environmentally scanning electron microscope. |
| FeS | Iron sulfide. |
| GPC | Gel permeation chromatography. |
| gpt | Gallons per thousand gallons. |
| H2S | Hydrogen sulfide. |
| HP/HT | High-pressure/high-temperature. |
| HPG | Hydroxypropyl guar. |
| MBI | Monoborate ions. |
| mm | Millimeter. |
| Mp | Peak molecular weight. |
| PDI | Polydispersity index. |
| ppm | Parts per million. |
| ppt | Pounds per thousand gallons. |
| PSC1 | Polymeric clay stabilizer 1. |
| PSC2 | Polymeric clay stabilizer 2. |
| Room temperature | 77 °F. |
References
- Clark, J. A hydraulic process for increasing the productivity of wells. J. Pet. Technol. 1949, 1, 1–8. [Google Scholar] [CrossRef]
- Farris, R.F. Fracturing Formations in Wells. US Patent No. US23733, 10 November 1953. [Google Scholar]
- Al-Muntasheri, G.A. A critical review of hydraulic-fracturing fluids for moderate to ultralow-permeability formations over the last decade. SPE Prod. Oper. 2014, 29, 243–260. [Google Scholar] [CrossRef]
- Yongjun, L.; Yandong, C.; Zhongyang, Z.; Zhenduo, W. A case study of boron crosslinked fracturing fluid in ultra-deep wall. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 16–19 February 1999. SPE-50787-MS. [Google Scholar] [CrossRef]
- Ding, Y.; Jiang, T.; Wang, Y.; Sun, P.; Xu, Z.; Wang, X.; Zeng, B. A case study of massive hydraulic fracturing in an ultra-deep gas well. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Perth, Australia, 18–20 October 2004. SPE-88613-MS. [Google Scholar] [CrossRef]
- Gupta, D.S.; Carman, P.S.; Venugopal, R.A. Stable fracturing fluid for produced water applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 8–10 October 2012. SPE-159837-MS. [Google Scholar] [CrossRef]
- Li, L.; Al-Muntasheri, G.A.; Liang, F. A review of crosslinked fracturing fluids prepared with produced water. Petroleum 2016, 2, 313–323. [Google Scholar] [CrossRef]
- Almubarak, T.; Alkhaldi, M.; Ng, J.H.; Nasr-El-Din, H.A. Design and application of high-temperature raw-seawater-based fracturing fluids. SPE J. 2019, 24, 1929–1946. [Google Scholar] [CrossRef]
- Rae, P.; Lullo, G.D. Fracturing fluids and breaker systems–A review of the state-of-the-art. In Proceedings of the SPE Eastern Regional Meeting, Columbus, OH, USA, 23–25 October 1996. SPE-37359-MS. [Google Scholar] [CrossRef]
- Howard, G.C.; Fast, C.R. Hydraulic Fracturing; Society of Petroleum Engineers of AIME: New York, NY, USA, 1970. [Google Scholar]
- Harms, W. Application of chemistry in oil and gas well fracturing. In Oil-Field Chemistry; Borchardt, J., Yen, T., Eds.; American Chemical Society: Washington, DC, USA, 1989; Volume 2, pp. 55–100. [Google Scholar] [CrossRef]
- Maberry, L.; McConnell, S.; Hinkel, J. New complexation chemistry provides improved continuous-mix gelled oil. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 18–21 February 1997. SPE-37227-MS. [Google Scholar] [CrossRef]
- Thompson, J.E.; DeVine, C.S.; Delorey, J.R. State-of-the-art nonphosphate design revolutionizes hydrocarbon-based completion technologies. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 1–4 October 2000. SPE-63244-MS. [Google Scholar] [CrossRef]
