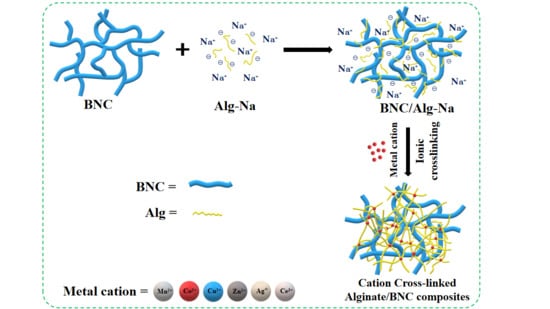
Bacterial Nanocellulose-Enhanced Alginate Double-Network Hydrogels Cross-Linked with Six Metal Cations for Antibacterial Wound Dressing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Strains
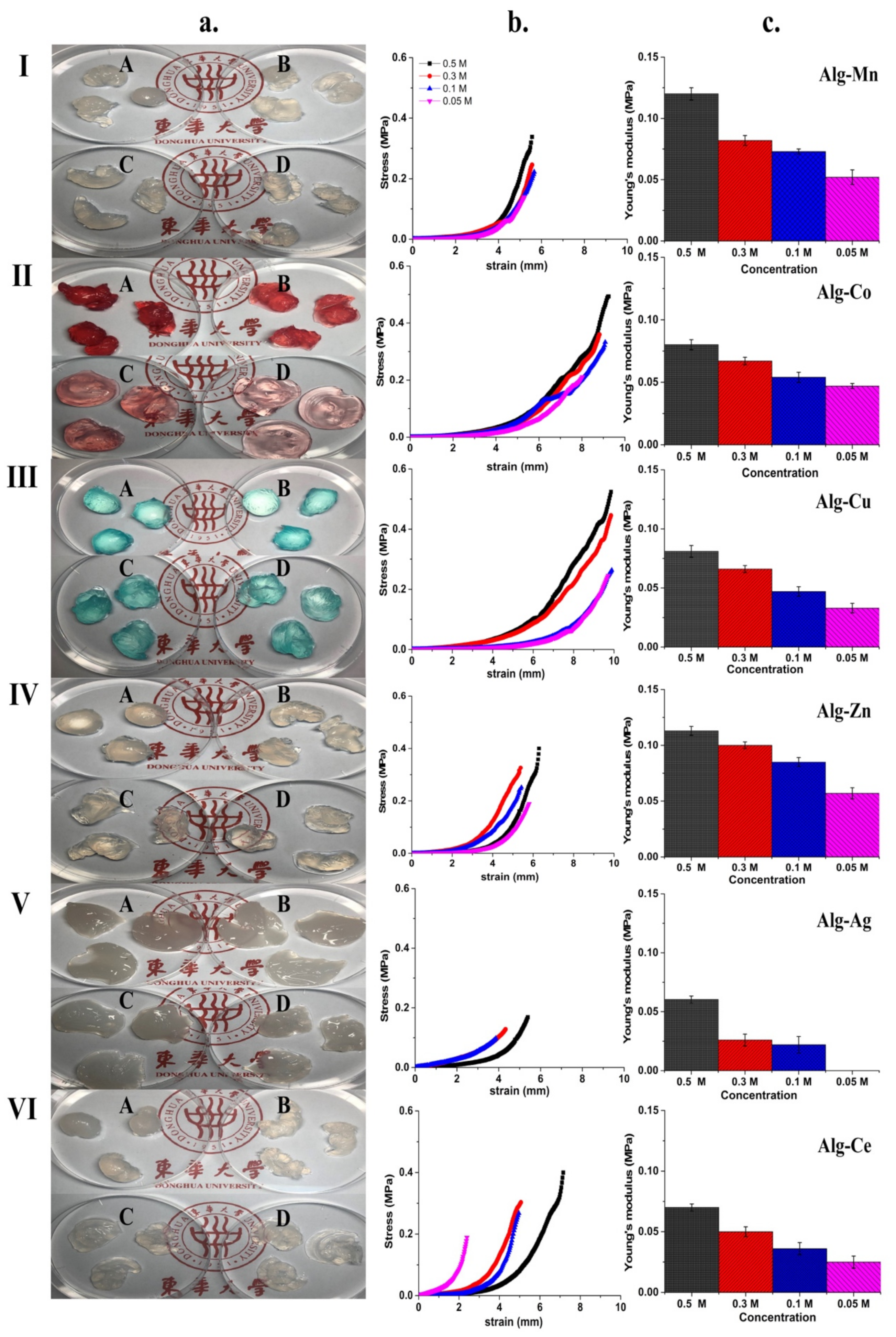
2.2. Effect of Cationic Concentration on the Physical Appearance and Hardness of Alginate Hydrogels
2.3. Preparation of Bacterial Nanocellulose
2.4. Fabrication of Dressing Material
2.5. Macro and Micro Morphology of Cation Cross-Linked Alginate/BNC Composites
2.6. Measurement of Alginate Content in the Alg/BNC Composites
2.7. Water Holding and Absorption Capacity of Composite Dressings
2.8. Antibacterial Activity
2.9. Mechanical Properties
2.10. pH-Responsive Release of Cations
2.11. Kinetic Analysis of Cation Release
2.12. In Vitro Whole-Blood Clotting Evaluation
2.13. In Vitro Hemolytic Rate
2.14. Cytotoxicity Test of Composite Dressings
2.15. In Vivo Healing Test
2.16. Statistical Analysis
3. Results and Discussion
3.1. Effect of Cation Concentration on Appearance and Hardness of Alginate Hydrogels
3.2. Macro and Micro Morphology of Different Cation Cross-Linked Alg/BNC Composites
3.3. Water and Alginate Content in Dressing Material
3.4. Water Holding and Absorption Capacity of Dressings
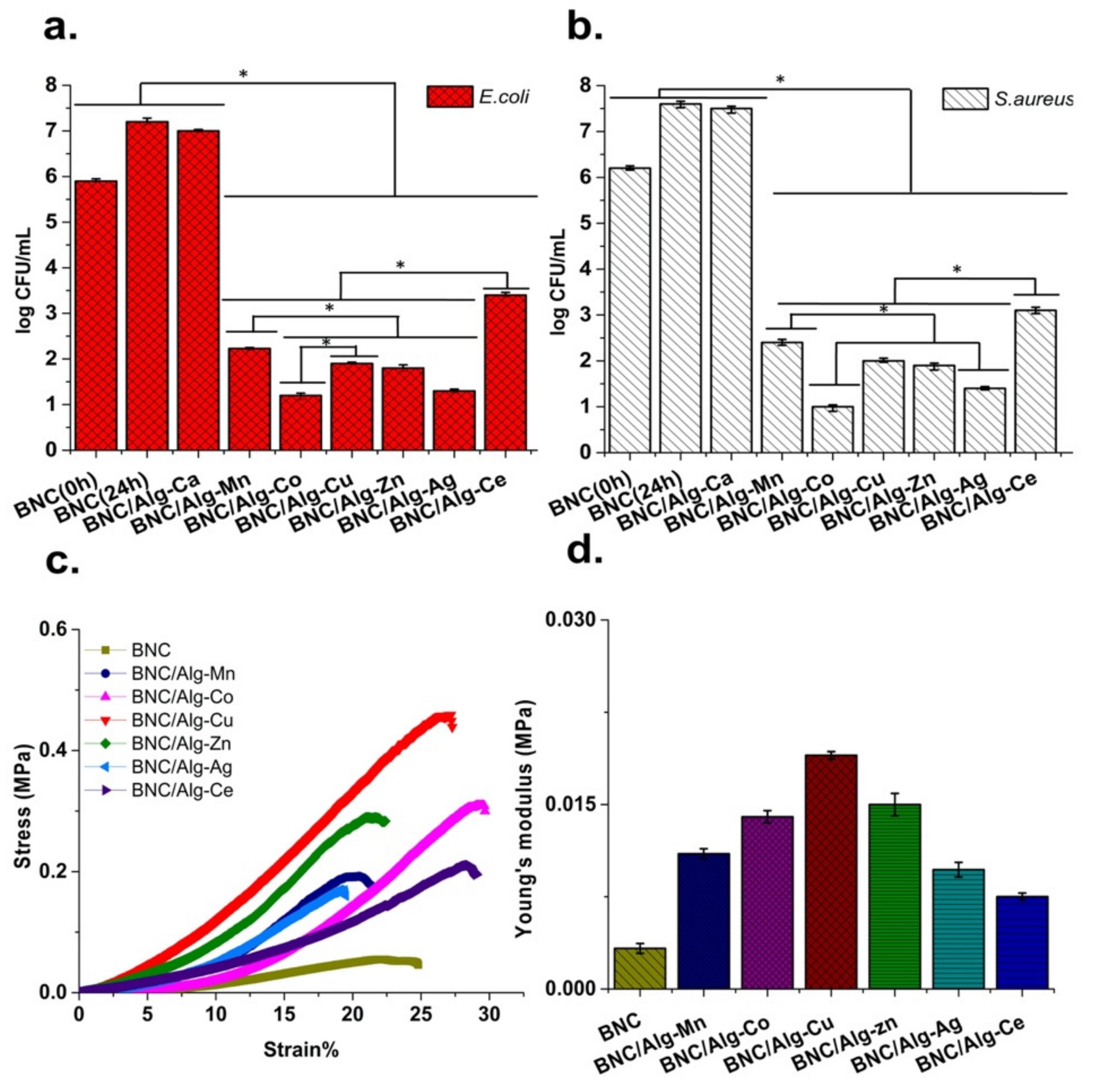
3.5. Antibacterial Activity
3.6. Mechanical Properties
3.7. pH-Responsive Release of Cations
3.8. Kinetic Analysis of Cations Release
3.9. Whole Blood Clotting Evaluation
3.10. In Vitro Hemolytic Rate
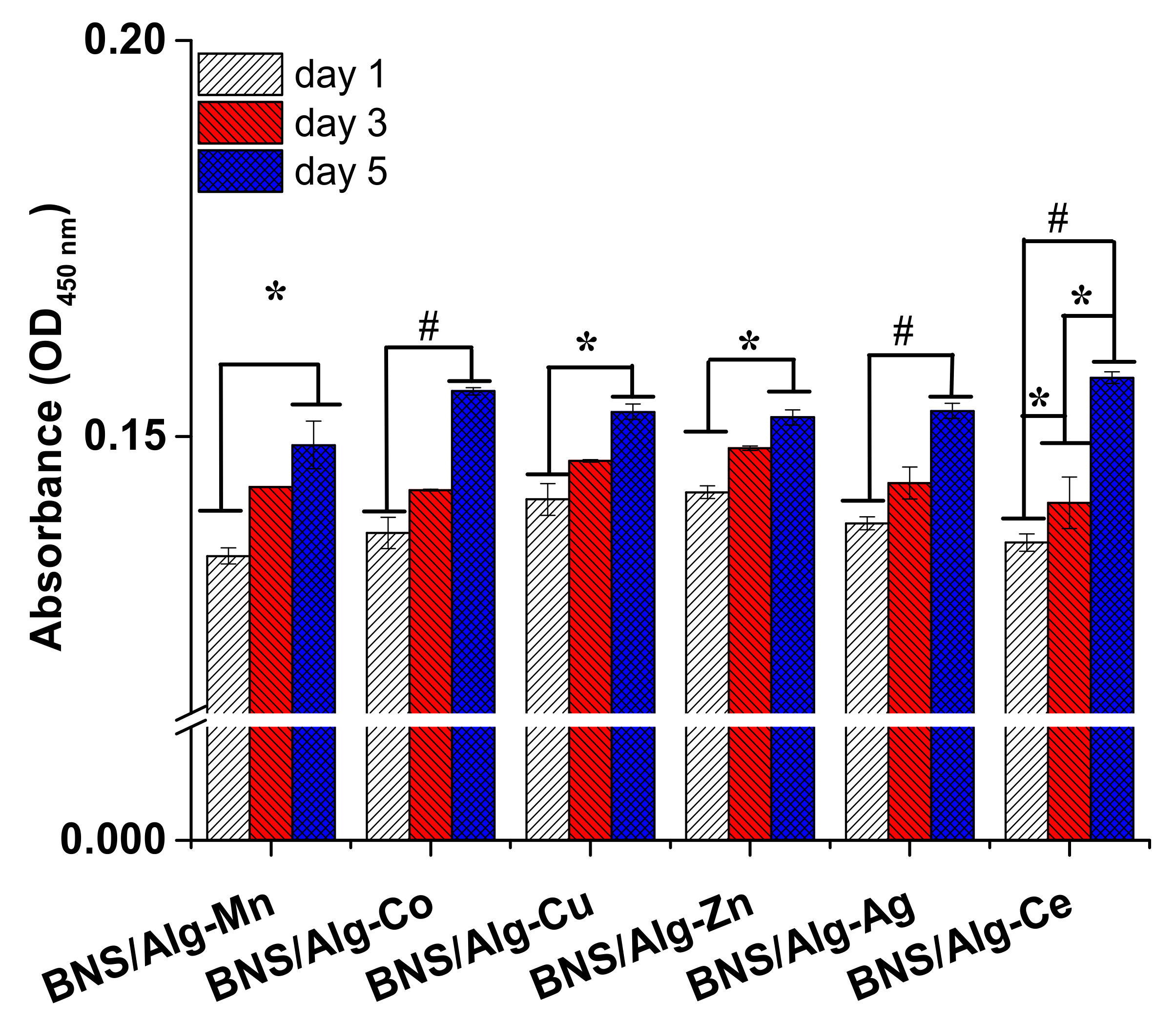
3.11. Cytotoxicity Test
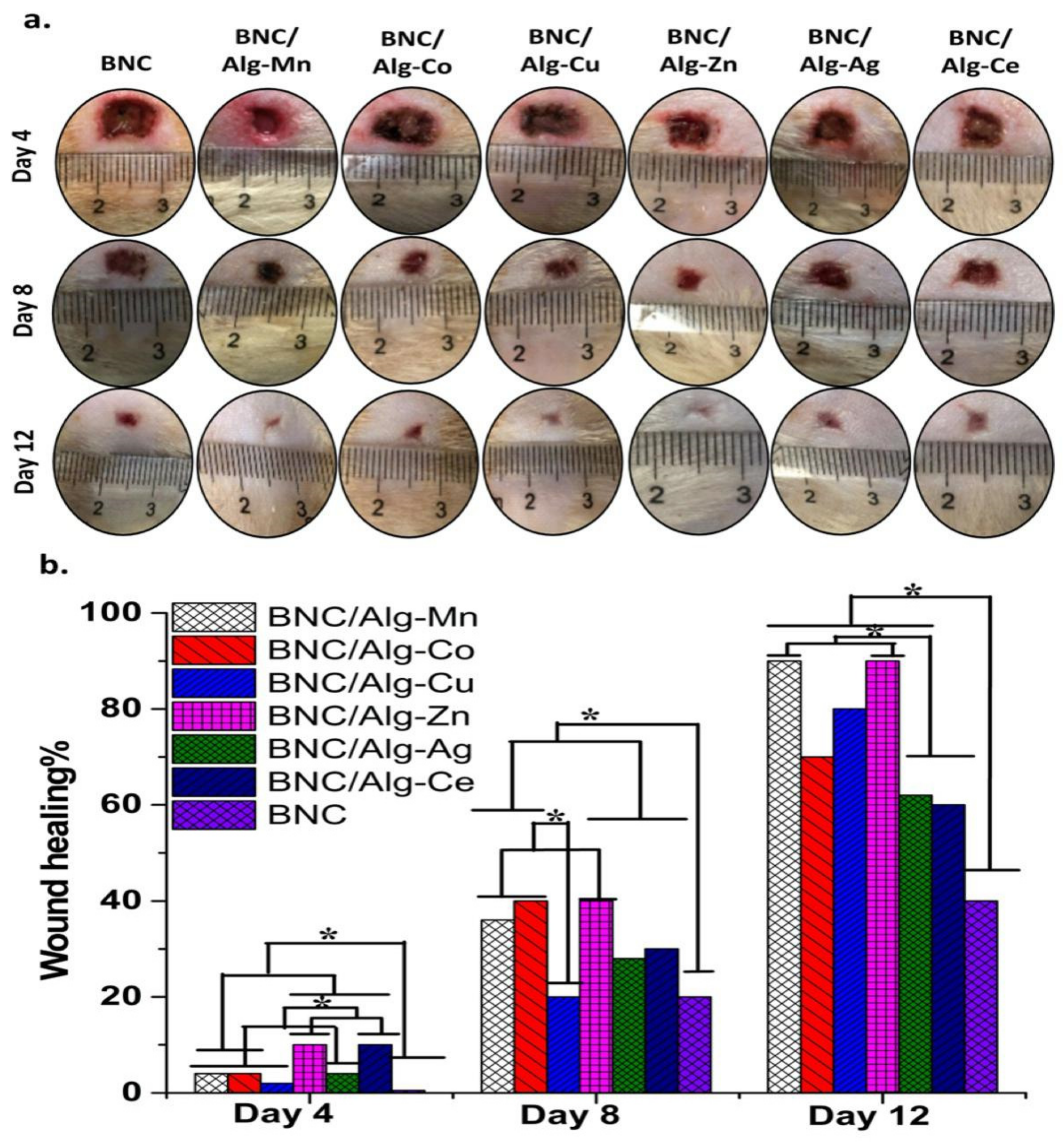
