Nanocomposite Materials with Poly(l-lactic Acid) and Transition-Metal Dichalcogenide Nanosheets 2D-TMDCs WS2
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Processing
2.2. Characterization Studies
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Wide-Angle X-ray Diffraction (WAXS)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Biodegradation Tests
3. Results
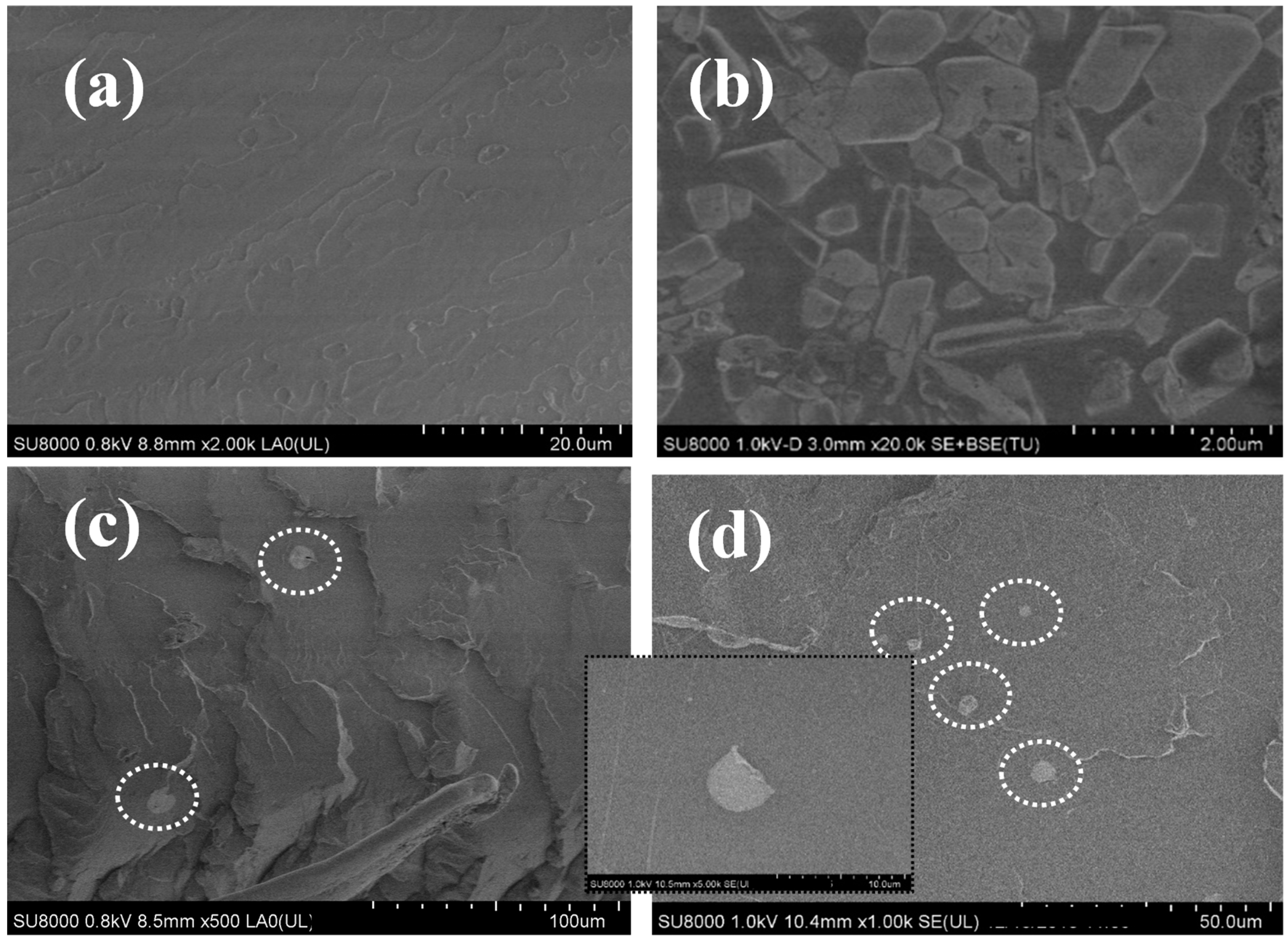
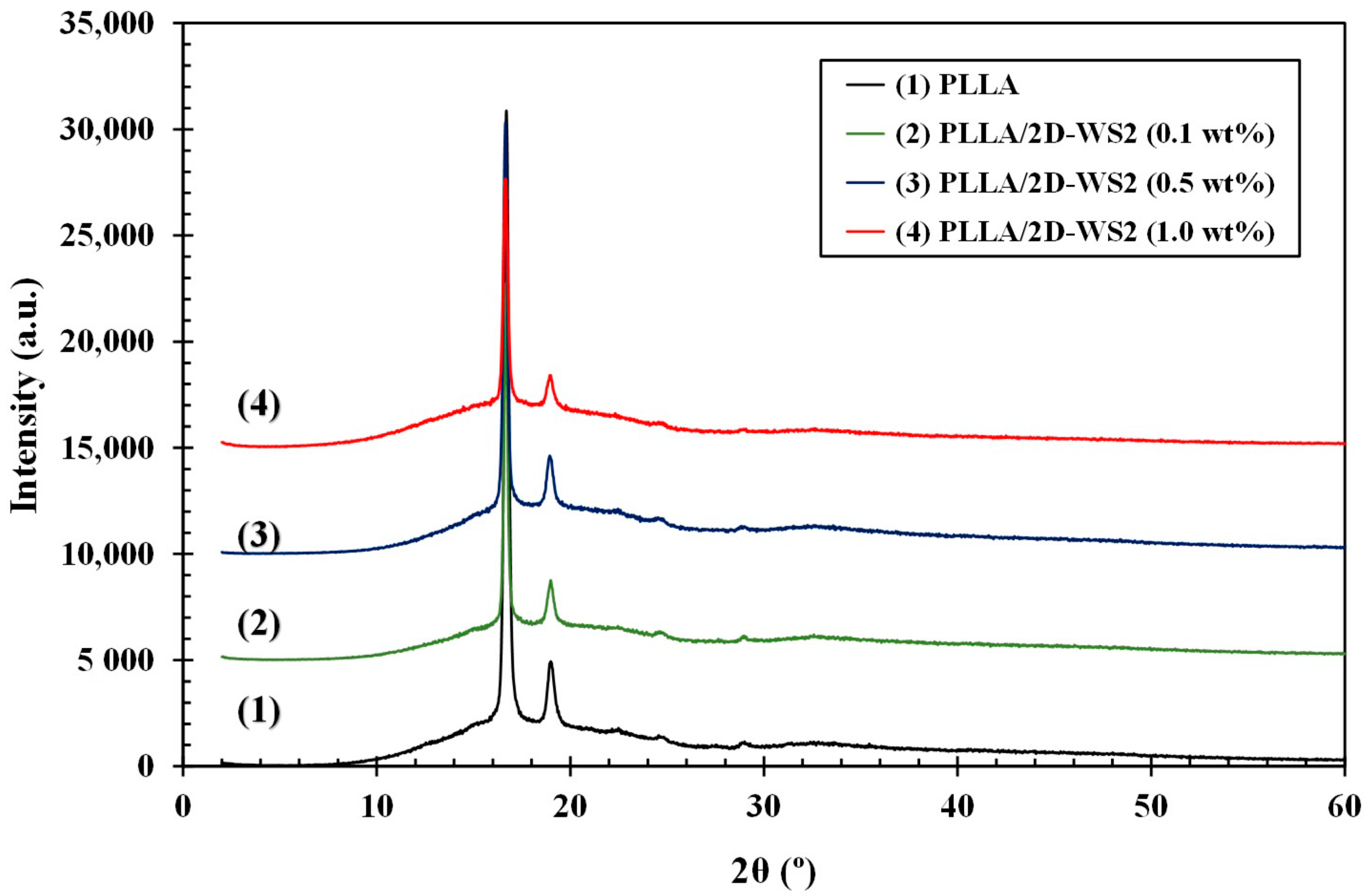
3.1. Morphology and Structure
3.2. Non-Isothermal Crystallization
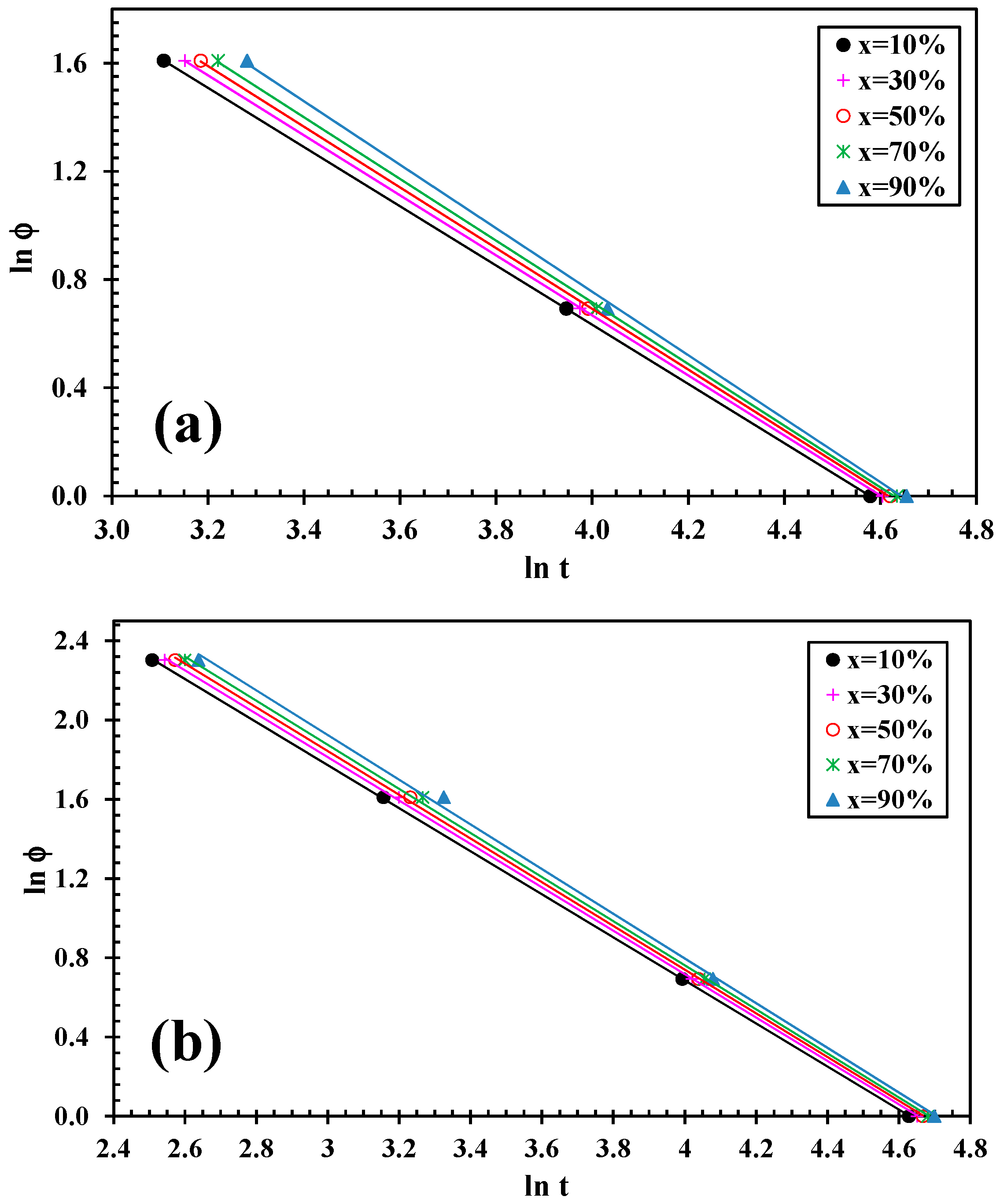
3.3. Lui Model
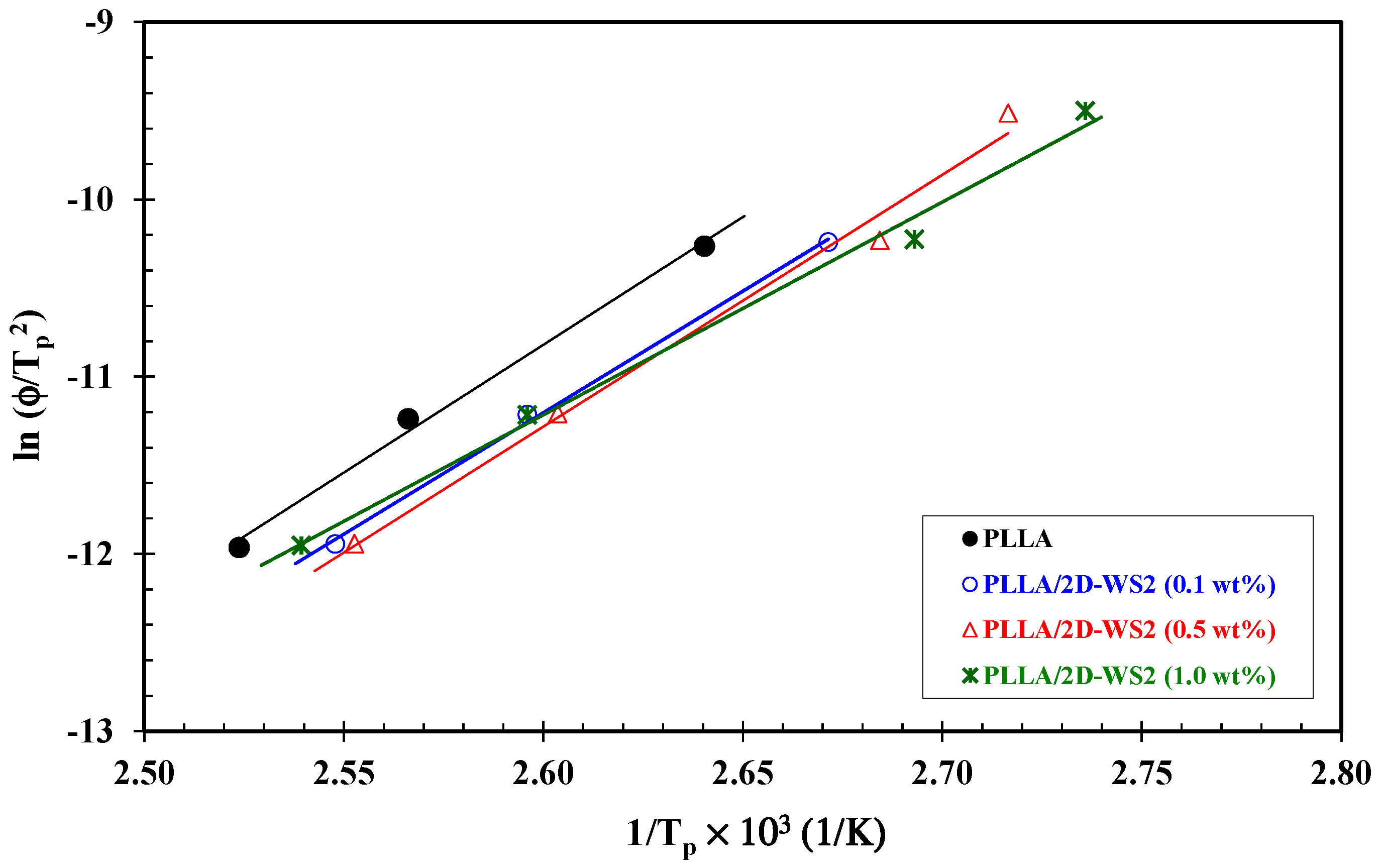
3.4. Effective Energy Barrier
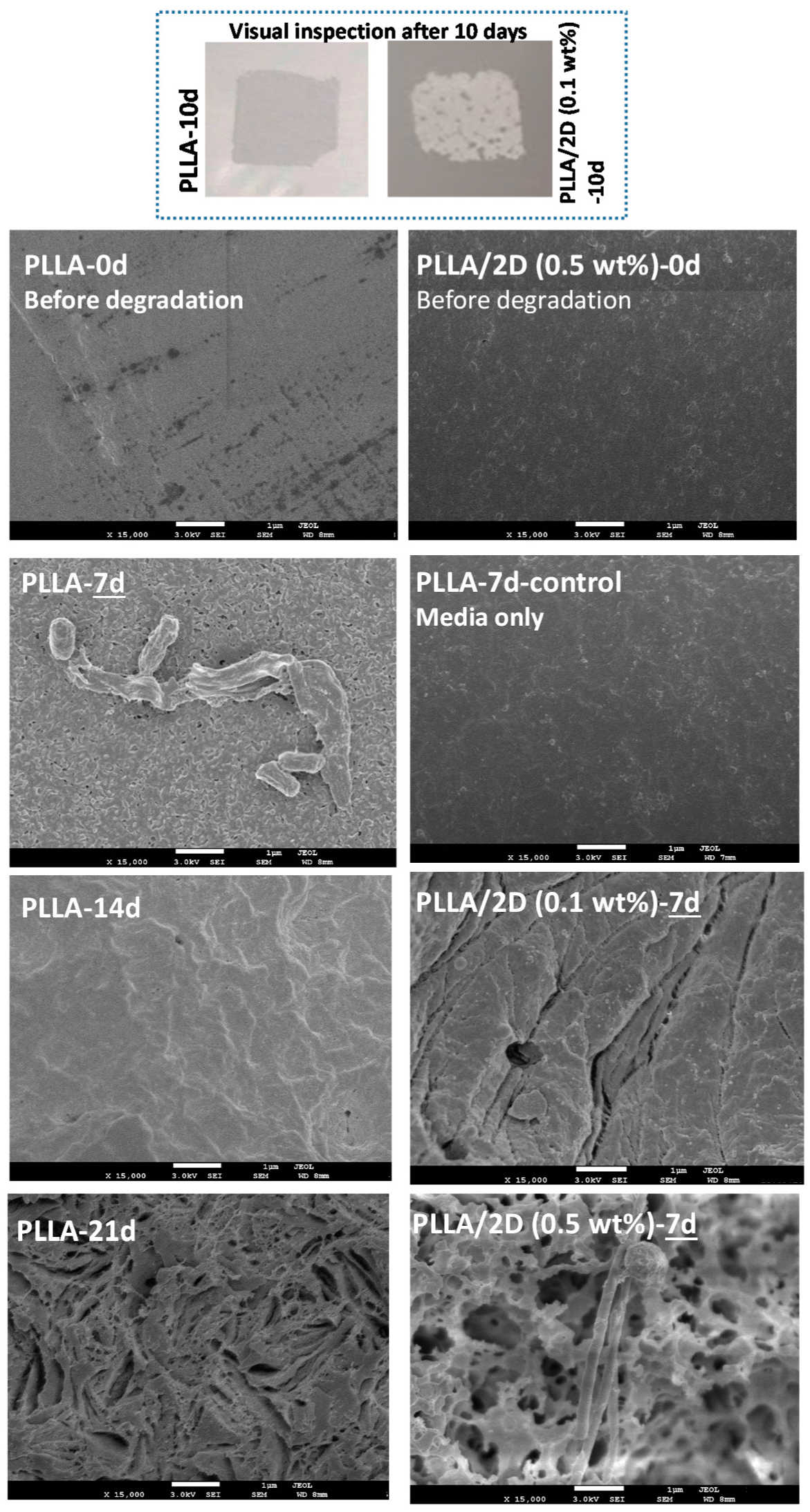
3.5. Biodegradation Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic acid technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Sinclair, R.G. The case for the polylactic acid as a commodity packaging plastic. Pure Appl. Chem. A 1996, 33, 585–597. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Rhim, J.W.; Mohanty, A.K.; Singh, S.P.; Ng, P.K.W. Effect of the processing methods on the performance of polylactide films: Thermocompression versus solvent casting. J. Appl. Polym. Sci. 2006, 101, 3736–3742. [Google Scholar] [CrossRef]