- Taylor, R.S.; Fyten, G.; Romanson, R.; Mclntosh, G.; Litun, R.; Munn, D.; Bennion, B.; Piwowar, M.; Hoch, O. Montney Fracturing-fluid considerations. J. Can. Pet. Technol. 2010, 49, 28–36. [Google Scholar] [CrossRef]
- Li, L.; Ozden, A.; Zhang, J.; Liang, F. Enhanced gelled hydrocarbon well treatment fluids. Petroleum 2020, 6, 177–181. [Google Scholar] [CrossRef]
- Almubarak, M.; Almubarak, T.; Ng, J.C.; Nasr-El-Din, H.A. Recent advances in waterless fracturing fluids: A review. In Proceedings of the Abu Dhabi International Petroleum and Exhibition, Abu Dhabi, UAE, 9–12 November 2020. SPE-202981-MS. [Google Scholar] [CrossRef]
- Montgomery, C. Fracturing Fluid Components. In Effective and Sustainable Hydraulic Fracturing; Jeffrey, R., Mclennan, J., Bunger, A., Eds.; Intechopen Limited: London, UK, 2013; Volume 2, pp. 26–44. [Google Scholar] [CrossRef]
- Azizov, E.; Quintero, H.J.; Saxton, K.; Sessarego, S. Carboxymethylcellulose a cost effective alternative to guar, CMHPG and surfactant-based fluid systems. In Proceedings of the SPE/CSUR Unconventional Resources Conference, Calgary, AL, Canada, 20–22 October 2015. SPE-175904-MS. [Google Scholar] [CrossRef] [Green Version]
- Ming, H.; Lu, Y.; Qiu, X.; Shu, Y.; Wang, S. Development and field application of a novel cellulose fracturing fluid. In Proceedings of the SPE Asia Pacific Hydraulic Fracturing Conference, Beijing, China, 24–26 August 2016. SPE-181778-MS. [Google Scholar] [CrossRef]
- White, J.; Means, J. Polysaccharide derivatives provide high viscosity and low friction at low surface fluid temperatures. J. Pet. Technol. 1975, 27, 1067–1073, SPE-4936-PA. [Google Scholar] [CrossRef]
- Venkataiah, S.; Mahadevan, E. Rheological properties of hydroxypropyl- and sodium carboxymethyl-substituted guar gums in aqueous solution. J. Appl. Polym. Sci. 1982, 27, 1533–1548. [Google Scholar] [CrossRef]
- Weaver, J.; Gdanski, R.; Karcher, A. Guar gum degradation: A kinetic study. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. SPE-80226-MS. [Google Scholar] [CrossRef]
- Lei, C.; Clark, P.E. Crosslinking of guar and guar derivatives. SPE J. 2007, 12, 316–321. [Google Scholar] [CrossRef]
- Pasha, M.; Ngn, S. Derivatization of guar to sodium carboxymethyl hydroxypropyl derivative: Characterization and evaluation. Pak. J. Pharm. Sci. 2008, 21, 40–44. [Google Scholar]
- Hu, Y.T.; Chung, H.; Maxey, J.E. What is more important for proppant transport, viscosity or elasticity? In Proceedings of the SPE Hydraulic Fracturing Technology Conference, The Woodlands, TX, USA, 3–5 February 2015. SPE-173339-MS. [Google Scholar] [CrossRef]
- Nickerson, R. Thickening of poly(vinyl alcohol) by borate. J. Appl. Polym. Sci. 1971, 15, 111–116. [Google Scholar] [CrossRef]
- Kramer, J.; Prud’homme, R.K.; Norman, L.R.; Sandy, J.M. Characteristics of metal-polymer interactions in fracturing fluid systems. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 27–30 September 1987. SPE-16914-MS. [Google Scholar] [CrossRef]
- Kramer, J.; Prud’homme, R.; Wiltzius, P.; Mirau, P.; Knoll, S. Comparison of galactomannan crosslinking with organotitanates and borates. Colloid Polym. Sci. 1988, 266, 145–155. [Google Scholar] [CrossRef]
