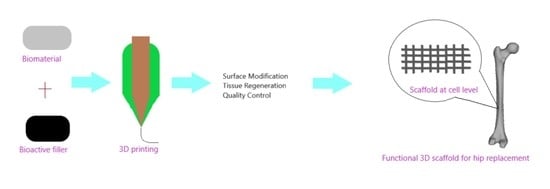
3D Printing for Hip Implant Applications: A Review
Abstract
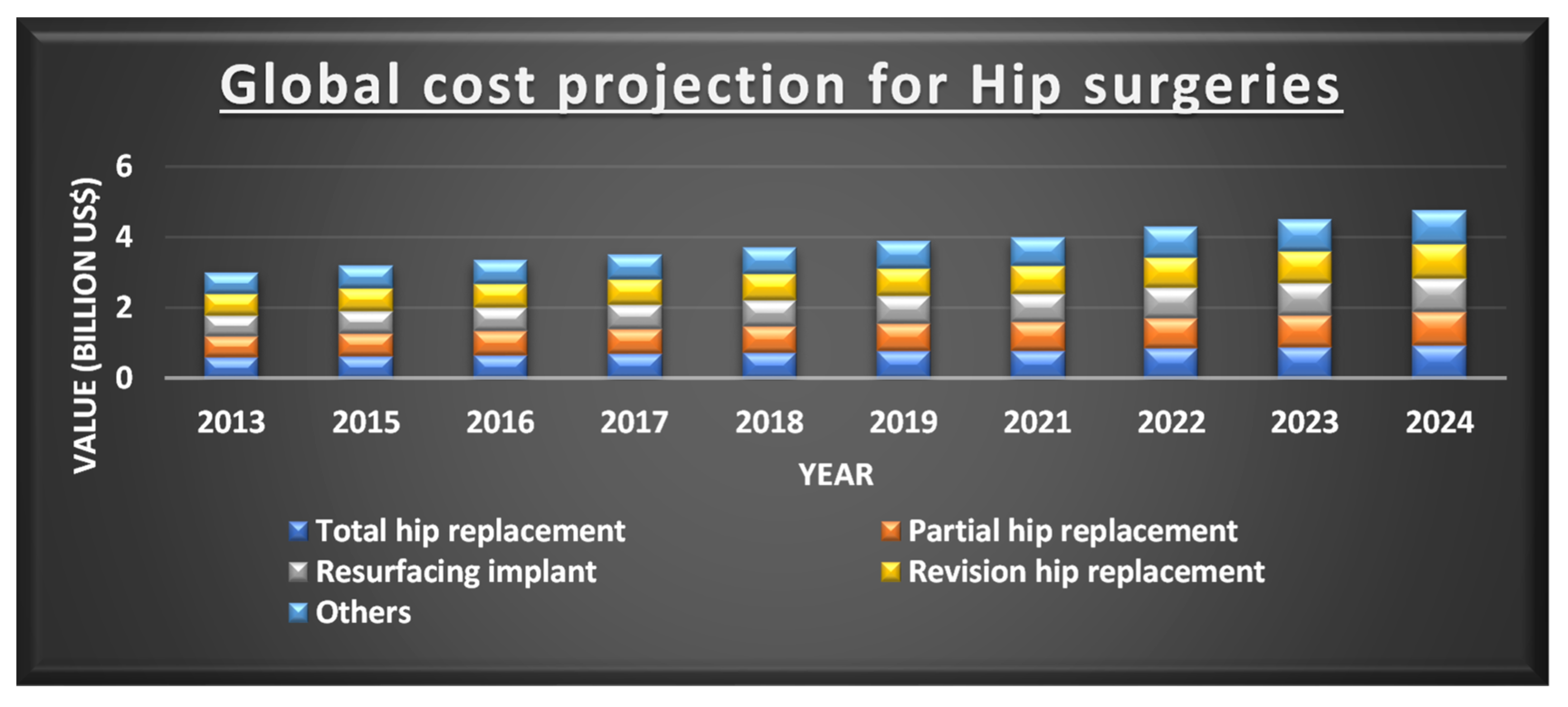
1. Introduction
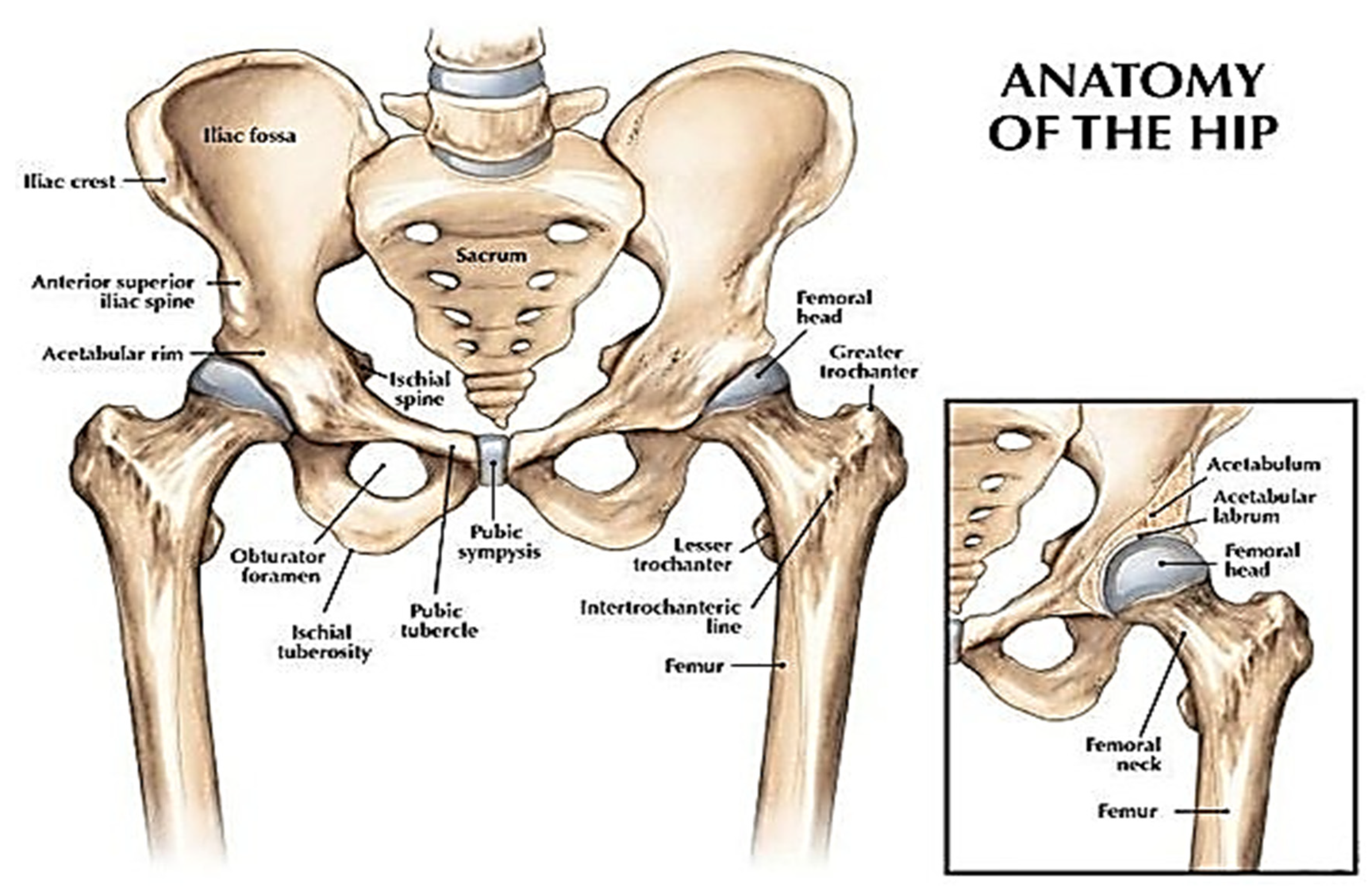
2. 3D Printing for Hip Replacement
2.1. Direct 3D Printing for Hip Replacement
2.2. Fused Deposition Modelling (FDM) for Hip Replacement
2.3. Selective Laser Sintering (SLS) for Hip Replacement
2.4. Stereolithography (SLA) for Hip Replacement
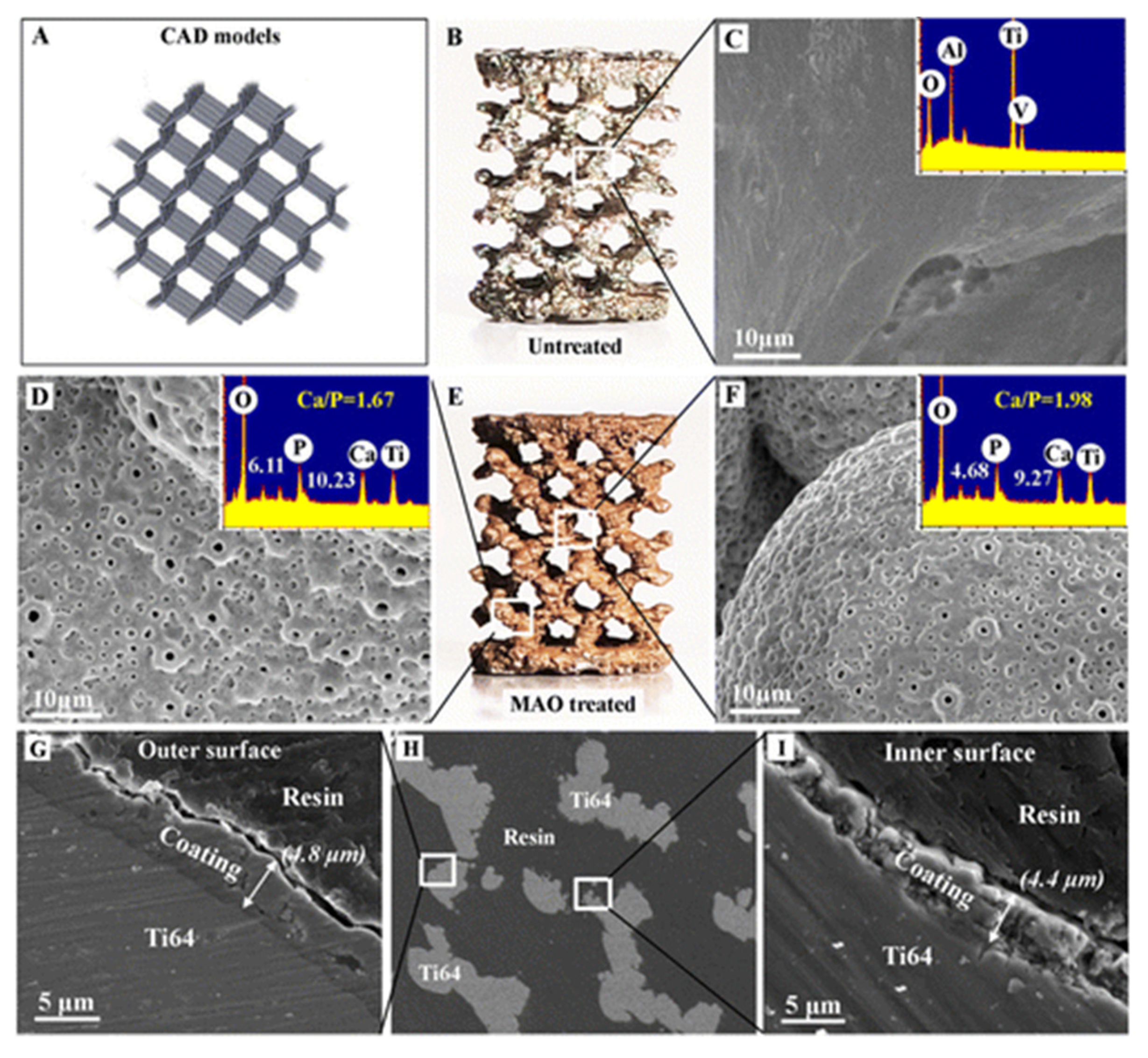
2.5. Surface Modifications of 3D Printed Implants
3. 3D Hip Tissue Regeneration
- (i)
- Should be porous (to ensure nutrient movement, removal of waste and cell growth), biocompatibility, reproducibility, cell/tissue compatibility, easy preparation and biodegradable.
- (ii)
- Lead to the reduced inflammatory reaction, therefore, decreases the possibility of immune system rejection.
- (iii)
- Advantageous if the biomaterial tissue scaffolds can act as substrates that support cellular fastening, growth and differentiation.
- (iv)
- The cells grow and differentiate, and this scaffold must have the ability to resist the forces put in by the cells else the scaffold disintegrates and causes dismal diffusion of nutrients, waste and oxygen.
- (v)
- The scaffold structure should be mechanically stable to be capable of maintaining load-bearing and varying body movements in daily activity on the joint.
4. Challenges, Ethics and Trends in 3D Printing of Implants
- Consideration of limits to bioprinting in medicine.
- Key risks of major harm on the human body because of 3D printing.
- Clinical trial process for bioprinting samples.
- The extent of replicability, irreversibility damage and loss of treatment opportunity during surgery.
- Current ethical laws are guarding 3D bioprinting for bio application.
- The clinically proven advancement of 3D printing over conventional treatments with significant success rates.
- The assurance of 3D printing efficiency in the human body risk to benefit ratio.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Do, A.V.; Khorsand, B.; Geary, S.M.; Salem, A.K. 3D printing of scaffolds for tissue regeneration applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar] [CrossRef] [PubMed]