3.12. In Vivo Healing Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 136. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine (Taipei) 2015, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.T.P.; Huang, C.-M.; Huang, C.-J. Intelligent metal-phenolic metallogels as dressings for infected wounds. Sci. Rep. 2019, 9, 11562. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Qin, Y. Absorption characteristics of alginate wound dressings. J. Appl. Polym. Sci. 2003, 91, 953–957. [Google Scholar] [CrossRef]
- Sulaeva, I.; Hettegger, H.; Bergen, A.; Rohrer, C.; Kostic, M.; Konnerth, J.; Rosenau, T.; Potthast, A. Fabrication of bacterial cellulose-based wound dressings with improved performance by impregnation with alginate. Mater. Sci. Eng. C 2020, 110, 110619. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Özen, İ.; von Klitzing, R.; Erim, F.B. Antimicrobial cerium ion-chitosan crosslinked alginate biopolymer films: A novel and potential wound dressing. Int. J. Biol. Macromol. 2017, 105, 1161–1165. [Google Scholar] [CrossRef]
- Mohammed, A.; Rivers, A.; Stuckey, D.C.; Ward, K. Alginate extraction from Sargassum seaweed in the Caribbean region: Optimization using response surface methodology. Carbohydr. Polym. 2020, 245, 116419. [Google Scholar] [CrossRef]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of alginate gels: Interaction of diuronate units with divalent cations from density functional calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Siqueira, P.; Siqueira, É.; de Lima, A.E.; Siqueira, G.; Pinzón-Garcia, A.D.; Lopes, A.P.; Segura, M.E.C.; Isaac, A.; Pereira, F.V.; Botaro, V.R. Three-dimensional