- Prud’homme, R.; Constien, V.; Knoll, S. The effects of shear history on the rheology of hydroxypropyl guar gels. Advan. Chem. 1989, 223, 89–112. [Google Scholar] [CrossRef]
- Brannon, H.; Ault, M. New, delayed borate-crosslinked fluid provides improved fracture conductivity in high-temperature applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 6–9 October 1991. SPE-22838-MS. [Google Scholar] [CrossRef]
- Dawson, J. A Thermodynamic study of borate complexation with guar and guar derivatives. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 6–9 October 1991. SPE-22837-MS. [Google Scholar] [CrossRef]
- Kesavan, S.; Prud’homme, R.; Parris, M. Crosslinked borate HPG equilibria and rheological characterization. In Proceedings of the SPE International Symposium on Oilfield Chemistry, New Orleans, LA, USA, 2–5 March 1993. SPE-25205-MS. [Google Scholar] [CrossRef]
- Harris, P.C. 1993. Chemistry and rheology of borate-crosslinked fluids at temperatures to 300F. J. Pet. Technol. 1993, 45, 264–269. [Google Scholar] [CrossRef]
- Moorhouse, R.; Harry, D.; Matthews, L.; Merchant, U. Inter-relationships between polymer/crosslinker chemistry and performance. In Proceedings of the SPE India Oil and Gas Conference and Exhibition, New Delhi, India, 17–19 February 1998. SPE-39531-MS. [Google Scholar] [CrossRef]
- Parris, M.D.; MacKay, B.A.; Rathke, J.W.; Klingler, R.J.; Gerald, R.E. Influence of pressure on boron cross-linked polymer gels. Macromolecules 2008, 41, 8181–8186. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Nasr-El-Din, H.A.; Almubarak, M.; Alkhaldi, M. Zirconium crosslinkers: Understanding performance variation in crosslinked fracturing fluids. In Proceedings of the Offshore Technology Conference Asia, Kuala Lumpur, Malaysia, 2–6 November 2020. OTC-30381-MS. [Google Scholar] [CrossRef]
- Yaritz, J.; Stegent, N.; Bailey, T.; Fritcher, E. Development of a dual crosslinker fracturing fluid system. In Proceedings of the Latin American and Caribbean Petroleum Engineering Conference, Rio de Janeiro, Brazil, 30 August–3 September 1997. SPE-38959-MS. [Google Scholar] [CrossRef]
- Driweesh, S.M.; Al-Atwi, M.A.; Malik, A.R.; Olarte, J.E.; Jauregui, J.A.; Bolarinwa, S.O. Successful implementation of zirconate borate based dual crosslinked gel and continuous mixing system during proppant fracturing treatment in a complex high temperature and high pressure sandstone gas reservoir in Saudi Arabia that exceeded the well objective and showed substantial increase in operational efficiency–A case study. In Proceedings of the International Petroleum Technology Conference, Beijing, China, 26–28 March 2013. IPTC-16664-MS. [Google Scholar] [CrossRef]
- Reddy, B.R. Laboratory Characterization of gel filter cake and development of nonoxidizing gel breakers for zirconium-crosslinked fracturing fluids. SPE J. 2014, 19, 662–673. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A.; Li, L.; Liang, F.; Gomaa, A.M. Concepts in cleanup of fracturing fluids used in conventional reservoirs: A literature review. SPE Prod. Oper. 2018, 33, 196–213. [Google Scholar] [CrossRef]
- Alterman, D.S.; Chun, K.W. Encapsulation Particles. US Patent No. US3983254, 28 September 1976. [Google Scholar]