- The Editors of Encyclopaedia Britannica. Pelvis, Encyclopædia Britannica. Encyclopædia Britannica, Inc. Available online: https://www.britannica.com/science/pelvis (accessed on 11 November 2020).
- Rodriguez, D. The Basics of Hip Pain, Everyday Health. 2009. Available online: https://www.everydayhealth.com/pain-management/Hip-pain/understanding/index.aspx (accessed on 11 November 2020).
- Commitee, N.S. National Joint Registry for England and Wales: 14th Annual Report; Northern Ireland and the Isle of Man (NJR): Hemel Hempstead, UK, 2018. [Google Scholar]
- Jain, A.; Clamp, K. Adult Hip Pain. InnovAiT 2019, 13, 21–27. [Google Scholar] [CrossRef]
- Older, J. Charnley low-friction arthroplasty: A worldwide retrospective review at 15 to 20 years. J. Arthroplast. 2002, 17, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Furnes, O.; Lie, S.A.; Espehaug, B.; Vollset, S.E.; Engesaeter, L.B.; Havelin, L.I. Hip disease and the prognosis of total Hip replacements: A review of 53,698 primary total Hip replacements reported to the Norwegian arthroplasty register 1987–1999. J. Bone Joint Surg. Br. Vol. 2001, 83, 579. [Google Scholar] [CrossRef]
- Hughes, A.J.; DeBuitleir, C.; Soden, P.; O’Donnchadha, B.; Tansey, A.; Abdulkarim, A.; McMahon, C.; Hurson, C.J. 3D printing aids acetabular reconstruction in complex revision Hip arthroplasty. Adv. Orthop. 2017, 8925050. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, L.; Casey, J.; Gee, M.; Palmer, C.; Sinha, J.; Moxham, J.; Colegate-Stone, T.J. Value-based healthcare analysis of joint replacement surgery for patients with primary Hip osteoarthritis. BMJ Open Qual. 2019, 8, e000549. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.J.; Hart, A.; Panagiotopoulou, V.; Henckel, J. Personalised orthopaedics–using 3D printing for tailor-made technical teaching, pre-operative planning and precise placement of implants. Orthop Prod. News 2017, 178, 22–26. [Google Scholar]
- Polaris Market Research. Polaris Market Research. Available online: https://www.polarismarketresearch.com/industry-analysis/orthopaedic-implants-market (accessed on 11 November 2020).