- Bayer, I.S. Thermomechanical properties of polylactic acid-graphene composites: A state-of-the-art review for biomedical applications. Materials 2017, 10, 748. [Google Scholar] [CrossRef]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Maitra, U.; Waghmare, U.V. Extraordinary attributes of 2-dimensional MoS2 nanosheets. Chem. Phys. Lett. 2014, 609, 172–183. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Yeoh, G.H.; Hu, Y. Enhanced mechanical and barrier properties of polyurethane nanocomposite films with randomly distributed molybdenum disulfide nanosheets. Compos. Sci. Technol. 2016, 127, 142–148. [Google Scholar] [CrossRef]
- Chen, P.; Liang, X.; Xu, Y.; Zhou, Y.; Nie, W. Enhanced thermal and mechanical properties of PLA/MoS2 nanocomposites synthesized via the in-situ ring-opening polymerization. Appl. Surf. Sci. 2018, 440, 1143–1149. [Google Scholar] [CrossRef]
- Feng, X.; Wang, B.; Wang, X.; Wen, P.; Cai, W.; Hu, Y.; Liew, K.M. Molybdenum disulfide nanosheets as barrier enhancing nanofillers in thermal decomposition of polypropylene composites. Chem. Eng. J. 2016, 295, 278–287. [Google Scholar] [CrossRef]