- Nolte, K.G. Fracture Fluid Breaker System Which is Activated by Fracture Closure. US Patent No. US4506734, 26 March 1985. [Google Scholar]
- Gulbis, J.; King, M.T.; Hawkins, G.W.; Brannon, H.D. Encapsulated breaker for aqueous polymeric fluids. SPE Prod. Eng. 1992, 7, 9–14. [Google Scholar] [CrossRef]
- Manalastas, P.V.; Drake, E.N.; Kresge, E.N.; Thaler, W.A.; Mcdougall, L.A.; Newlove, J.C.; Swarup, V.; Geiger, A.J. Breaker Chemical Encapsulation with a crosslinked Elastomer Coating. US Patent No. US5110486, 5 May 1992. [Google Scholar]
- Lo, S.; Miller, M.J.; Li, J. Encapsulated breaker release rate at hydrostatic pressure and elevated temperatures. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 29 September–2 October 2002. SPE-77744-MS. [Google Scholar] [CrossRef]
- Barati, R.; Johnson, S.; Mccool, S.; Green, D.W.; Willhite, G.P.; Liang, J.T. Polyelectrolyte complex nanoparticles for protection and delayed release of enzymes in alkaline pH and at elevated tempearature during hydraulic fracturing oil wells. J. Appl. Polym. Sci. 2012, 126, 587–592. [Google Scholar] [CrossRef]
- Gall, B.; Raible, C. Molecular size studies of degraded fracturing fluid polymers. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Phoenix, AZ, USA, 9–11 March 1985. SPE-13566-MS. [Google Scholar] [CrossRef]
- Brannon, H.; Tjon-Joe-Pin, R. Biotechnological breakthrough improves performance of moderate to high-temperature fracturing applications. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 25–28 September 1994. SPE-28513-MS. [Google Scholar] [CrossRef]
- Economides, M.; Nolte, K. Reservoir Stimulation, 3rd ed.; John Wiley and Sons Limited: Chichester, UK, 2000. [Google Scholar]
- Gupta, D.V.S.; Pakulski, M.K.; Prasek, B.; Franklin, V.F. High-pH-tolerant enzyme breaker for oilfield applications. In Proceedings of the Permian Basin Oil and Gas Recovery Conference, Midland, TX, USA, 18–20 March 1992. SPE-23986-MS. [Google Scholar] [CrossRef]
- Cobianco, S.; Albonico, P.; Battistel, E.; Bianchi, D.; Fornaroli, M. Thermophilic enzymes for filtercake removal at high temperature. In Proceedings of the European Formation Damage Conference, Scheveningen, The Netherlands, 30 May–1 June 2007. SPE-107756-MS. [Google Scholar] [CrossRef]
- Li, L.; Lin, L.; Ezeokonkwo, C.; Boney, C.L.; Howard, P.R.; Shenoy, S.; Quintero, B.W.; Eliseeva, K. Treatment and Reuse of Oilfield Produced Water for Operations in a well. US Patent No. US8658574, 25 February 2014. [Google Scholar]
- Elkatatny, S.; Mahmoud, M.; Shawabkeh, R. Investigating the compatibility of enzyme with chelating agents for calcium carbonate-filter cake removal. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2017. SPE-187978-MS. [Google Scholar] [CrossRef]
- Nelson, C.M.; Schuppenhauer, M.R.; Clark, D.S. High-pressure, high-temperature bioreactor for comparing effects of hyperbaric and hydrostatic pressure on bacterial growth. Appl. Environ. Microbiol. 1992, 58, 1790–1793. [Google Scholar] [CrossRef] [Green Version]
- Samuel, M.; Mohsen, A.H.; Ejan, A.B.; Ooi, Y.S.; Ashraf, S.; Nasr-el-din, H.A. A novel alpha-amylase enzyme stabilizer for applications at high temperatures. SPE Prod. Oper. 2010, 25, 398–408. [Google Scholar] [CrossRef]