- Kang, C.W.; Fang, F.Z. State of the art of bioimplants manufacturing: Part I. Adv. Manuf. 2018, 6, 20–40. [Google Scholar] [CrossRef]
- Kang, C.W.; Fang, F.Z. State of the art of bioimplants manufacturing: Part II. Adv. Manuf. 2018, 6, 137–154. [Google Scholar] [CrossRef]
- Gharde, S.; Goud, R.; Nimje, S.; Kandasubramanian, B. Aggrandized flexural properties of assorted natural biological materials. In Biodegradable Composites: Materials, Manufacturing and Engineering; Kaushik, K., Davim Paulo, J., Eds.; De Gruyter: Berlin, Germany, 2020; Chapter 6. [Google Scholar] [CrossRef]
- Gharde, S.; Surendren, A.; Korde, J.M.; Saini, S.; Deoray, N.; Goud, R.; Nimje, S.; Kandasubramanian, B. Recent Advances in Additive Manufacturing of Bio-Inspired Materials. In Biomanufacturing; Prakash, C., Singh, S., Singh, R., Ramakrishna, S., Pabla, B.S., Puri, S., Uddin, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 35–68. [Google Scholar] [CrossRef]
- Martinez-Marquez, D.; Mirnajafizadeh, A.; Carty, C.P.; Stewart, R.A. Application of quality by design for 3D printed bone prostheses and scaffolds. PLoS ONE 2018, 13, e0195291. [Google Scholar] [CrossRef]
- Mai, J.G.; Gu, C.; Lin, X.Z.; Li, T.; Huang, W.Q.; Wang, H.; Tan, X.Y.; Lin, H.; Wang, Y.M.; Yang, Y.Q.; et al. Application of three-dimensional printing personalized acetabular wing-plate in treatment of complex acetabular fractures via lateral-rectus approach. Zhonghua wai ke za zhi [Chin. J. Surg.] 2017, 55, 172. [Google Scholar] [PubMed]
- Xia, R.Z.; Zhai, Z.J.; Chang, Y.Y.; Li, H.W. Clinical Applications of 3-Dimensional Printing Technology in Hip Joint. Orthop. Surg. 2019, 11, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, H.E.; Fahimipour, F.; Dashtimoghadam, E.; Tayebi, L. Collagen Grafted 3D-Printed Polycaprolactone Scaffolds for Craniomaxillofacial Bone Regeneration. J. Oral Maxillofac. Surg. 2017, 75, e402. [Google Scholar] [CrossRef]
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current biomedical applications of 3D printing and additive manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef]
- Szymczyk, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Biocybern. Biomed. Eng. 2020, 75. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef]
- EU. Article 2, Comma 3 of the Regulation (EU); 2017/745; Official Journal of the European Union: Brussels, Belgium, 2017. [Google Scholar]
- EU. Article 20, Comma 1 of the Regulation (EU); 2017/745; Official Journal of the European Union: Brussels, Belgium, 2017. [Google Scholar]
- Bartolo, P.; Kruth, J.P.; Silva, J.; Levy, G.; Malshe, A.; Rajurkar, K.; Mitsuishi, M.; Ciurana, J.; Leu, M. Biomedical production of implants by additive electro-chemical and physical processes. CIRP Ann. 2012, 61, 635–655. [Google Scholar] [CrossRef]
- Sachs, E.M.; Haggerty, J.S.; Cima, M.J.; Williams, P.A. Massachusetts Institute of Technology. Three-Dimensional Printing Techniques. U.S. Patent 5,204,055, 20 April 1993. [Google Scholar]
- Milazzo, M.; ContessiNegrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29, 1903055. [Google Scholar] [CrossRef]
- Walczak, R.; Adamski, K.; Pokrzywnicka, A.; Kubicki, W. Inkjet 3D printing–studies on applicability for lab-on-a-cHip technique. Procedia Eng. 2016, 168, 1362–1365. [Google Scholar] [CrossRef]
- Krivec, M.; Roshanghias, A.; Abram, A.; Binder, A. Exploiting the combination of 3D polymer printing and inkjet Ag-nanoparticle printing for advanced packaging. Microelectron. Eng. 2017, 176, 1–5. [Google Scholar] [CrossRef]
- Zhang, B.; Pei, X.; Song, P.; Sun, H.; Li, H.; Fan, Y.; Jiang, Q.; Zhou, C.; Zhang, X. Porous bioceramics produced by inkjet 3D printing: Effect of printing ink formulation on the ceramic macro and micro porous architectures control. Compos. Part B Eng. 2018, 155, 112–121. [Google Scholar] [CrossRef]
- Cui, X.; Dean, D.; Ruggeri, Z.M.; Boland, T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol. Bioeng. 2010, 106, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Kyobula, M.; Adedeji, A.; Alexander, M.R.; Saleh, E.; Wildman, R.; Ashcroft, I.; Gellert, P.R.; Roberts, C.J. 3D inkjet printing of Tablets exploiting bespoke complex geometries for controlled and tuneable drug release. J. Control. Release 2017, 261, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef]
- Sinha, S.K. Additive manufacturing (AM) of medical devices and scaffolds for tissue engineering based on 3D and 4D printing. In 3D and 4D Printing of Polymer Nanocomposite Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–160. [Google Scholar]