stable alginate-nanocellulose gels for biomedical applications: Towards tunable mechanical properties and cell growing. Nanomaterials 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, K.M.; Gea, S.; Ilyas, S.; Tamrin, T.; Radecka, I. Characterization of bacterial cellulose-based wound dressing in different order impregnation of chitosan and collagen. Biomolecules 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ward, J.; Choy, K.-L. Nature-inspired bacterial cellulose/methylglyoxal (BC/MGO) nanocomposite for broad-spectrum antimicrobial wound dressing. Macromol. Biosci. 2020, 20, e2000070. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application: A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Chuang, J.-J.; Huang, Y.-Y.; Lo, S.-H.; Hsu, T.-F.; Huang, W.-Y.; Huang, S.-L.; Lin, Y.-S. Effects of pH on the shape of alginate particles and its release behavior. J. Drug Deliv. Sci. Technol. 2019, 51, 3902704. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zhang, Z.; Liu, C.; Sun, C.; Zhang, W.; Marhaba, T. pH effect on heavy metal release from a polluted sediment. J. Chem. 2018, 2018, 7597640. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, L.; Hong, F.F.; Zhu, M. Evaluation of nanocellulose carriers produced by four different bacterial strains for laccase immobilization. Carbohydr. Polym. 2018, 196, 457–464. [Google Scholar] [CrossRef]
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]