- Zhang, B.; Davenport, A.H.; Whipple, L.; Urbina, H.; Barrett, K.; Wall, M.; Hutchins, R.; Mirakyan, A. A superior, high-performance enzyme for breaking borate crosslinked fracturing fluids under extreme well conditions. SPE Prod. Oper. 2013, 28, 210–216. [Google Scholar] [CrossRef]
- Al-Khaldi, M.H.; Ghosh, B.; Ghosh, D. A novel enzyme breaker for mudcake removal in high temperature horizontal and multi-lateral wells. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Jakarta, Indonesia, 20–22 September 2011. SPE-147863-MS. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-Mohammed, A.M.; Al-Aamri, A.D.; Al-Fuwaires, O. A study of gel degradation, surface tension, and their impact on the productivity of hydraulically fractured gas wells. In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November 2007. SPE-109690-MS. [Google Scholar] [CrossRef]
- Maley, D.M.; O’Neil, B.J. Breaker enhancer for crosslinked borates: Novel self generating acid. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AL, Canada, 19–21 October 2010. SPE-137490-MS. [Google Scholar] [CrossRef]
- Reinicke, A.; Blöcher, G.; Zimmermann, G.; Huenges, E.; Dresen, G.; Stanchits, S.; Legarth, B.A.; Makurat, A. Mechanically induced fracture-face skin—Insights from laboratory testing and modeling approaches. SPE Prod. Oper. 2012, 28, 26–35. [Google Scholar] [CrossRef]
- Almond, S.; Bland, W. The effect of break mechanism on gelling agent residue and Flow impairment in 20/40 mesh sand. In Proceedings of the SPE Formation Damage Control Symposium, Bakersfield, CA, USA, 13–14 February 1984. SPE-12485-MS. [Google Scholar] [CrossRef]
- ISO. ISO 13503-1. Petroleum and natural gas industries-completion fluids and materials-Part 1. Measurement of Viscous Properties of Completion Fluids, 2nd ed.; ISO: Geneva, Switzerland, 2011. [Google Scholar]
- Jackson, C.; Larsen, B.; McEwen, C. Comparison of peak molecular weight values as measured for polymer molecular weight distribution by MALDI mass spectrometry and by size exclusion chromatography. Anal. Chem. 1996, 68, 1303–1308. [Google Scholar] [CrossRef]
- Chorom, M.; Rengasamy, P. Dispersion and zeta potential of pure clays as related to net particle charge under varying pH, electrolyte concentration and cation type. Eur. J. Soil Sci. 1995, 46, 657–665. [Google Scholar] [CrossRef]
- Almubarak, T.; AlDajani, O.; AlMubarak, M. A collective clay stabilizers review. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 6–9 December 2015. IPTC-18394-MS. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, J.H. Hybrid Enhanced Oil Recovery Using Smart Waterflooding, 1st ed.; Elsevier Science & Technology: New York, NY, USA, 2019. [Google Scholar]
- Ogunsanya, T.; Li, L. Safe boundaries of high-temperature fracturing fluids. In Proceedings of the SPE Western Regional Meeting, Garden Grove, CA, USA, 22–26 April 2018. SPE-190029-MS. [Google Scholar] [CrossRef]
- Cadena, F.; Peters, R. Evaluation of chemical oxidizers for hydrogen sulfide control. J. Water Pollut. Control Fed. 1988, 60, 1259–1263. Available online: https://www.jstor.org/stable/25043633 (accessed on 16 November 2020).