- Danforth, S.C.; Agarwala, M.; Bandyopadghyay, A.; Langrana, N.; Jamalabad, V.R.; Safari, A.; Van Weeren, R. Rutgers State University of New Jersey. Solid Freeform Fabrication Methods. U.S. Patent 5,738,817, 14 April 1998. [Google Scholar]
- Prasad, A.; Kandasubramanian, B. Fused Deposition Processing Polycaprolactone of Composites for Biomedical Applications. Polym. Plast. Technol. Mater. 2019, 58, 1365–1398. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Bagaria, V.; Chaudhary, K. A paradigm shift in surgical planning and simulation using 3Dgraphy: Experience of first 50 surgeries done using 3D-printed biomodels. Injury 2017, 48, 2501–2508. [Google Scholar] [CrossRef]
- Hong, J.M.; Kim, B.J.; Shim, J.H.; Kang, K.S.; Kim, K.J.; Rhie, J.W.; Cha, H.J.; Cho, D.W. Enhancement of bone regeneration through facile surface functionalization of solid freeform fabrication-based three-dimensional scaffolds using mussel adhesive proteins. Acta Biomater. 2012, 8, 2578–2586. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A review of additive manufacturing. ISRN Mech. Eng. 2012, 2012, 208760. [Google Scholar] [CrossRef]
- Novakova-Marcincinova, L.; Kuric, I. Basic and advanced materials for fused deposition modeling rapid prototyping technology. Manuf. Ind. Eng. 2012, 11, 24–27. [Google Scholar]
- Korpela, J.; Kokkari, A.; Korhonen, H.; Malin, M.; Närhi, T.; Seppälä, J. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.F.; Vaquette, C.; Zhang, Q.; Reis, R.L.; Ivanovski, S.; Hutmacher, D.W. Advanced tissue engineering scaffold design for regeneration of the complex hierarchical periodontal structure. J. Clin. Periodontol. 2014, 41, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.A.; Choudhury, D.; Zou, M. 3D printed PCU/UHMWPE polymeric blend for artificial knee meniscus. Tribol. Int. 2018, 122, 1–7. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704. [Google Scholar]
- Yorukoglu, A.C.; Kiter, A.H.M.E.T.; Akkaya, S.; Satiroglu-Tufan, N.L.; Tufan, A.C. A concise review on the use of mesenchymal stem cells in cell sheet-based tissue engineering with special emphasis on bone tissue regeneration. Stem Cells Int. 2017, 2017, 2374161. [Google Scholar] [CrossRef] [PubMed]
- Pollak, F.H.; Shen, H. Modulation spectroscopy of semiconductors: Bulk/thin film, microstructures, surfaces/interfaces and devices. Mater. Sci. Eng. R Rep. 1993, 10, 275–374. [Google Scholar] [CrossRef]
- Eosoly, S.; Brabazon, D.; Lohfeld, S.; Looney, L. Selective laser sintering of hydroxyapatite/poly-ε-caprolactone scaffolds. Acta Biomater. 2010, 6, 2511–2517. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, P.; Cheng, X.; Xie, Y.; Liang, C.; Li, C.; Xu, S. Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue engineering applications. Int. J. Nanomed. 2013, 8, 4197. [Google Scholar]
- Chung, H.; Jee, H.; Das, S. Selective laser sintering of PCL/TCP composites for tissue engineering scaffolds. J. Mech. Sci. Technol. 2010, 24, 241–244. [Google Scholar] [CrossRef]
- Doyle, H.; Lohfeld, S.; McHugh, P. Predicting the elastic properties of selective laser sintered PCL/β-TCP bone scaffold materials using computational modelling. Ann. Biomed. Eng. 2014, 42, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Sudarmadji, N.; Tan, J.Y.; Leong, K.F.; Chua, C.K.; Loh, Y.T. Investigation of the mechanical properties and porosity relationsHips in selective laser-sintered polyhedral for functionally graded scaffolds. Acta Biomater. 2011, 7, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Lohfeld, S.; Tyndyk, M.A.; Cahill, S.; Flaherty, N.; Barron, V.; McHugh, P.E. A method to fabricate small features on scaffolds for tissue engineering via selective laser sintering. J. Biomed. Sci. Eng. 2010, 3, 138. [Google Scholar] [CrossRef]
- Ghita, O.R.; James, E.; Trimble, R.; Evans, K.E. Physico-chemical behaviour of poly (ether ketone) (PEK) in high temperature laser sintering (HT-LS). J. Mater. Process. Technol. 2014, 214, 969–978. [Google Scholar] [CrossRef]
- Melchels, F.P.; Feijen, J.; Grijpma, D.W. A poly (D, L-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials 2009, 30, 3801–3809. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F.; Tan, K.H.; Wiria, F.E.; Cheah, C.M. Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J. Mater. Sci. Mater. Med. 2004, 15, 1113–1121. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Wang, Y.; Li, Z.; Huang, S. Study of the selective laser sintering of polycarbonate and postprocess for parts reinforcement. Proceedings of the Institution of Mechanical Engineers, Part L. J. Mater. Des. Appl. 2007, 221, 37–42. [Google Scholar]