- Portela, R.; Leal, C.R.; Almeida, P.L.; Sobral, R.G. Bacterial cellulose: A versatile biopolymer for wound dressing applications. Microb. Biotechnol. 2019, 12, 586–610. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, C.; Hong, F.; Yang, X.; Cao, Z. Preparation and characterization of BC/PAM-AgNPs nanocomposites for antibacterial applications. Carbohydr. Polym. 2015, 115, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, L.; Zhang, Q.; Hong, F.F. Using in situ dynamic cultures to rapidly biofabricate fabric-reinforced composites of chitosan/bacterial nanocellulose for antibacterial wound dressings. Front. Microbiol. 2016, 7, 260. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, S.; Chen, L.; Hu, J.; Hong, F.F. Determination of live and dead Komagataeibacter xylinus cells and first attempt at precise control of inoculation in nanocellulose production. Microb. Biotechnol. 2019, 13, 458–469. [Google Scholar] [CrossRef]
- Chen, G.; Wu, G.; Chen, L.; Wang, W.; Hong, F.F.; Jönsson, L. Comparison of productivity and quality of bacterial nanocellulose synthesized using culture media based on seven sugars from biomass. Microb. Biotechnol. 2019, 12, 677–687. [Google Scholar] [CrossRef]
- Vyas, K.S.; Vasconez, H.C. Wound healing: Biologics, skin substitutes, biomembranes and scaffolds. Healthcare 2014, 2, 356–400. [Google Scholar] [CrossRef]
- Kaygusuz, H.; Evingür, G.A.; Pekcan, Ö.; von Klitzing, R.; Erim, F.B. Surfactant and metal ion effects on the mechanical properties of alginate hydrogels. Int. J. Biol. Macromol. 2016, 92, 220–224. [Google Scholar] [CrossRef]
- Qin, Y. The gel swelling properties of alginate fibers and their applications in wound management. Polym. Adv. Technol. 2008, 19, 6–14. [Google Scholar] [CrossRef]