- Zhou, K.; Gao, R.; Gui, Z.; Hu, Y. The effective reinforcements of functionalized MoS2 nanosheets in polymer hybrid composites by sol-gel technique. Compos. Part A 2017, 94, 1–9. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. Opportunities and challenges in the use of inorganic fullerene-like nanoparticles to produce advanced polymer nanocomposites. Prog. Polym. Sci. 2013, 38, 1163–1231. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M. Thermoplastic polymer nanocomposites based on inorganic fullerene-like nanoparticles and inorganic nanotubes. Inorganics 2014, 2, 291–312. [Google Scholar] [CrossRef]
- Lalwani, G.; Henslee, A.M.; Farshid, B.; Parmar, P.; Lin, L.; Qin, Y.-X.; Kasper, F.K.; Mikos, A.G.; Sitharaman, B. Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering. Acta Biomater. 2013, 9, 8365–8373. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Marco, C.; Ellis, G.; Cohen, S.R.; Laikhtman, A.; Rapoport, L.; Zak, A. Novel poly(3-hydroxybutyrate) nanocomposites containing WS2 inorganic nanotubes with improved thermal, mechanical and tribological properties. Mater. Chem. Phys. 2014, 147, 273–284. [Google Scholar] [CrossRef]
- Naffakh, N.; Díez-Pascual, A.M. Nanocomposite biomaterials based on poly(ether-ether-ketone) (PEEK) and WS2 inorganic nanotubes. J. Mater. Chem. B 2014, 2, 4509–4520. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G. Development of novel melt-processable biopolymer nanocomposites based on poly(l-lactic acid) and WS2 inorganic nanotubes. CrystEngComm 2014, 16, 5062–5072. [Google Scholar] [CrossRef]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar]