- Bracaglia, L.G.; Smith, B.T.; Watson, E.; Arumugasaamy, N.; Mikos, A.G.; Fisher, J.P. 3D printing for the design and fabrication of polymer-based gradient scaffolds. Acta Biomater. 2017, 56, 3–13. [Google Scholar] [CrossRef]
- Matsuda, T.; Mizutani, M. Liquid acrylate-endcapped poly (e-caprolactone-co-trimethylene carbonate). II. Computer-aided stereolithographic microarchitectural surface photoconstructs. J. Biomed. Mater. Res. 2002, 62, 395–403. [Google Scholar] [CrossRef]
- Griffith, M.L.; Halloran, J.W. Freeform fabrication of ceramics via stereolithography. J. Am. Ceram. Soc. 1996, 79, 2601–2608. [Google Scholar] [CrossRef]
- Dhariwala, B.; Hunt, E.; Boland, T. Rapid prototyping of tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 2004, 10, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Vahabzadeh, S.; Shivaram, A.; Bose, S. Three-dimensional printing of biomaterials and soft materials. MRS Bull. 2015, 40, 1162–1169. [Google Scholar] [CrossRef]
- Kondiah, P.J.; Kondiah, P.P.; Choonara, Y.E.; Marimuthu, T.; Pillay, V. A 3D Bioprinted Pseudo-Bone Drug Delivery Scaffold for Bone Tissue Engineering. Pharmaceutics 2020, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- De Momi, E.L.; Pavan, E.; Motyl, B.; Bandera, C.; Frigo, C. Hip joint anatomy virtual and stereolithographic reconstruction for preoperative planning of total Hip replacement. In International Congress Series; Elsevier: Amsterdam, The Netherlands, 2005; Volume 1281, pp. 708–712. [Google Scholar]
- Lee, K.W.; Wang, S.; Fox, B.C.; Ritman, E.L.; Yaszemski, M.J.; Lu, L. Poly (propylene fumarate) bone tissue engineering scaffold fabrication using stereolithography: Effects of resin formulations and laser parameters. Biomacromolecules 2007, 8, 1077–1084. [Google Scholar] [CrossRef]
- Cooke, M.N.; Fisher, J.P.; Dean, D.; Rimnac, C.; Mikos, A.G. Use of stereolithography to manufacture critical-sized 3D biodegradable scaffolds for bone ingrowth. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 64, 65–69. [Google Scholar] [CrossRef]
- Akopova, T.A.; Demina, T.S.; Bagratashvili, V.N.; Bardakova, K.N.; Novikov, M.M.; Selezneva, I.I.; Istomin, A.V.; Svidchenko, E.A.; Cherkaev, G.V.; Surin, N.M.; et al. Solid state synthesis of chitosan and its unsaturated derivatives for laser microfabrication of 3D scaffolds. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2015; Volume 87, p. 012079. [Google Scholar]
- Qiu, Y.; Zhang, N.; Kang, Q.; An, Y.; Wen, X. Chemically modified light-curable chitosans with enhanced potential for bone tissue repair. J. Biomed. Mater. Res. Part A 2009, 89, 772–779. [Google Scholar] [CrossRef]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef]
- Chan, V.; Zorlutuna, P.; Jeong, J.H.; Kong, H.; Bashir, R. Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab CHip 2010, 10, 2062–2070. [Google Scholar] [CrossRef]
- Gauvin, R.; Chen, Y.C.; Lee, J.W.; Soman, P.; Zorlutuna, P.; Nichol, J.W.; Bae, H.; Chen, S.; Khademhosseini, A. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials 2012, 33, 3824–3834. [Google Scholar] [CrossRef]
- Elomaa, L.; Kokkari, A.; Närhi, T.; Seppälä, J.V. Porous 3D modeled scaffolds of bioactive glass and photocrosslinkable poly (ε-caprolactone) by stereolithography. Compos. Sci. Technol. 2013, 74, 99–106. [Google Scholar] [CrossRef]
- Ni, J.; Ling, H.; Zhang, S.; Wang, Z.; Peng, Z.; Benyshek, C.; Zan, R.; Miri, A.K.; Li, Z.; Zhang, X.; et al. Three-dimensional printing of metals for biomedical applications. Mater. Today Bio 2019, 3, 100024. [Google Scholar] [CrossRef] [PubMed]
- Peltola, S.M.; Melchels, F.P.; Grijpma, D.W.; Kellomäki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hagg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; Newman, P.; Zreiqat, H. Design and fabrication of 3D printed scaffolds with a mechanical strength comparable to cortical bone to repair large bone defects. Sci. Rep. 2016, 6, 19468. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Park, S.H.; Park, D.S.; Shin, J.W.; Kang, Y.G.; Kim, H.K.; Yoon, T.R.; Shin, J.W. Scaffolds for bone tissue engineering fabricated from two different materials by the rapid prototyping technique: PCL versus PLGA. J. Mater. Sci. Mater. Med. 2012, 23, 2671–2678. [Google Scholar] [CrossRef]
- Haberstroh, K.; Ritter, K.; Kuschnierz, J.; Bormann, K.H.; Kaps, C.; Carvalho, C.; Mülhaupt, R.; Sittinger, M.; Gellrich, N.C. Bone repair by cell-seeded 3D-bioplotted composite scaffolds made of collagen treated tricalciumphosphate or tricalciumphosphate-chitosan-collagen hydrogel or PLGA in ovine critical-sized calvarial defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 93, 520–530. [Google Scholar] [CrossRef]