- Voo, W.-P.; Ooi, C.-W.; Islam, A.; Tey, B.-T.; Chan, E.-S. Calcium alginate hydrogel beads with high stiffness and extended dissolution behaviour. Eur. Polym. J. 2016, 75, 343–353. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Li, Y.; Pan, Y.; Jin, Y.; Xiao, H. Controlled release of agrochemicals and heavy metal ions capture dual-functional redox-responsive hydrogel for soil remediation. Chem. Commun. 2018, 54, 13714–13717. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lin, H.; Peng, B.; Huang, Q.; Shuai, F.; Xie, Y. Design and evaluation of hydrophilic matrix system for pH-independent sustained release of weakly acidic poorly soluble drug. AAPS PharmSciTech 2018, 19, 2144–2154. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-F.; Shau, M.-D.; Chang, M.-Y.; Chiou, S.-K.; Chang, J.-K.; Cherng, J.-Y. Platelet adsorption and hemolytic properties of liquid crystal/composite polymers. Int. J. Pharm. 2006, 327, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef] [PubMed]
- Kühbeck, D.; Mayr, J.; Häring, M.; Hofmann, M.; Quignard, F.; Díaz, D. Evaluation of the nitroaldol reaction in the presence of metal ion-crosslinked alginates. New J. Chem. 2015, 39, 2306–2315. [Google Scholar] [CrossRef]
- Zhang, J.; Ke, J.; Zhu, Y.; Song, J.; Yang, J.; Wen, C.; Zhang, L. Influence of divalent cations on the biofouling behaviors of alginate hydrogels. Biomed. Mater. 2019, 15, 015003. [Google Scholar] [CrossRef]