- Jabbarzadeh, A. The origins of enhanced and retarded crystallization in nanocomposite polymers. Nanomaterials 2019, 9, 1472. [Google Scholar] [CrossRef]
- Kumar, S.K.; Ganesan, V.; Riggleman, R.A. Perspective: Outstanding theoretical questions in polymer-nanoparticle hybrids. J. Chem. Phys. 2017, 147, 020901. [Google Scholar] [CrossRef]
- Jabbarzadeh, A.; Halfina, B. Unravelling the effects of size, volume fraction and shape of nanoparticle additives on crystallization of nanocomposite polymers. Nanoscale Adv. 2019, 1, 4704–4721. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, Z.; Guo, G. Biodegradation assessment of poly (lactic acid) filled with functionalized titania nanoparticles (PLA/TiO2) under compost conditions. Nanoscale Res. Lett. 2019, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Camino, G. Biodegradation of poly(lactic acid) and its nanocomposites. Polym. Degrad. Stab. 2009, 94, 1646–1655. [Google Scholar] [CrossRef]
- Someya, Y.; Kondo, N.; Shibata, M. Biodegradation of poly(butylene adipate-co-butylene terephthalate)/layered-silicate nanocomposites. J. Appl. Polym. Sci. 2007, 106, 730–736. [Google Scholar] [CrossRef]