- Miller, H.; Barceloux, D.G.; Krenzelok, E.P.; Olson, K.; Watson, W. American academy of clinical toxicology practice guidelines on the treatment of ethylene glycol poisoning. J. Toxicol. Clin. Toxicol. 1999, 37, 537–560. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed Tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.; Kalyon, D.M.; Yu, X.; Valdevit, A. Segmental bone replacement via patient-specific, three-dimensional printed bioresorbable graft substitutes and their use as templates for the culture of mesenchymal stem cells under mechanical stimulation at various frequencies. Biotechnol. Bioeng. 2018, 115, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Nam, J.; Sun, W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Eng. Part A 2008, 14, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.J.; Khang, G.; Lee, H.B. Interaction of fibroblasts on polycarbonate membrane surfaces with different micropore sizes and hydrophilicity. J. Biomater. Sci. Polymer Ed. 1999, 10, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Barata, D.; Dias, P.; Wieringa, P.; van Blitterswijk, C.; Habibovic, P. Cell-instructive high-resolution micropatterned polylactic acid surfaces. Biofabrication 2017, 9, 035004. [Google Scholar] [CrossRef]
- Singhvi, R.; Stephanopoulos, G.; Wang, D.I. Effects of substratum morphology on cell physiology. Biotechnol. Bioeng. 1994, 43, 764–771. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokosz, K.; Rokicki, R.; Prima, F. Nanoindentation and XPS studies of Titanium TNZ alloy after electrochemical polishing in a magnetic field. Materials 2015, 8, 205–215. [Google Scholar] [CrossRef]
- Habibzadeh, S.; Li, L.; Shum-Tim, D.; Davis, E.C.; Omanovic, S. Electrochemical polishing as a 316L stainless steel surface treatment method: Towards the improvement of biocompatibility. Corros. Sci. 2014, 87, 89–100. [Google Scholar] [CrossRef]
- Chan, K.H.; Zhuo, S.; Ni, M. Priming the surface of orthopaedic implants for osteoblast attachment in bone tissue engineering. Int. J. Med. Sci. 2015, 12, 701. [Google Scholar] [CrossRef][Green Version]
- Bellino, M.G.; Golbert, S.; De Marzi, M.C.; Soler-Illia, G.J.; Desimone, M.F. Controlled adhesion and proliferation of a human osteoblastic cell line by tuning the nanoporosity of titania and silica coatings. Biomater. Sci. 2013, 1, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Balasundaram, G.; Sato, M.; Webster, T.J. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials 2006, 27, 2798–2805. [Google Scholar] [CrossRef] [PubMed]
- Dieudonne, S.C.; Van Den Dolder, J.; De Ruijter, J.E.; Paldan, H.; Peltola, T.; Van’t Hof, M.A.; Happonen, R.P.; Jansen, J.A. Osteoblast differentiation of bone marrow stromal cells cultured on silica gel and sol–gel-derived titania. Biomaterials 2002, 23, 3041–3051. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-printed magnetic Fe 3 O 4/MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.J.; Patrick, P.S.; Page, K.; Powell, M.J.; Lythgoe, M.F.; Miodownik, M.A.; Parkin, I.P.; Carmalt, C.J.; Kalber, T.L.; Bear, J.C. Chemically treated 3D printed polymer scaffolds for biomineral formation. ACS Omega 2018, 3, 4342–4351. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F.; et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef]
- Kosik-Kozioł, A.; Graham, E.; Jaroszewicz, J.; Chlanda, A.; Kumar, P.S.; Ivanovski, S.; Swieszkowski, W.; Vaquette, C. Surface modification of 3D printed polycaprolactone constructs via a solvent treatment: Impact on physical and osteogenic properties. ACS Biomater. Sci. Eng. 2018, 5, 318–328. [Google Scholar] [CrossRef]
- Zhang, Y. Post-printing surface modification and functionalization of 3D-printed biomedical device. Int. J. Bioprint. 2017, 3, 93–99. [Google Scholar] [CrossRef]
- An, J.; Chua, C.K.; Mironov, V. A perspective on 4D bioprinting. Int. J. Bioprint. 2016, 2, 3–5. [Google Scholar] [CrossRef]
- Tibbits, S. 4D printing: Multi-material shape change. Archit. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Grimes, A.; Breslauer, D.N.; Long, M.; Pegan, J.; Lee, L.P.; Khine, M. Shrinky-Dink microfluidics: Rapid generation of deep and rounded patterns. Lab Chip 2008, 8, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Roohani-Esfahani, S.I.; Wang, G.; Zreiqat, H. Bone biomimetic microenvironment induces osteogenic differentiation of adipose tissue-derived mesenchymal stem cells. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.V.; Martins, A.; Leonor, I.B.; Pinho, E.D.; Reis, R.L.; Neves, N.M. Surface controlled biomimetic coating of polycaprolactone nanofiber meshes to be used as bone extracellular matrix analogues. J. Biomater. Sci. Polym. Ed. 2008, 19, 1261–1278. [Google Scholar] [CrossRef] [PubMed]