- Zhou, Q.; Kang, H.; Bielec, M.; Wu, X.; Cheng, Q.; Wei, W.; Dai, H. Influence of different divalent ions cross-linking sodium alginate-polyacrylamide hydrogels on antibacterial properties and wound healing. Carbohydr. Polym. 2018, 197, 292–304. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, R.; Zhao, X.; Zhang, Y.; Tam, A.; Yan, Y.; Shen, H.; Zhang, Y.S.; Qi, J.; Feng, Y.; et al. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019, 11, 3. [Google Scholar] [CrossRef]
- Wichai, S.; Chuysinuan, P.; Chaiarwut, S.; Ekabutr, P.; Supaphol, P. Development of bacterial cellulose/alginate/chitosan composites incorporating copper (II) sulfate as an antibacterial wound dressing. J. Drug Deliv. Sci. Technol. 2019, 51, 662–671. [Google Scholar] [CrossRef]
- Vu, T.T.; Fredenburgh, J.C.; Weitz, J.I. Zinc: An important cofactor in haemostasis and thrombosis. Thromb. Haemost. 2013, 109, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Garner, J.; Heppell, S. Cerium nitrate in the management of burns. Burns J. Int. Soc. Burn Inj. 2005, 31, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Li, M.; Yu, P.; Chu, X.; Li, L.; Tan, G.; Wang, Y.; Chen, X.; Zhang, Y.; et al. The synergistic antibacterial activity and mechanism of multicomponent metal ions-containing aqueous solutions against Staphylococcus aureus. J. Inorg. Biochem. 2016, 163, 214–220. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, R.; Hu, X.; Luo, G.; Wu, J.; He, W. A systematic and quantitative method for wound-dressing evaluation. Burn. Trauma 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The effect of pH on the extracellular matrix and biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Bhasarkar, J.; Bal, D.J. Kinetic investigation of a controlled drug delivery system based on alginate scaffold with embedded voids. J. Appl. Biomater. Funct. Mater. 2019, 17. [Google Scholar] [CrossRef]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef]
