An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic
Abstract
1. Introduction
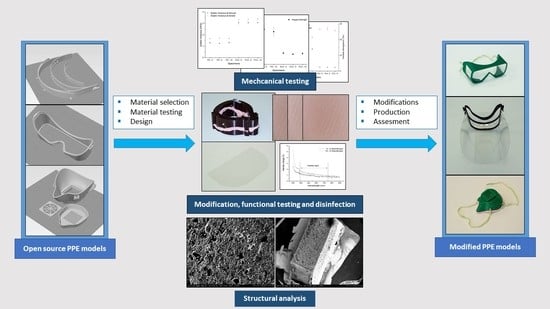
2. Materials and Methods
2.1. 3D Printing Technology, Materials, and Disinfection Protocol
2.2. Mechanical and Structural Comparison of PLA, PA, and Silicone Materials
2.3. Spectrophotometry
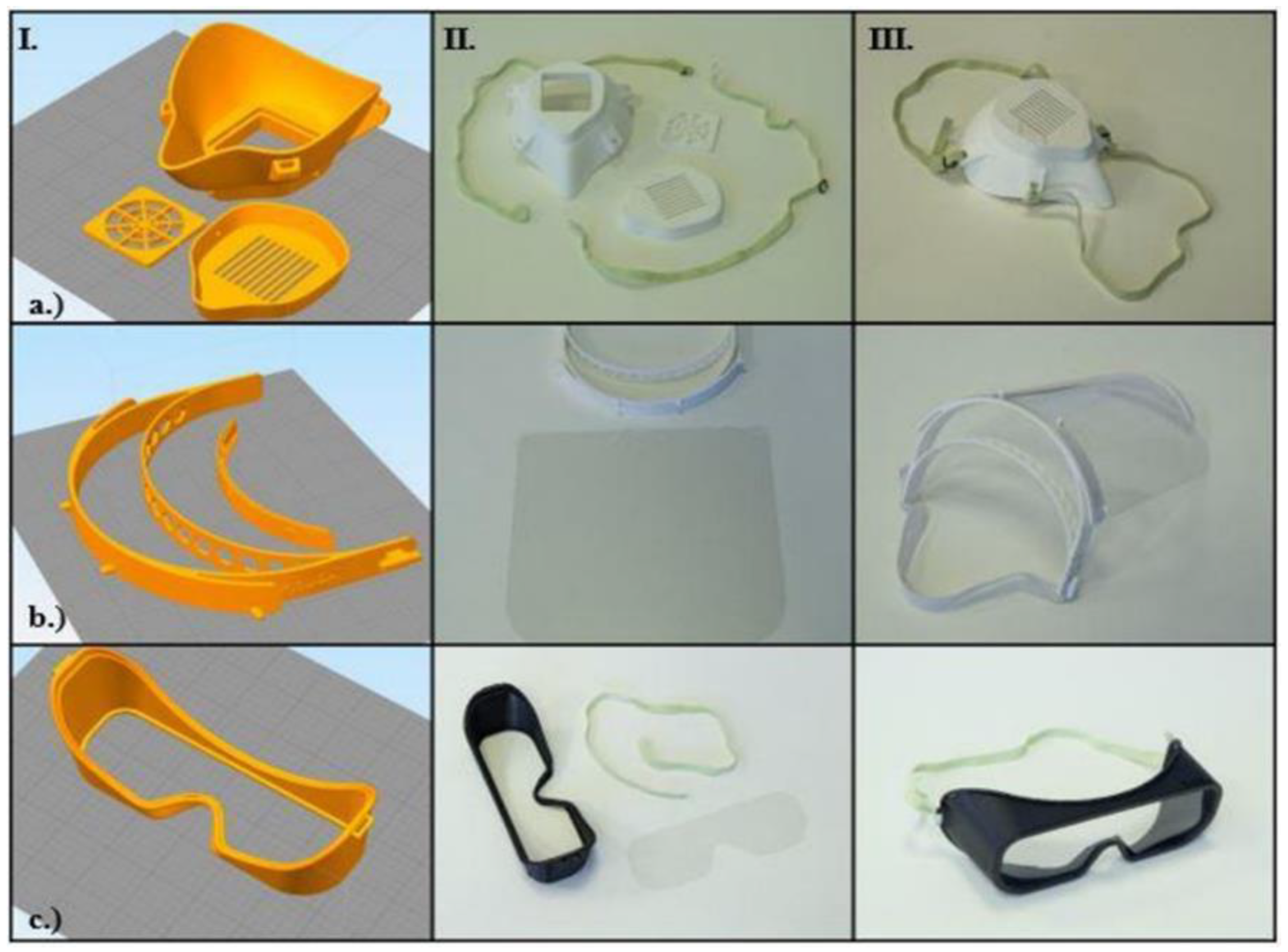
2.4. Introduction of the Open-Source Models
3. Results
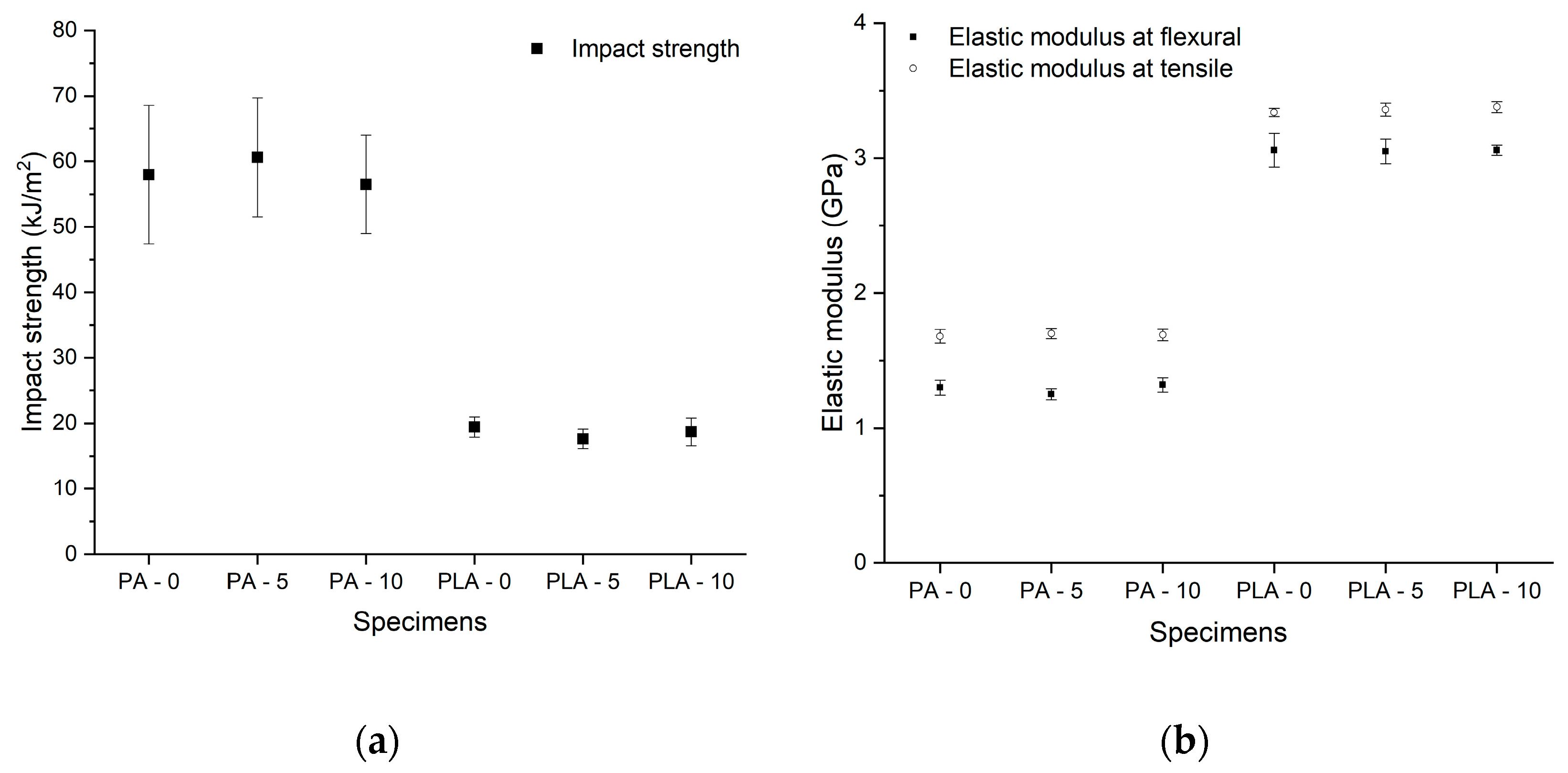
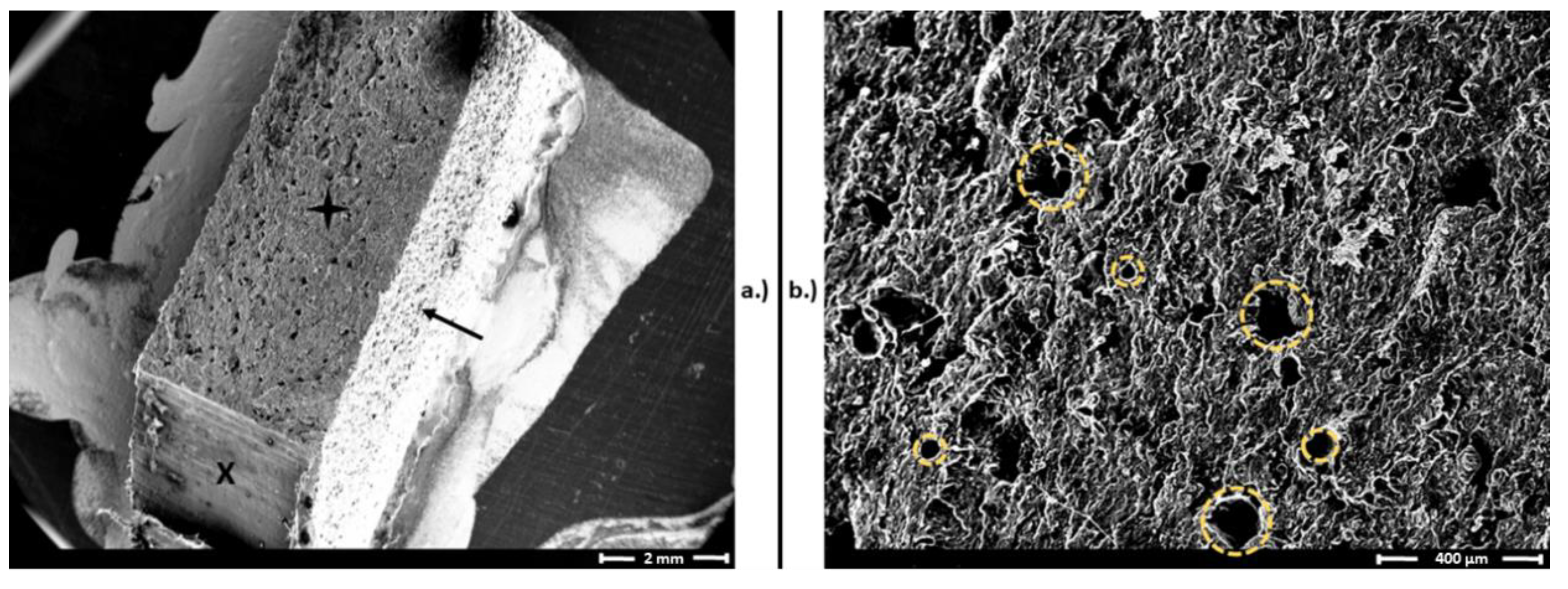
3.1. Results of Mechanical and Structural Analyses before and after Disinfection
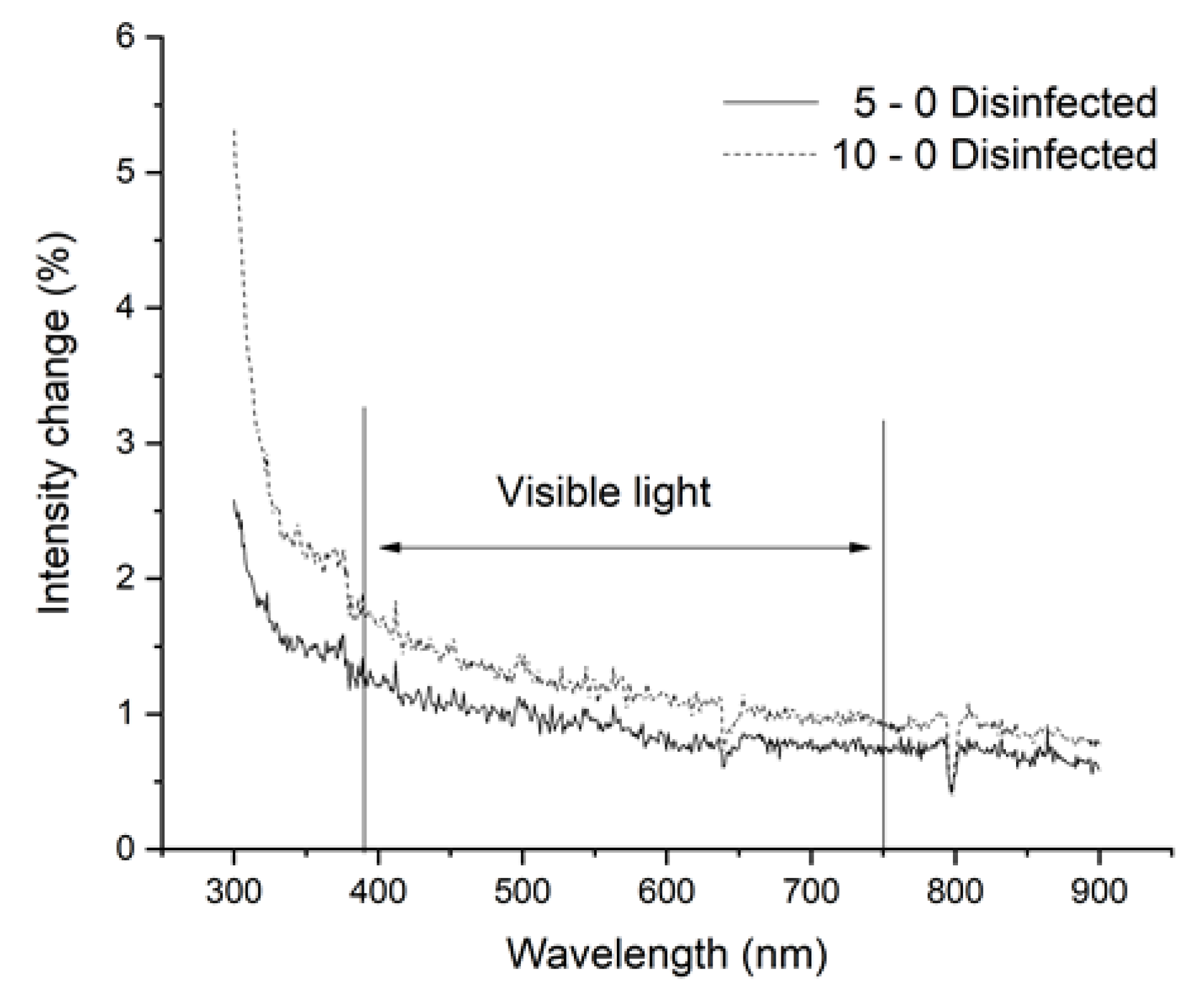
3.2. Results of Spectrophotometry
3.3. Possible Threats, Hazards, and Practical Aspects of OS Models
- OS Shield: The initial design has three main disadvantages: it does not protect the top of the head, it has a relatively high printing time (100 min with FFF and 51 min with SLS/piece) and high material usage, and the shield part is not long enough to protect the whole face.