- Mavis, B.; Demirtaş, T.T.; Gümüşderelioğlu, M.; Gündüz, G.; Çolak, Ü. Synthesis, characterization and osteoblastic activity of polycaprolactone nanofibers coated with biomimetic calcium phosphate. Acta Biomater. 2009, 5, 3098–3111. [Google Scholar] [CrossRef]
- Polini, A.; Pisignano, D.; Parodi, M.; Quarto, R.; Scaglione, S. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Den Braber, E.D.; De Ruijter, J.E.; Jansen, J.A. The effect of a subcutaneous silicone rubber implant with shallow surface microgrooves on the surrounding tissues in rabbits. J. Biomed. Mater. Res. 1997, 37, 539–547. [Google Scholar] [CrossRef][Green Version]
- Taguchi, T.; Muraoka, Y.; Matsuyama, H.; Kishida, A.; Akashi, M. Apatite coating on hydrophilic polymer-grafted poly (ethylene) films using an alternate soaking process. Biomaterials 2000, 22, 53–58. [Google Scholar] [CrossRef]
- Jovanović, M.T.; Tadić, S.; Zec, S.; Mišković, Z.; Bobić, I. The effect of annealing temperatures and cooling rates on microstructure and mechanical properties of investment cast Ti–6Al–4V alloy. Mater. Des. 2006, 27, 192–199. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Monje, A.; Jiao, Y.; Hao, J.; Kruger, L.; Flanagan, C.L.; Hollister, S.J.; Giannobile, W.V. Integration of 3D printed and micropatterned polycaprolactone scaffolds for guidance of oriented collagenous tissue formation in vivo. Adv. Healthc. Mater. 2016, 5, 676–687. [Google Scholar] [CrossRef]
- Ran, Q.; Yang, W.; Hu, Y.; Shen, X.; Yu, Y.; Xiang, Y.; Cai, K. Osteogenesis of 3D printed porous Ti6Al4V implants with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Ma, B.; Zhu, H.; Huan, Z.; Ma, N.; Wu, C.; Chang, J. 3D-printed bioactive Ca3SiO5 bone cement scaffolds with nano surface structure for bone regeneration. ACS Appl. Mater. Interfaces 2017, 9, 5757–5767. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef] [PubMed]
- Castilho, M.; Moseke, C.; Ewald, A.; Gbureck, U.; Groll, J.; Pires, I.; Teßmar, J.; Vorndran, E. Direct 3D powder printing of biphasic calcium phosphate scaffolds for substitution of complex bone defects. Biofabrication 2014, 6, 015006. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the hydrophilicity and cell attachment of 3D printed PCL/graphene scaffolds for bone tissue engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Chatterjee, K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016, 46, 256–265. [Google Scholar] [CrossRef]
- Visscher, L.E.; Dang, H.P.; Knackstedt, M.A.; Hutmacher, D.W.; Tran, P.A. 3D printed Polycaprolactone scaffolds with dual macro-microporosity for applications in local delivery of antibiotics. Mater. Sci. Eng. C 2018, 87, 78–89. [Google Scholar] [CrossRef]
- Xiu, P.; Jia, Z.; Lv, J.; Yin, C.; Cheng, Y.; Zhang, K.; Song, C.; Leng, H.; Zheng, Y.; Cai, H.; et al. Tailored surface treatment of 3D printed porous Ti6Al4V by microarc oxidation for enhanced osseointegration via optimized bone in-growth patterns and interlocked bone/implant interface. ACS Appl. Mater. Interfaces 2016, 8, 17964–17975. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef]
- Pierantozzi, D.; Scalzone, A.; Jindal, S.; Stīpniece, L.; Šalma-Ancāne, K.; Dalgarno, K.; Gentile, P.; Mancuso, E. 3D printed Sr-containing composite scaffolds: Effect of structural design and material formulation towards new strategies for bone tissue engineering. Compos. Sci. Technol. 2020, 191, 108069. [Google Scholar] [CrossRef]
- Golzar, H.; Mohammadrezaei, D.; Yadegari, A.; Rasoulianboroujeni, M.; Hashemi, M.; Omidi, M.; Yazdian, F.; Shalbaf, M.; Tayebi, L. Incorporation of functionalized reduced graphene oxide/magnesium nanohybrid to enhance the osteoinductivity capability of 3D printed calcium phosphate-based scaffolds. Compos. Part B Eng. 2020, 185, 107749. [Google Scholar] [CrossRef]
- Alksne, M.; Kalvaityte, M.; Simoliunas, E.; Rinkunaite, I.; Gendviliene, I.; Locs, J.; Rutkunas, V.; Bukelskiene, V. In vitro comparison of 3D printed polylactic acid/hydroxyapatite and polylactic acid/bioglass composite scaffolds: Insights into materials for bone regeneration. J. Mech. Behav. Biomed. Mater. 2020, 104, 103641. [Google Scholar] [CrossRef] [PubMed]
- Piticescu, R.M.; Cursaru, L.M.; Negroiu, G.; Ciobota, C.F.; Neagoe, C.; Safranchik, D. Innovative Hybrid Materials with Improved Tensile Strength Obtained by 3D Printing. In Biomaterials; IntechOpen: London, UK, 2020. [Google Scholar]