- Kotronarou, A.; Hoffmann, M. Peroxymonosulfate: An alternative to hydrogen peroxide for the control of hydrogen sulfide. Res. J. Water Pollut. Control Fed. 1991, 63, 965–970. [Google Scholar]
- Parker, M. Method for Removing Hydrogen Sulfide from Sour Gas and Converting it to Hydrogen and Sulfuric Acid. Ph.D. Thesis, Stanford University, Palo Alto, CA, USA, 2010. [Google Scholar]
- Benchoam, D.; Cuevasanta, E.; Möller, M.; Alvarez, B. Hydrogen sulfide and persulfides oxidation by biologically relevant oxidizing species. Antioxidants 2019, 8, 48. [Google Scholar] [CrossRef] [Green Version]





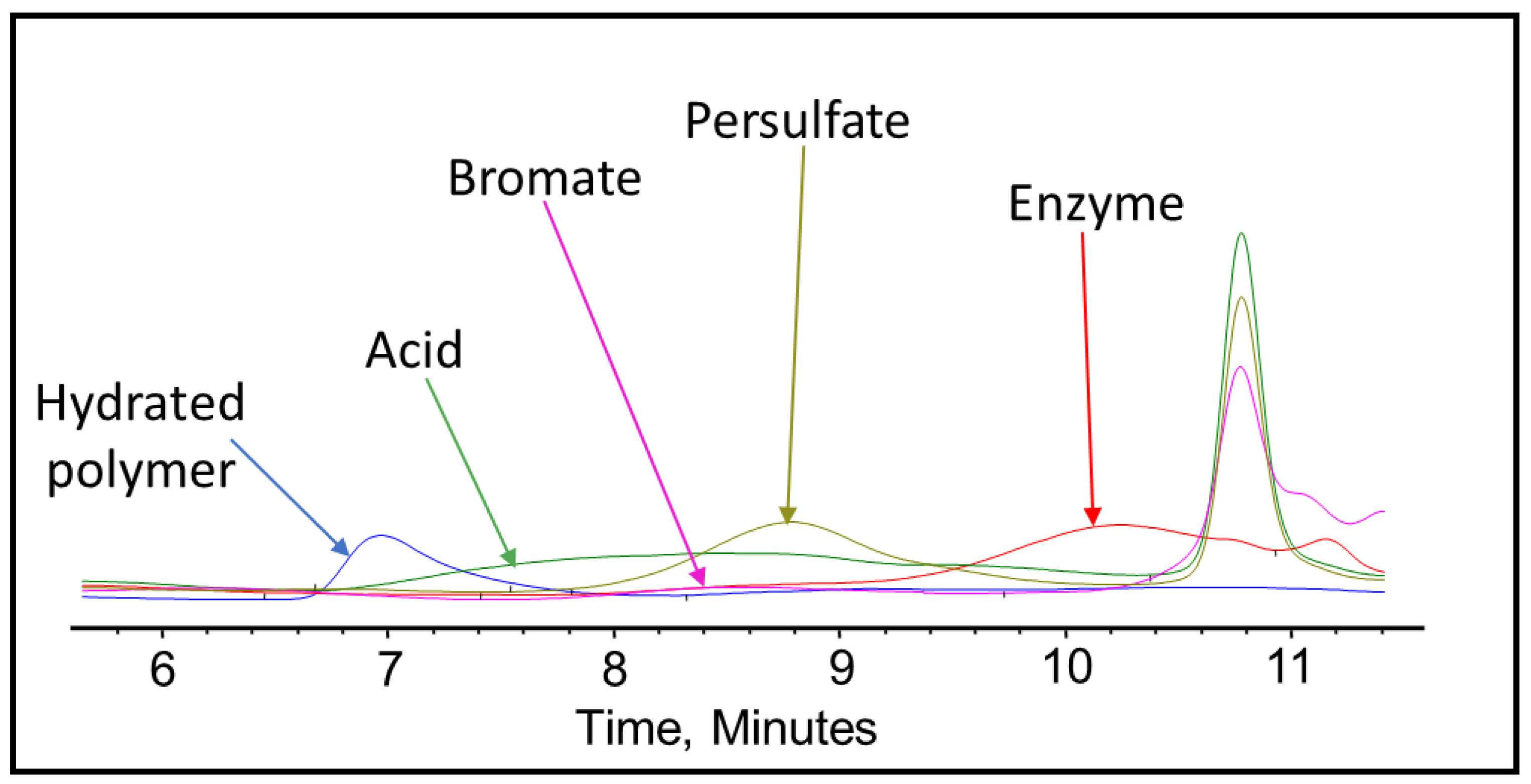


| Chemical | Concentration |
|---|---|
| Polymer (CMHPG) | 45 ppt |
| High-temperature stabilizer (Sodium Thiosulfate) | 9 gpt |
| Zr-crosslinker (Zirconium Triethanolamine) | 0.8 gpt |
| B-crosslinker (Potassium Metaborate) | 0.1 gpt |
| Additive | Main Component |
|---|---|
| Acid breaker | Chlorous acid |
| Bromate gel breaker | Sodium bromate |
| Persulfate gel breaker | Diammonium peroxidisulfate |
| Enzyme gel breaker | Mixture of 1,6-α-D-galactosidase and endo-1,4-β-mannosidase |
| Polymeric clay stabilizer 1 | Hydroxyalkyl alkylammonium chloride |
| Polymeric clay stabilizer 2 | Polyquaternary amine |
| Salt clay stabilizer | KCl |
| Sample, Concentration | Mp 0.5 Hours (g/mol) | Mp 2 Hours (g/mol) | Mp 4 Hours (g/mol) | Mp 24 Hours (g/mol) |
|---|---|---|---|---|
| Persulfate gel breaker (8 ppt) @200 °F | 1,518,323 | 501,571 | 516,813 | 244,990 |
| Bromate gel breaker (8 ppt) @300 °F | >2,350,000 | >2,350,000 | 386,069 | 383,142 |
| Acid gel breaker (2 gpt) @300 °F | >2,350,000 | >2,350,000 | 1,142,500 | 458,188 |
| Enzyme gel breaker (5 gpt) @300 °F | 234,276 | 248,669 | 1,651,134 | 12,428 |
| Hydrated Polymer @77 °F | >2,350,000 | |||