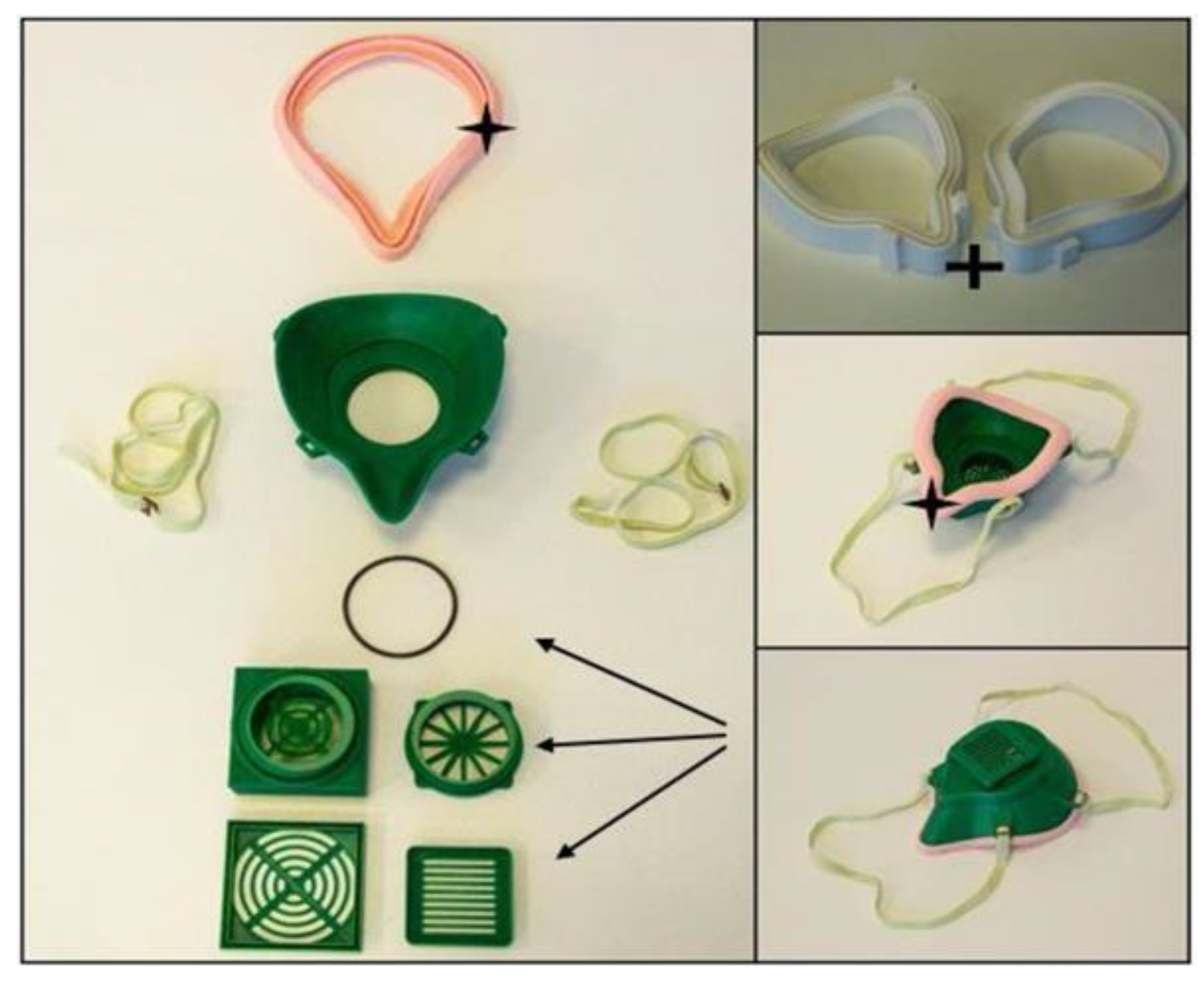
- OS Half Mask: The available open-source model has severe fitting problems if it is printed using PLA or other relatively rigid thermopolymers. A specific size filter with an unknown origin can be fitted inside, which reduces the compatibility with other products. In this form, the design has several air leakage possibilities around the filter holder. With certain design modifications, a more compact configuration could provide better safety features. Furthermore, using SLS technology would make it possible to reduce the material’s overall weight (one piece) to 59.09% and the cost to 78.73% of the original values.
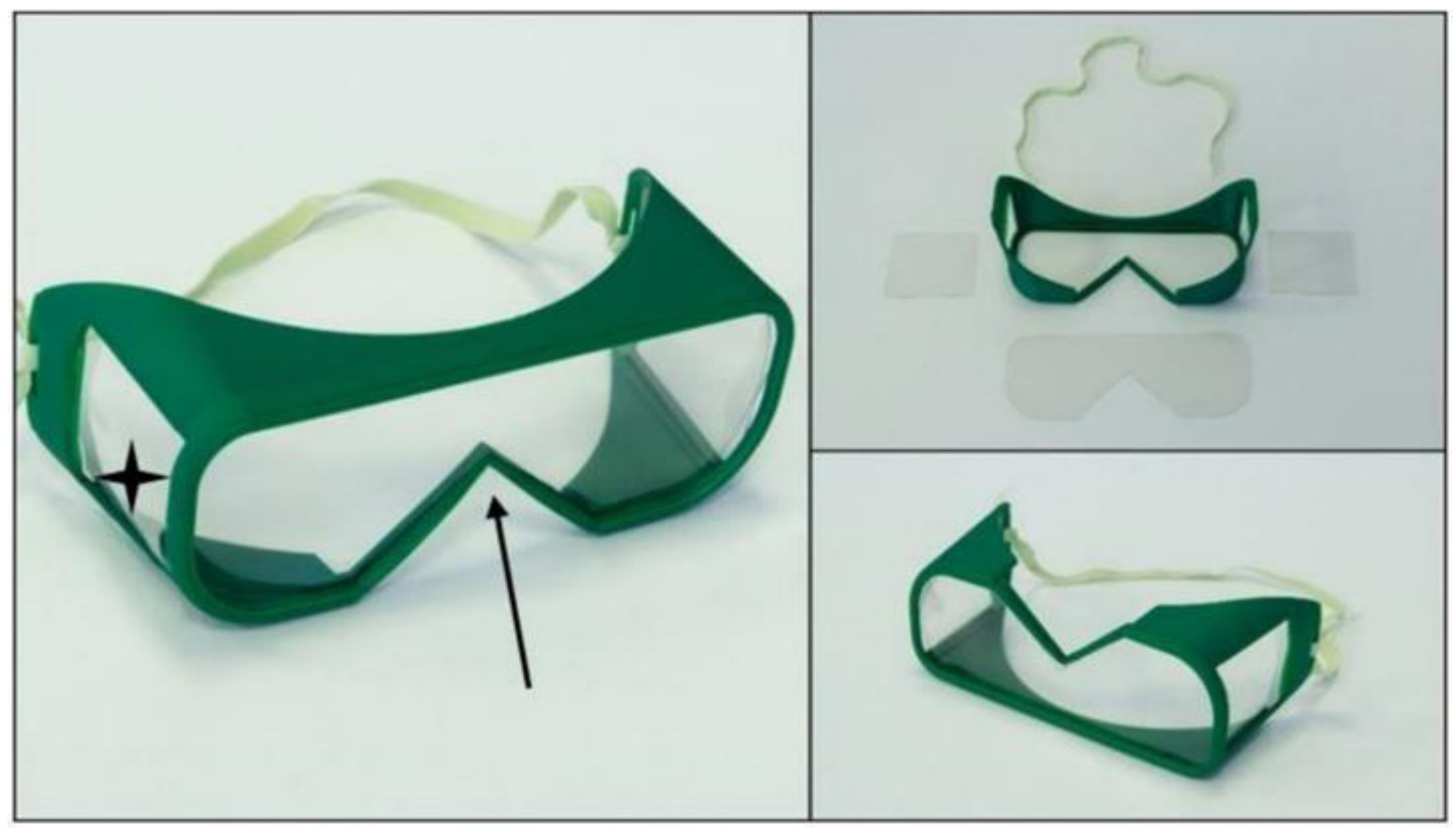
- OS Safety Goggles: The initial model is promising, but the application with a half mask is fairly difficult. Safety goggles reduce the peripheral viewing angle, which could be dangerous in a clinical environment. Furthermore, adding a poly (methyl methacrylate) (PMMA) or polyethylene terephthalate glycol (PETG) sheet is not practical, which reduces the possibility of applying disinfection measures.
3.4. Potential Development Aspects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitsouras, D.; Liacouras, P.; Imanzadeh, A.; Giannopoulos, A.A.; Cai, T.; Kumamaru, K.K.; George, E.; Wake, N.; Caterson, E.J.; Pomahac, B.; et al. Medical 3D Printing for the Radiologist. Radiographics 2015, 35, 1965–1988. [Google Scholar] [CrossRef] [PubMed]
- Ballard, D.H.; Trace, A.P.; Ali, S.; Hodgdon, T.; Zygmont, M.E.; DeBenedectis, C.M.; Smith, S.E.; Richardson, M.L.; Patel, M.J.; Decker, S.J.; et al. Clinical Applications of 3D Printing. Acad. Radiol. 2018, 25, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Öblom, H.; Zhang, J.; Pimparade, M.; Speer, I.; Preis, M.; Repka, M.; Sandler, N. 3D-Printed Isoniazid Tablets for the Treatment and Prevention of Tuberculosis—Personalized Dosing and Drug Release. AAPS PharmSciTech 2019, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.-C.; Wang, Y.; Dai, J.; Ren, C.-W.; Li, J.-H.; Lai, Y.-Q. Application of 3D printing in the surgical planning of hypertrophic obstructive cardiomyopathy and physician-patient communication: A preliminary study. J. Thorac. Dis. 2018, 10, 867–873. [Google Scholar] [CrossRef]
- Atalay, H.A.; Canat, H.L.; Ülker, V.; Alkan, I.; Özkuvanci, Ü.; Altunrende, F. Impact of personalized three-dimensional (3D) printed pelvicalyceal system models on patient information in percutaneous nephrolithotripsy surgery: A pilot study. Int. Braz. Urol. 2017, 43, 470–475. [Google Scholar] [CrossRef]
- Juneja, M.; Thakur, N.; Jindal, P.; Gupta, A.; Bajwa, B.; Jindal, P. Accuracy in dental surgical guide fabrication using different 3-D printing techniques. Addit. Manuf. 2018, 22, 243–255. [Google Scholar] [CrossRef]
- Chen, J.; Ahmad, R.; Suenaga, H.; Li, W.; Sasaki, K.; Swain, M.; Li, Q. Shape Optimization for Additive Manufacturing of Removable Partial Dentures—A New Paradigm for Prosthetic CAD/CAM. PLoS ONE 2015, 10, e0132552. [Google Scholar] [CrossRef]
- Deng, A.; Xiong, R.; He, W.; Wei, D.; Zeng, C. Postoperative rehabilitation strategy for acetabular fracture: Application of 3D printing technique. J. South. Med. Univ. 2014, 34, 591–593. [Google Scholar]
- Shuxian, Z.; Wanhua, Z.; Bingheng, L. 3D reconstruction of the structure of a residual limb for customising the design of a prosthetic socket. Med. Eng. Phys. 2005, 27, 67–74. [Google Scholar] [CrossRef]