- Fukushima, K.; Abbate, C.; Tabuani, D.; Gennari, M.; Rizzarelli, P.; Camino, G. Biodegradation trend of poly(ε-caprolactone) and nanocomposites. Mater. Sci. Eng. C 2010, 30, 566–574. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Jarerat, A.; Pranamuda, H.; Tokiwa, Y. Poly(L-lactide)-degrading activity in various actinomycetes. Macromol. Biosci. 2002, 2, 420–428. [Google Scholar] [CrossRef]
- Krikorian, V.; Pochan, D.J.; Krikorian, V.; Pochan, D.J. Unusual crystallization behavior of organoclay reinforced poly(l-lactic acid) nanocomposites. Macromolecules 2005, 38, 6520–6527. [Google Scholar] [CrossRef]
- Wu, C.H.; Eder, G.; Janeschitz-Kriegl, H. Polymer crystallization dynamics, as reflected by differential scanning calorimetry. Part 2: Numerical simulations. Colloid Polym. Sci. 1993, 271, 1116–1128. [Google Scholar] [CrossRef]
- Burzic, I.; Pretschuh, C.; Kaineder, D.; Eder, G.; Smilek, J.; Masilko, J.; Kateryna, W. Impact modification of PLA using biobased biodegradable PHA biopolymers. Eur. Polym. J. 2019, 114, 32–38. [Google Scholar] [CrossRef]
- Ning, N.; Fu, S.; Zhang, W.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Realizing the enhancement of interfacial interaction in semicrystalline polymer/filler composites via interfacial crystallization. Prog. Polym. Sci. 2012, 37, 1425–1455. [Google Scholar] [CrossRef]