| Samples | Peak Molecular Weight (Mp) (g/mol) |
|---|---|
| PCS1 @ 77 °F | 5786 |
| PCS1 + Heat @300 °F | 5085 |
| PCS1 + Enzyme gel breaker (5 gpt) @ 300 °F | 4950 |
| PCS1 + Bromate gel breaker (8 ppt) @ 300 °F | 4241 |
| PCS1 + Persulfate gel breaker (8 ppt) @ 200 °F | 4130 |
| PCS1 + 2 gpt Acid gel breaker (2 gpt) @ 300 °F | 4021 |
| Samples | Peak Molecular Weight (Mp) (g/mol) |
|---|---|
| PSC2 @ 77 °F | 107,594 |
| PSC2 + Persulfate gel breaker (8 ppt) @ 200 °F | 46,810 |
| PSC2 + Bromate gel breaker (8 ppt) @ 300 °F | 17,011 |
| PSC2 + Acid gel breaker (2 gpt) @ 300 °F | 16,101 |
| PSC2 + Enzyme gel breaker (5 gpt) @ 300 °F | 11,691 |
| PSC2 + Heat @300 °F | 11,519 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almubarak, T.; Ng, J.H.C.; AlKhaldi, M.; Panda, S.; Nasr-El-Din, H.A. Insights on Potential Formation Damage Mechanisms Associated with the Use of Gel Breakers in Hydraulic Fracturing. Polymers 2020, 12, 2722. https://doi.org/10.3390/polym12112722
Almubarak T, Ng JHC, AlKhaldi M, Panda S, Nasr-El-Din HA. Insights on Potential Formation Damage Mechanisms Associated with the Use of Gel Breakers in Hydraulic Fracturing. Polymers. 2020; 12(11):2722. https://doi.org/10.3390/polym12112722
Chicago/Turabian StyleAlmubarak, Tariq, Jun Hong C. Ng, Mohammed AlKhaldi, Saroj Panda, and Hisham A. Nasr-El-Din. 2020. "Insights on Potential Formation Damage Mechanisms Associated with the Use of Gel Breakers in Hydraulic Fracturing" Polymers 12, no. 11: 2722. https://doi.org/10.3390/polym12112722
APA StyleAlmubarak, T., Ng, J. H. C., AlKhaldi, M., Panda, S., & Nasr-El-Din, H. A. (2020). Insights on Potential Formation Damage Mechanisms Associated with the Use of Gel Breakers in Hydraulic Fracturing. Polymers, 12(11), 2722. https://doi.org/10.3390/polym12112722