- Kate, J.T.; Smit, G.; Breedveld, P. 3D-printed upper limb prostheses: A review. Disabil. Rehabil. Assist. Technol. 2017, 12, 300–314. [Google Scholar] [CrossRef]
- Maroti, P.; Varga, P.; Abraham, H.; Falk, G.; Zsebe, T.; Meiszterics, Z.; Mano, S.; Csernatony, Z.; Rendeki, S.; Nyitrai, M. Printing orientation defines anisotropic mechanical properties in additive manufacturing of upper limb prosthetics. Mater. Res. Express 2018, 6, 035403. [Google Scholar] [CrossRef]
- Farzadi, A.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Abu Osman, N.A. Effect of Layer Thickness and Printing Orientation on Mechanical Properties and Dimensional Accuracy of 3D Printed Porous Samples for Bone Tissue Engineering. PLoS ONE 2014, 9, e108252. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Tarfaoui, M.; Nachtane, M.; Goda, I.; Qureshi, Y.; Benyahia, H. Additive manufacturing in fighting against novel coronavirus COVID-19. Int. J. Adv. Manuf. Technol. 2020, 110, 2913–2927. [Google Scholar] [CrossRef] [PubMed]
- Tarfaoui, M.; Nachtane, M.; Goda, I.; Qureshi, Y.; Benyahia, H. 3D Printing to Support the Shortage in Personal Protective Equipment Caused by COVID-19 Pandemic. Materials 2020, 13, 3339. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.L.; Shi, Y.; Zeng, F.D.; Huang, S.-H. Microstructure of selective laser sintered polyamide. J. Wuhan Univ. Technol. 2003, 18, 60–63. [Google Scholar]
- Pallari, J.H.P.; Dalgarno, K.W.; Woodburn, J. Mass Customization of Foot Orthoses for Rheumatoid Arthritis Using Selective Laser Sintering. IEEE Trans. Biomed. Eng. 2010, 57, 1750–1756. [Google Scholar] [CrossRef]
- Schmidt, M.; Pohle, D.; Rechtenwald, T. Selective Laser Sintering of PEEK. CIRP Ann. 2007, 56, 205–208. [Google Scholar] [CrossRef]
- Mazzoli, A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256. [Google Scholar] [CrossRef]
- Wong, J.Y. On-Site 3D Printing of Functional Custom Mallet Splints for Mars Analogue Crewmembers. Aerosp. Med. Hum. Perform. 2015, 86, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Pfahnl, A.C. 3D Printing of Surgical Instruments for Long-Duration Space Missions. Aviat. Space Environ. Med. 2014, 85, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Loy, J.; Tatham, P.; Healey, R.; Tapper, C.L. 3D Printing Meets Humanitarian Design Research. In Advances in Media, Entertainment, and the Arts; IGI Global: Hershey, PA, USA, 2016; pp. 54–75. [Google Scholar]
- Pavlosky, A.; Glauche, J.; Chambers, S.; Al-Alawi, M.; Yanev, K.; Loubani, T. Validation of an effective, low cost, Free/open access 3D-printed stethoscope. PLoS ONE 2018, 13, e0193087. [Google Scholar] [CrossRef] [PubMed]
- Saripalle, S.; Maker, H.; Bush, A.; Lundman, N. 3D printing for disaster preparedness: Making life-saving supplies on-site, on-demand, on-time. In Proceedings of the 2016 IEEE Global Humanitarian Technology Conference (GHTC), Seattle, WA, USA, 13–16 October 2016; pp. 205–208. [Google Scholar]
- Lacaze, A.; Murphy, K.; Mottern, E.; Corley, K. 3D-printed rapid disaster response. In Proceedings of the 2015 IEEE International Symposium on Technologies for Homeland Security (HST), Waltham, MA, USA, 14–16 April 2015. [Google Scholar]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sanna, M.; Tsai, M.K.; Wen, C.P. Geo-temporal distribution of 1688 Chinese healthcare workers infected with COVID-19 in severe conditions—A secondary data analysis. PLoS ONE 2020, 15, e0233255. [Google Scholar] [CrossRef]