- Schmidlin, P.R.; Tchouboukov, A.; Wegehaupt, F.J.; Weber, F.E. Effect of cerium chloride application on fibroblast and osteoblast proliferation and differentiation. Arch. Oral Biol. 2012, 57, 892–897. [Google Scholar] [CrossRef]
- Raman, S.P.; Keil, C.; Dieringer, P.; Hübner, C.; Bueno, A.; Gurikov, P.; Nissen, J.; Holtkamp, M.; Karst, U.; Haase, H.; et al. Alginate aerogels carrying calcium, zinc and silver cations for wound care: Fabrication and metal detection. J. Supercrit. Fluids 2019, 153, 104545. [Google Scholar] [CrossRef]




| Dressing Material * | WHC * (g/g) | WAC * (g/g) | WBF * (g/g) | WDWM * (g/g) |
|---|---|---|---|---|
| Control (BNC) | 20.54 ± 0.15 | 15.24 ± 0.04 | 13.24 ± 0.03 | 5.14 ± 0.04 |
| BNC/Alg-Mn | 42.25 ± 0.05 c | 31.38 ± 0.1 c | 26.61 ± 0.04 c | 5.31 ± 0.01 a |
| BNC/Alg-Co | 23.34 ± 0.02 c | 19.26 ± 0.03 c | 17.22 ± 0.08 c | 8.32 ± 0.04 a |
| BNC/Alg-Cu | 20.16 ± 0.09 a | 17.51 ± 0.04 b | 14.63 ± 0.05 c | 6.24 ± 0.03 a |
| BNC/Alg-Zn | 23.66 ± 0.04 c | 21.26 ± 0.05 b | 20.52 ± 0.08 c | 15.45 ± 0.07 c |
| BNC/Alg-Ag | 49.55 ± 0.06 c | 46.52 ± 0.14 c | 37.13 ± 0.1 c | 5.13 ± 0,02 a |
| BNC/Alg-Ce | 37.38 ± 0.07 c | 33.89 ± 0.04 c | 31.94 ± 0.08 b | 15.89 ± 0.065 b |
| Cations | pH | Zero-Order | First-Order | Higuchi |
|---|---|---|---|---|
| r2 | r2 | r2 | ||
| Mn2+ | 5.5 | 0.9958 | 0.9993 | 0.9482 |
| 7.4 | 0.9739 | 0.9686 | 0.9972 | |
| 8.4 | 0.9818 | 0.9938 | 9.9945 | |
| Co2+ | 5.5 | 0.9781 | 0.9714 | 0.9502 |
| 7.4 | 0.9922 | 0.9994 | 0.9717 | |