- Jing, M.; Jiang, H.; Guo, Y.; Wu, Z.; Fu, Q. Transcrystallization of poly(l-lactic acid) on the surface of reduced graphene oxide fibers. RSC Adv. 2016, 6, 100090–100097. [Google Scholar] [CrossRef]
- Pluta, M.; Galeski, A.; Alexandre, M.; Paul, M.A.; Dubois, P. Polylactide/montmorillonite nanocomposites and microcomposites prepared by melt blending: Structure and some physical properties. J. Appl. Polym. Sci. 2002, 86, 1497–1506. [Google Scholar] [CrossRef]
- Safandowsk, M.; Rozanski, A.; Galeski, A. Plasticization of polylactide after solidification: An effectiveness and utilization for correct interpretation of thermal properties. Polymers 2020, 12, 561. [Google Scholar] [CrossRef]
- Liu, T.; Mo, Z.; Wang, S.; Zhang, H. Nonisothermal melt and cold crystallization kinetics of poly(aryl ether ether ketone ketone). Polym. Eng. Sci. 1997, 37, 568–575. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase changes 1. General theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of phase change. III. Granulation, phase change, and microstructure. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 128, 150–158. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Gómez, M.A.; Jiménez, I. Unique nucleation activity of inorganic fullerene-like WS2 nanoparticles in polyphenylene sulfide nanocomposites: Isokinetic and isoconversional study of dynamic crystallization kinetics. J. Phys. Chem. B 2009, 113, 7107–7115. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of peak temperature with heating rate in differential thermal analysis. J. Res. Natl. Bur. Stand. 1956, 57, 217–221. [Google Scholar] [CrossRef]




| 2D-WS2 | φ (°C/min) | Tp (°C) | (1−λ) c (%) | x a (%) | α b | f(T) b | ΔE c (kJ/mol) |
|---|---|---|---|---|---|---|---|
| 0 | 1 | 123.1 | 57.7 | 10 | 1.09 | 5.01 | −119.9 |
| 2 | 116.5 | 53.2 | 30 | 1.11 | 5.1 | ||
| 5 | 105.6 | 45.9 | 50 | 1.12 | 5.18 | ||
| 10 | 94.3 | 4 | 70 | 1.14 | 5.27 | ||
| 20 | - | - | 90 | 1.17 | 5.45 | ||
| 0.1 | 1 | 119.3 | 56.1 | 10 | 1.1 | 5.08 | −114.1 |
| 2 | 112.1 | 51.4 | 30 | 1.11 | 5.13 | ||
| 5 | 101.2 | 40.9 | 50 | 1.13 | 5.24 | ||
| 10 | - | - | 70 | 1.15 | 5.35 | ||
| 20 | - | - | 90 | 1.18 | 5.5 | ||
| 0.5 | 1 | 118.6 | 55.7 | 10 | 1.09 | 5.03 | −118.2 |
| 2 | 110.9 | 51.1 | 30 | 1.1 | 5.1 | ||
| 5 | 99.4 | 36.9 | 50 | 1.1 | 5.16 | ||
| 10 | 93.4 | 3.5 | 70 | 1.11 | 5.21 | ||
| 20 | - | - | 90 | 1.13 | 5.31 | ||
| 1 | 1 | 120.7 | 57.6 | 10 | 1.09 | 5.01 | −99.9 |