- World Health Organisation. Coronavirus Disease 2019 (COVID-19) Situation Report–70. 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/331683/nCoVsitrep30Mar2020-eng.pdf (accessed on 27 October 2020).
- World Health Organisation. COVID-19 Weekly Epidemiological Update. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20201020-weekly-epi-update-10.pdf (accessed on 27 October 2020).
- Esakandari, H.; Nabi-Afjadi, M.; Fakkari-Afjadi, J.; Farahmandian, N.; Miresmaeili, S.-M.; Bahreini, E. A comprehensive review of COVID-19 characteristics. Biol. Proced. Online 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Ahn, D.-G.; Shin, H.-J.; Kim, M.-H.; Lee, S.; Kim, H.-S.; Myoung, J.; Kim, B.-T.; Kim, S.-J. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324. [Google Scholar] [CrossRef]
- World Health Organisation. Coronavirus Disease (COVID-19) Advice for the Public. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (accessed on 6 October 2020).
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19)—How to Protect Yourself. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html (accessed on 6 October 2020).
- World Health Organization. Advice on the Use of Masks in the Context of COVID-19: Interim Guidance, 5 June 2020; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/332293/WHO-2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y (accessed on 6 October 2020).
- Centers for Disease Control and Prevention. Considerations on Wearing Masks. 2020; Help Slow the Spread of COVID-19; 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html (accessed on 6 October 2020).
- World Health Organization. Coronavirus Disease (COVID-19) Advice for the Public: When and How to Use Masks. 2020. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks (accessed on 6 October 2020).
- World Health Organization. Cleaning and Disinfection of Environmental Surfaces in the Context of COVID-19–Interim Guidance 15 May 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/332096/WHO-2019-nCoV-Disinfection-2020.1-eng.pdf?sequence=1&isAllowed=y (accessed on 6 October 2020).
- Fathizadeh, H.; Maroufi, P.; Momen-Heravi, M.; Dao, S.; Köse, Ş.; Ganbarov, K.; Pagliano, P.; Esposito, S.; Kafil, H.S. Protection and disinfection policies against SARS-CoV-2 (COVID-19). Infez. Med. 2020, 28, 185–191. [Google Scholar]
- Henwood, A.F. Coronavirus disinfection in histopathology. J. Histotechnol. 2020, 43, 102–104. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Patel, P.; Sanghvi, S.; Malik, K.; Khachemoune, A. Back to the basics: Diluted bleach for COVID-19. J. Am. Acad. Dermatol. 2020, 83, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Swennen, G.R.J.; Pottel, L.; Haers, P.E. Custom-made 3D-printed face masks in case of pandemic crisis situations with a lack of commercially available FFP2/3 masks. Int. J. Oral. Maxillofac. Surg. 2020, 49, 673–677. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health. Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers during the Coronavirus Disease 2019 (COVID-19) Public Health Emergency Guidance for Industry and Food and Drug Administration Staff. 2020. Available online: https://www.fda.gov/media/136533/download (accessed on 6 October 2020).