| 8.4 | 0.9798 | 0.9819 | 0.9692 | |
| Cu2+ | 5.5 | 0.9995 | 0.9819 | 0.9963 |
| 7.4 | 0.9958 | 0.9878 | 0.9839 | |
| 8.4 | 0.9948 | 0.9955 | 0.9981 | |
| Zn2+ | 5.5 | 0.9935 | 0.9986 | 0.9403 |
| 7.4 | 0.9819 | 0.9958 | 0.9324 | |
| 8.4 | 0.9925 | 0.9981 | 0.9381 | |
| Ag+ | 5.5 | 0.9819 | 0.9958 | 0.9742 |
| 7.4 | 0.9952 | 0.9162 | 0.9901 | |
| 8.4 | 0.9995 | 0.9695 | 0.9678 | |
| Ce3+ | 5.5 | 0.9922 | 0.9726 | 0.9624 |
| 7.4 | 0.9849 | 0.9894 | 0.9869 | |
| 8.4 | 0.9922 | 0.9977 | 0.9945 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahriari-Khalaji, M.; Hong, S.; Hu, G.; Ji, Y.; Hong, F.F. Bacterial Nanocellulose-Enhanced Alginate Double-Network Hydrogels Cross-Linked with Six Metal Cations for Antibacterial Wound Dressing. Polymers 2020, 12, 2683. https://doi.org/10.3390/polym12112683
Shahriari-Khalaji M, Hong S, Hu G, Ji Y, Hong FF. Bacterial Nanocellulose-Enhanced Alginate Double-Network Hydrogels Cross-Linked with Six Metal Cations for Antibacterial Wound Dressing. Polymers. 2020; 12(11):2683. https://doi.org/10.3390/polym12112683
Chicago/Turabian StyleShahriari-Khalaji, Mina, Siyi Hong, Gaoquan Hu, Ying Ji, and Feng F. Hong. 2020. "Bacterial Nanocellulose-Enhanced Alginate Double-Network Hydrogels Cross-Linked with Six Metal Cations for Antibacterial Wound Dressing" Polymers 12, no. 11: 2683. https://doi.org/10.3390/polym12112683
APA StyleShahriari-Khalaji, M., Hong, S., Hu, G., Ji, Y., & Hong, F. F. (2020). Bacterial Nanocellulose-Enhanced Alginate Double-Network Hydrogels Cross-Linked with Six Metal Cations for Antibacterial Wound Dressing. Polymers, 12(11), 2683. https://doi.org/10.3390/polym12112683