| 2 | 112.1 | 56.2 | 30 | 1.11 | 5.11 | ||
| 5 | 98.2 | 45.7 | 50 | 1.11 | 5.17 | ||
| 10 | - | 3 | 70 | 1.12 | 5.22 | ||
| 20 | - | - | 90 | 1.12 | 5.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naffakh, M.; Fernández, M.; Shuttleworth, P.S.; García, A.M.; Moreno, D.A. Nanocomposite Materials with Poly(l-lactic Acid) and Transition-Metal Dichalcogenide Nanosheets 2D-TMDCs WS2. Polymers 2020, 12, 2699. https://doi.org/10.3390/polym12112699
Naffakh M, Fernández M, Shuttleworth PS, García AM, Moreno DA. Nanocomposite Materials with Poly(l-lactic Acid) and Transition-Metal Dichalcogenide Nanosheets 2D-TMDCs WS2. Polymers. 2020; 12(11):2699. https://doi.org/10.3390/polym12112699
Chicago/Turabian StyleNaffakh, Mohammed, Miriam Fernández, Peter S. Shuttleworth, Ana M. García, and Diego A. Moreno. 2020. "Nanocomposite Materials with Poly(l-lactic Acid) and Transition-Metal Dichalcogenide Nanosheets 2D-TMDCs WS2" Polymers 12, no. 11: 2699. https://doi.org/10.3390/polym12112699
APA StyleNaffakh, M., Fernández, M., Shuttleworth, P. S., García, A. M., & Moreno, D. A. (2020). Nanocomposite Materials with Poly(l-lactic Acid) and Transition-Metal Dichalcogenide Nanosheets 2D-TMDCs WS2. Polymers, 12(11), 2699. https://doi.org/10.3390/polym12112699