- U.S. Environmental Protection Agency. List N: Disinfectants for Coronavirus (COVID-19). 2020. Available online: https://www.epa.gov/pesticide-registration/list-n-advanced-search-page-disinfectants-coronavirus-covid-19 (accessed on 6 October 2020).
- Ishack, S.; Lipner, S.R. Applications of 3D Printing Technology to Address COVID-19—Related Supply Shortages. Am. J. Med. 2020, 133, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Vordos, N.; Gkika, D.A.; Maliaris, G.; Tilkeridis, K.; Antoniou, A.; Bandekas, D.V.; Mitropoulos, A.C. How Social Media and 3D Printing Tackles the PPE Shortage during Covid–19 Pandemi. medRxiv 2020. [Google Scholar] [CrossRef]
- Makowski, K.; Okrasa, M. Application of 3D scanning and 3D printing for designing and fabricating customized half-mask facepieces: A pilot study. Work 2019, 63, 125–135. [Google Scholar] [CrossRef]
- Wesemann, C.; Pieralli, S.; Fretwurst, T.; Nold, J.; Nelson, K.; Schmelzeisen, R.; Hellwig, E.; Spies, B.C. 3-D Printed Protective Equipment during COVID-19 Pandemic. Materials 2020, 13, 1997. [Google Scholar] [CrossRef]
- Livingston, E.H.; Desai, A.; Berkwits, M. Sourcing Personal Protective Equipment During the COVID-19 Pandemic. JAMA 2020, 323, 1912–1914. [Google Scholar] [CrossRef]
- Derraik, J.G.B.; Anderson, W.; Connelly, E.A.; Anderson, Y.C. Rapid evidence summary on SARS-CoV-2 survivorship and disinfection, and a reusable PPE protocol using a double-hit process. medRxiv 2020, 2020. [Google Scholar] [CrossRef]
- Çelik, E.U.; Türkün, M.; Yapar, A.G.D. Oxygen release of tetra acetyl ethylene diamine (TAED) and sodium perborate combination. Int. Endod. J. 2008, 41, 571–576. [Google Scholar] [CrossRef]
- Kerekes, T.W.; Lim, H.; Joe, W.Y.; Yun, G.J. Characterization of process-deformation/damage property relationship of fused deposition modeling (FDM) 3D-printed specimens. Addit. Manuf. 2019, 25, 532–544. [Google Scholar] [CrossRef]
- Varga, P.; Lorinczy, D.; Tóth, L.; Pentek, A.; Nyitrai, M.; Maroti, P. Novel PLA-CaCO3 composites in additive manufacturing of upper limb casts and orthotics–A feasibility study. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Somireddy, M.; Singh, C.V.; Czekanski, A. Analysis of the Material Behavior of 3D Printed Laminates via FFF. Exp. Mech. 2019, 59, 871–881. [Google Scholar] [CrossRef]
- Toth-Tascau, M.; Raduta, A.; Stoia, D.I.; Locovei, C. Influence of the Energy Density on the Porosity of Polyamide Parts in SLS Process. Solid State Phenom. 2012, 188, 400–405. [Google Scholar] [CrossRef]
- World Health Organization. Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease (COVID-19). 2020. Available online: https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages (accessed on 6 October 2020).
- World Health Organization. Modes of Transmission of Virus Causing COVID-19: Implications for IPC Precaution Recommendations. 2020. Available online: https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (accessed on 6 October 2020).
- Plasma Protein Therapeutics Association. New Coronavirus (SARS-CoV-2) and the Safety Margins of Plasma Protein Therapies. 2020. Available online: https://www.pptaglobal.org/media-and-information/ppta-statements/1055-2019-novel-coronavirus-2019-ncov-and-plasma-protein-therapies (accessed on 6 October 2020).
- Ye, G.; Lin, H.; Chen, L.-J.; Wang, S.; Zeng, Z.; Wang, W.; Zhang, S.; Rebmann, T.; Li, Y.; Pan, Z.; et al. Environmental contamination of the SARS-CoV-2 in healthcare premises: An urgent call for protection for healthcare workers. medRxiv 2020, 2020. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Belter, B.M.J.T.; Dollar, A.M. Strengthening of 3D Printed Fused Deposition Manufactured Parts Using the Fill Compositing Technique. PLoS ONE 2015, 10, e0122915. [Google Scholar] [CrossRef]
- Goyanes, A.; Fabrizio, F.; Gaisford, S.; Basit, A. (Eds.) Manufacture of Modified Release Medicines by Desktop Selective Laser Sintering (SLS) 3D Printing. 2017. Available online: https://www.semanticscholar.org/paper/Manufacture-of-Modified-Release-Medicines-by-Laser-Goyanes-Fabrizio/0f9c3cc385ecbd069b0bc7a6d0aa7f0bee8dea8d?p2df (accessed on 6 October 2020).
- Rindelaub, J.D.; Baird, Z.; Lindner, B.A.; Strantz, A.A. Identifying extractable profiles from 3D printed medical devices. PLoS ONE 2019, 14, e0217137. [Google Scholar] [CrossRef]
- Kubyshkina, G.; Župančić, B.; Štukelj, M.; Grošelj, D.; Marion, L.; Emri, I. Sterilization effect on structure, thermal and time-dependent properties of polyamides. In Proceedings of the Society for Experimental Mechanics Series; Springer: Berlin/Heidelberg, Germany, 2011; Volume 3, pp. 11–19. [Google Scholar]
- Athanasiou, K.A.; Niederauer, G.G.; Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/ polyglycolic acid copolymers. Biomaterials 1996, 17, 93–102. [Google Scholar] [CrossRef]
- McKeen, L. The Effect of Sterilization on Plastics and Elastomers, a Volume in Plastics Design Library, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Trik, S.; Bullemer, M.; Gessler, M.; Gilmour, L.J. Influence of Sterilization on Laser Sintered Polyamide Materials. EOS GmbH. Krailling, Germany, Technical University of Munich (EOS North America, Pflugerville, TX):2. Available online: https://3dagainstcorona.eos.info/subdomain/subdomain_corona/pdf/shield_influence-of-sterilization-on-laser-sintered-polyamide-materials.pdf (accessed on 6 October 2020).
- Savaris, M.; Braga, G.L.; Dos Santos, V.; Carvalho, G.A.; Falavigna, A.; Machado, D.C.; Viezzer, C.; Brandalise, R.N. Biocompatibility Assessment of Poly(lactic acid) Films after Sterilization with Ethylene Oxide in Histological Study In Vivo with Wistar Rats and Cellular Adhesion of Fibroblasts In Vitro. Int. J. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Memorandum of Understanding: Rapid Response to Covid-19 Using 3d Printing between National Institutes of Health within U.S. Department of Health and Human Services and Food and Drug Administration. S Department of Health and Human Services and Veterans Health Administration within the US Department of Veterans Affairs, FDA; 2020. Available online: https://www.fda.gov/about-fda/domestic-mous/mou-225-20-008 (accessed on 6 October 2020).
- Faith, R. FDA Approves First 3D-Printed Mask for COVID-19 Support, Technological Solutions Could Provide Relief to Medical Facilities in Urgent Need of Necessary Equipment. 2020. Available online: https://governmentciomedia.com/fda-approves-first-3d-printed-mask-covid-19-support (accessed on 6 October 2020).
- Informatics RaCCIo. CIIRC CTU Anti COVID-19. 2020. Available online: https://www.ciirc.cvut.cz/covid/ (accessed on 6 October 2020).
- Fagade, A.A.; Kazmer, D. Early cost estimation for injection molded components. J. Inject. Molding Technol. 2000, 4, 97–106. [Google Scholar]
- Franchetti, M.; Kress, C. An economic analysis comparing the cost feasibility of replacing injection molding processes with emerging additive manufacturing techniques. Int. J. Adv. Manuf. Technol. 2016, 88, 2573–2579. [Google Scholar] [CrossRef]
- Iannone, P.; Castellini, G.; Coclite, D.; Napoletano, A.; Fauci, A.J.; Iacorossi, L.; D’Angelo, D.; Renzi, C.; La Torre, G.; Mastroianni, C.M.; et al. The need of health policy perspective to protect Healthcare Workers during COVID-19 pandemic. A GRADE rapid review on the N95 respirators effectiveness. PLoS ONE 2020, 15, e0234025. [Google Scholar] [CrossRef] [PubMed]


| Product Time (1 pc) (min) | Material Weight for 1 Piece (g) | Material Cost for 1 Piece (EUR) | Pieces with Full Capacity (pcs) | Production Time for Full Volume (min) | Material Weight for full Capacity (g) | Material Cost for Full Capacity (EUR) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model Name | FFF | SLS * | FFF | SLS | FFF | SLS ** | FFF | SLS | FFF | SLS * | FFF | SLS | FFF | SLS ** |
| OS Shield | 100 | 51 | 47 | 43 | 0.29 | 6.49 | 2 | 18 | 200 | 919 | 94 | 1305 | 0.57 | 116.87 |
| Shield V.2.0 | 38 | 27 | 18 | 17 | 0.11 | 1.06 | 2 | 41 | 75 | 1125 | 36 | 488 | 0.22 | 43.66 |
| OS Half Mask | 157 | 75 | 120 | 111 | 0.73 | 6.11 | 3 | 14 | 470 | 1046 | 360 | 956 | 2.20 | 85.59 |
| Mask V.2.0 | 167 | 90 | 70 | 65 | 0.43 | 7.77 | 3 | 12 | 500 | 1083 | 210 | 1042 | 1.28 | 93.25 |
| OS Safety Googles | 80 | 54 | 30 | 28 | 0.18 | 2.25 | 4 | 20 | 320 | 1081 | 120 | 503 | 0.73 | 45.03 |
| Safety Goggles V.2.0 | 100 | 56 | 43 | 40 | 0.26 | 3.12 | 3 | 16 | 300 | 896 | 129 | 557 | 0.79 | 49.85 |
| Material cost estimation | PLA/kg: | EUR 22.04 | PLA/kg: | EUR 89.53 | EUR/HUF: | 363 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendeki, S.; Nagy, B.; Bene, M.; Pentek, A.; Toth, L.; Szanto, Z.; Told, R.; Maroti, P. An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic. Polymers 2020, 12, 2703. https://doi.org/10.3390/polym12112703
Rendeki S, Nagy B, Bene M, Pentek A, Toth L, Szanto Z, Told R, Maroti P. An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic. Polymers. 2020; 12(11):2703. https://doi.org/10.3390/polym12112703
Chicago/Turabian StyleRendeki, Szilard, Balint Nagy, Matyas Bene, Attila Pentek, Luca Toth, Zalan Szanto, Roland Told, and Peter Maroti. 2020. "An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic" Polymers 12, no. 11: 2703. https://doi.org/10.3390/polym12112703
APA StyleRendeki, S., Nagy, B., Bene, M., Pentek, A., Toth, L., Szanto, Z., Told, R., & Maroti, P. (2020). An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic. Polymers, 12(11), 2703. https://doi.org/10.3390/polym12112703