- Bulygina, I.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Kaloshkin, S.; Scholz, R.; Knyazeva, M.; Walther, F.; Anisimova, N.; Kiselevskiy, M. Biomimetic scaffold fabricated with a mammalian trabecular bone template. Polym. Degrad. Stab. 2020, 172, 109076. [Google Scholar] [CrossRef]
- Yi, T.; Zhou, C.; Ma, L.; Wu, L.; Xu, X.; Gu, L.; Fan, Y.; Xian, G.; Fan, H.; Zhang, X. Direct 3D printing of Ti-6Al-4V/HA composite porous scaffolds for customized mechanical properties and biological functions. J. Tissue Eng. Regen. Med. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- Nájera, S.E.; Michel, M.; Kim, N.S. 3D Printed PLA/PCL/TiO 2 Composite for Bone Replacement and Grafting. MRS Adv. 2018, 3, 2373–2378. [Google Scholar] [CrossRef]
- Correa, V.L.; Garza, K.M.; Murr, L.E. Vascularization in interconnected 3D printed Ti-6Al-4V foams with hydrogel matrix for biomedical bone replacement implants. Sci. China Mater. 2018, 61, 565–578. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef]
- Abbott, R.D.; Kaplan, D.L. Engineering biomaterials for enhanced tissue regeneration. Curr. Stem Cell Rep. 2016, 2, 140–146. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Zhou, Z.; Lai, H.; Xu, P.; Liao, L.; Wei, J. Biodegradable polymer membranes applied in guided bone/tissue regeneration: A review. Polymers 2016, 8, 115. [Google Scholar] [CrossRef]
- Ooi, H.W.; Hafeez, S.; Van Blitterswijk, C.A.; Moroni, L.; Baker, M.B. Hydrogels that listen to cells: A review of cell-responsive strategies in biomaterial design for tissue regeneration. Mater. Horiz. 2017, 4, 1020–1040. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, L.; Zhou, C.; Zhou, Y.; Bai, Y.; Lee, F.Y.; Mao, J.J. 3D printing for regenerative medicine: From bench to bedside. Mrs Bull. 2015, 40, 145–154. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Balla, V.K.; Bodhak, S.; Datta, P.; Kundu, B.; Das, M.; Bandyopadhyay, A.; Bose, S. Biointegration of three-dimensional–printed biomaterials and biomedical devices. In Biointegration of Medical Implant Materials; Woodhead Publishing: Cambridge, UK, 2020; pp. 433–482. [Google Scholar]
- Eingartner, C. Current trends in total Hip arthroplasty. Ortop. Traumatol. Rehabil. 2007, 9, 8–14. [Google Scholar] [PubMed]
- Korde, J.M.; Shaikh, M.; Kandasubramanian, B. Bionic Prototyping of Honeycomb Patterned Polymer Composite and Its Engineering Application. Polym. Plast. Technol. Eng. 2018, 57, 1828–1844. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Q.; Wang, M. Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication 2017, 9, 025031. [Google Scholar] [CrossRef]
- Tserovski, S.; Georgieva, S.; Simeonov, R.; Bigdeli, A.; Röttinger, H.; Kinov, P. Advantages and disadvantages of 3D printing for pre-operative planning of revision Hip surgery. J. Surg. Case Rep. 2019, 2019, rjz214. [Google Scholar] [CrossRef]
- Quality Assurance for Metal 3D Printing: Solving 3 Common Challenges. Autonomous Manufacturing. Available online: https://amfg.ai/2019/03/15/quality-assurance-for-metal-3d-printing-solving-3-common-challenges/ (accessed on 11 November 2020).
- Davies, E. How to Carry out Quality Control in Additive Manufacturing. The Institute of Materials, Minerals and Mining. Available online: https://www.iom3.org/materials-world-magazine/feature/2018/jul/01/how-carry-out-quality-control-additive-manufacturing (accessed on 22 June 2020).
- Gilbert, F.; O’Connell, C.D.; Mladenovska, T.; Dodds, S. Print me an organ? Ethical and regulatory issues emerging from 3D bioprinting in medicine. Sci. Eng. Ethics 2018, 24, 73–91. [Google Scholar] [CrossRef]
- Howard, J.J. Balancing innovation and medical device regulation: The case of modern metal-on-metal Hip replacements. Med. Devices 2016, 9, 267. [Google Scholar] [CrossRef] [PubMed]
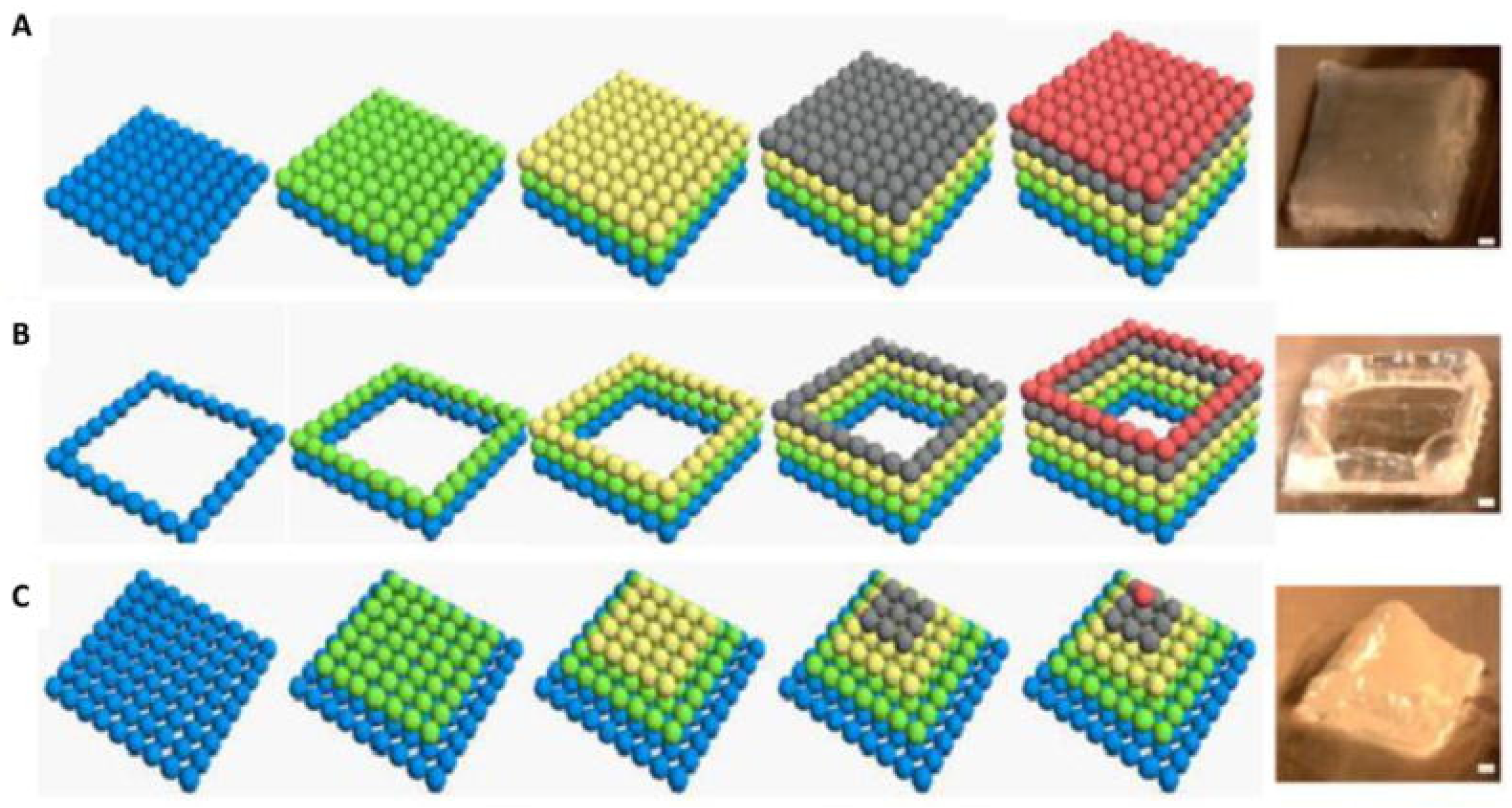
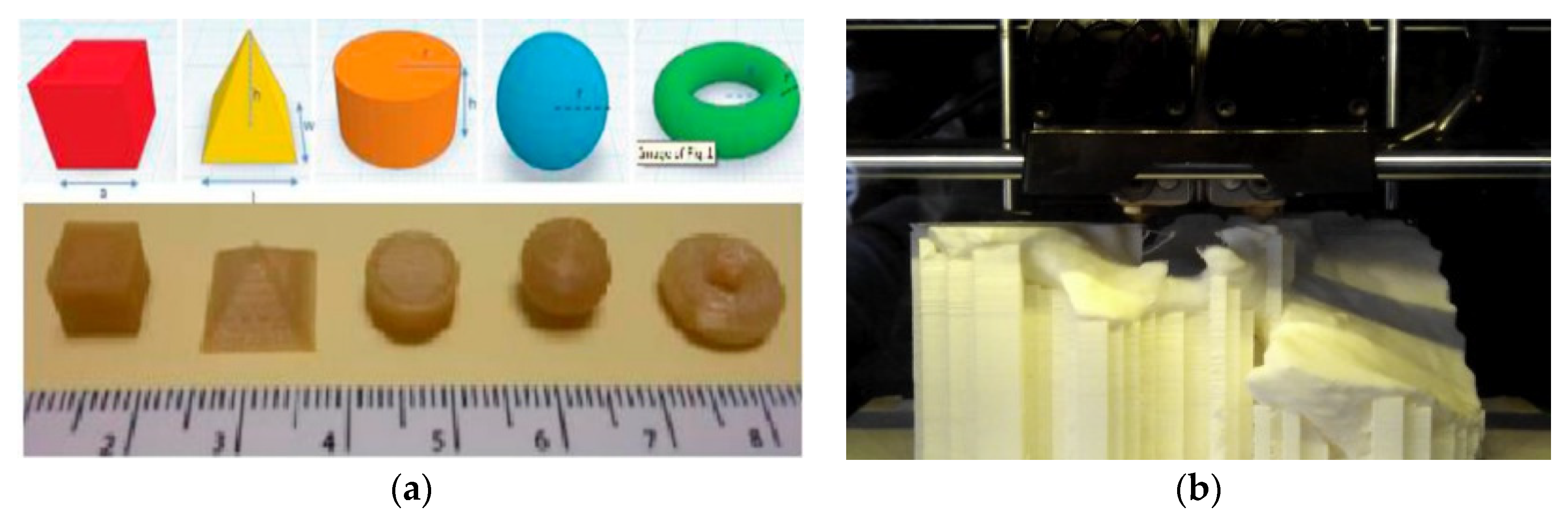
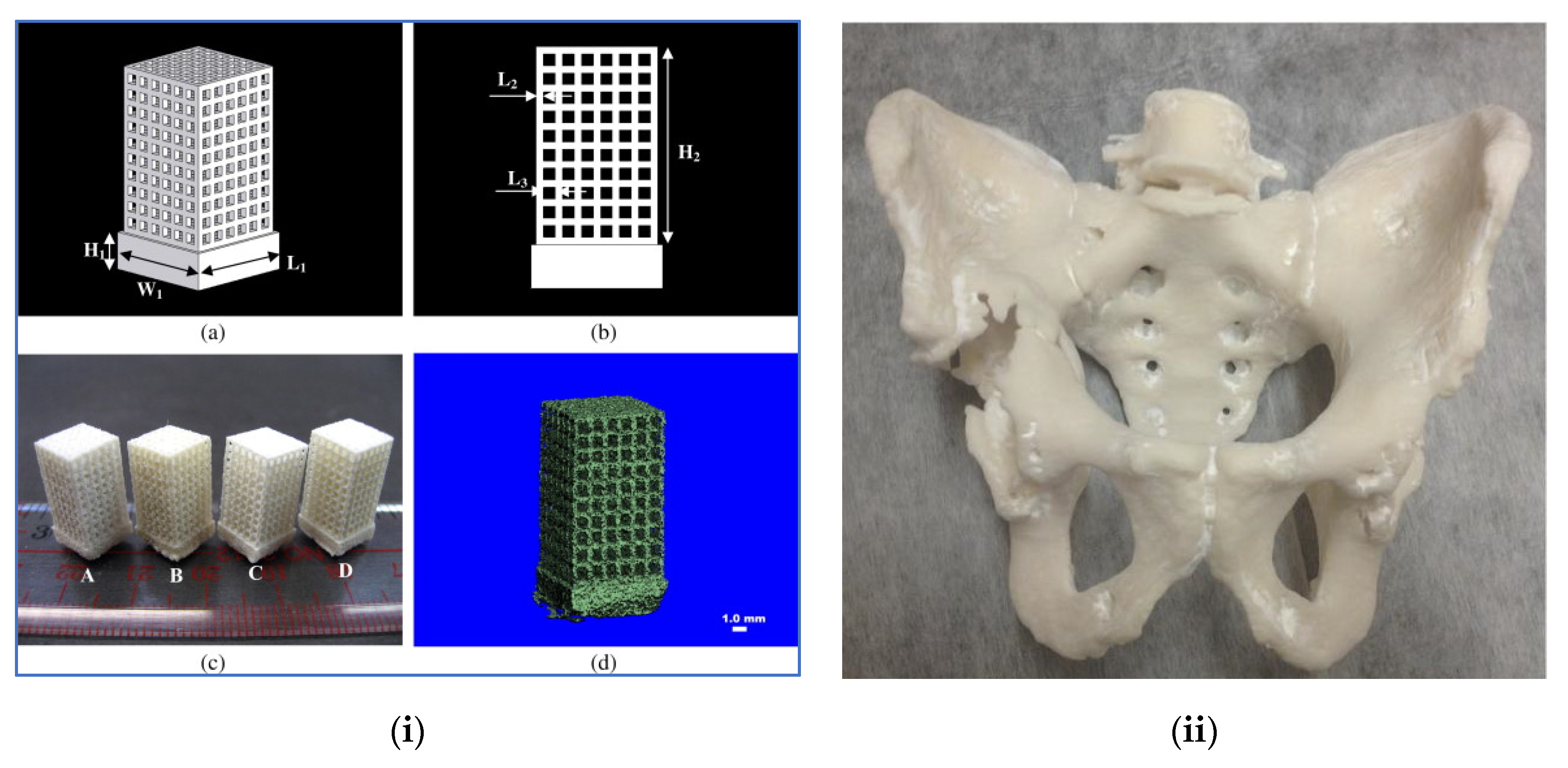
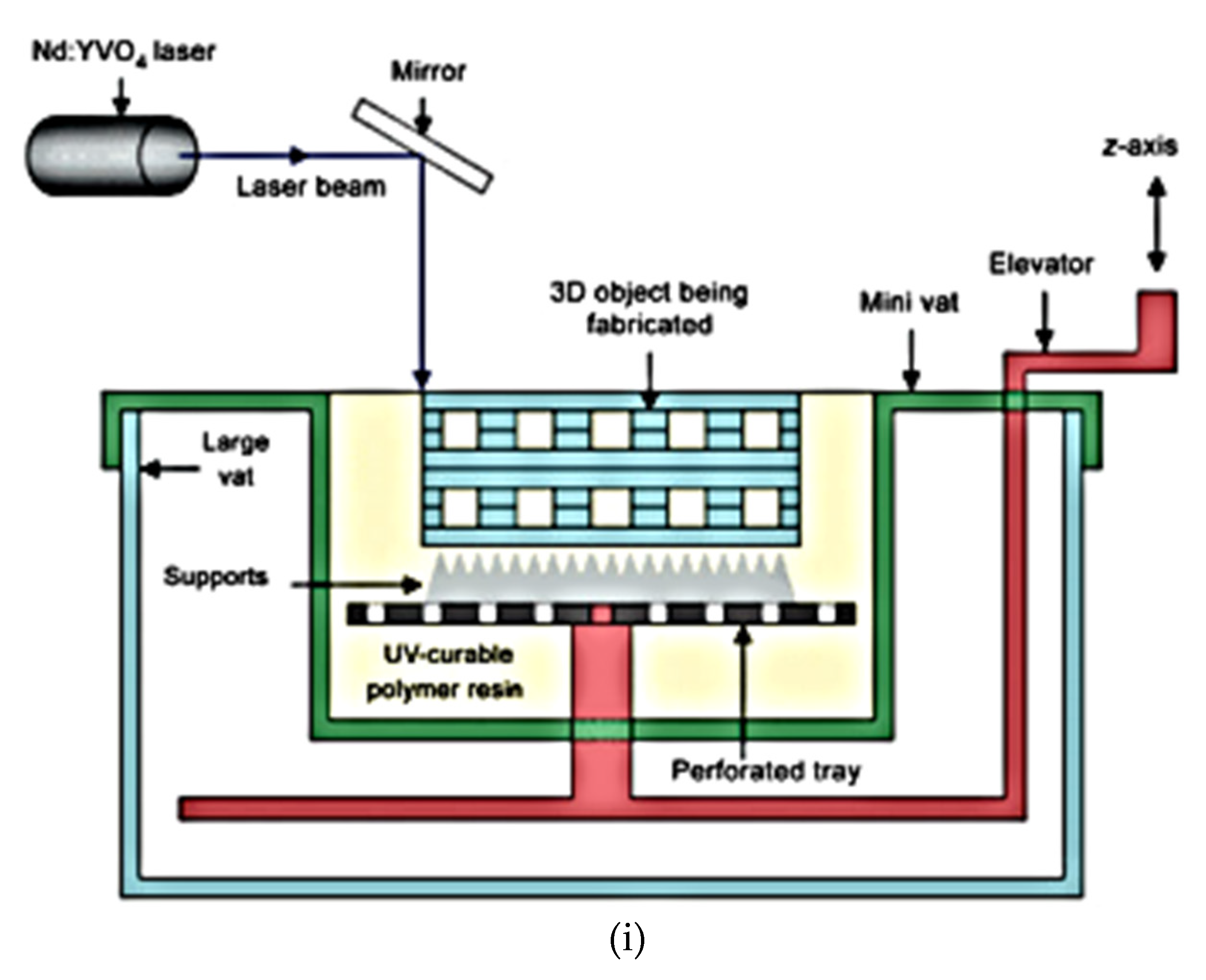
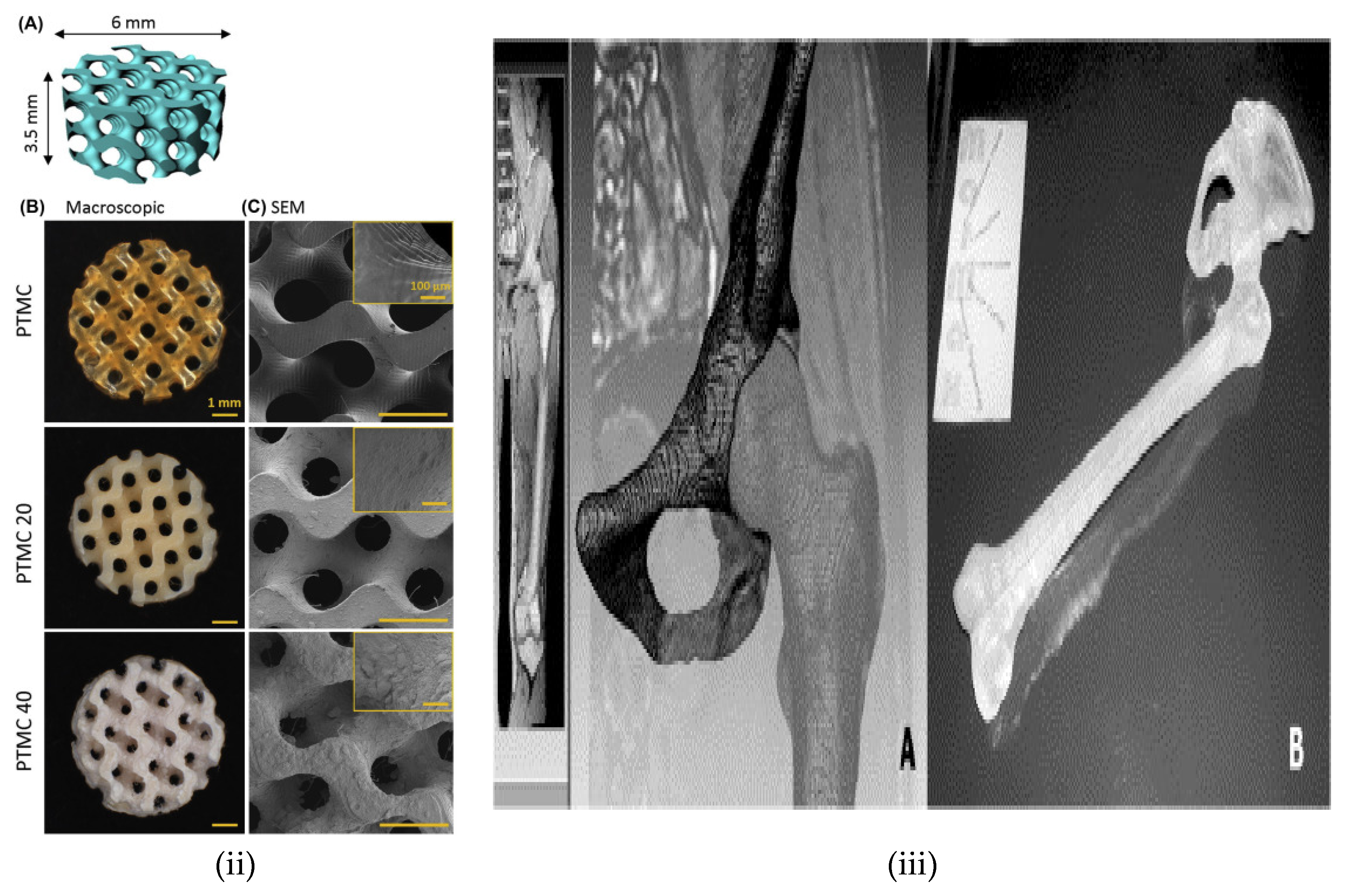
| 3D Printing Technique | Composition | Application | Advantages | Disadvantage | References |
|---|---|---|---|---|---|
| Direct 3D printing | Alginate/CaCl2; HA; Collagen/CaP; Ti alloys |
|
|
| [1,30,33,35,74] |
| Bioplotting | PCL; Nanocellulose-Alginate; Glass-ceramic; Nano CaP/ (PLLA); PLGA, TCP/COL |
|
|
| [1,74,75,76,77,78,79] |
| FDM | PLA copolymer, PLC copolymer, bioactive glass; PLGA; PU; PCL. HA/PCL, TCP/ PCL; PLGA and PCL; PEEK and ABS; PCL; PCU/UHMWPE |
|
|
| [1,40,43,45,80,81,82,83,84] |
| SLS | NanoHA/PCL; PCL/TCP and β-TCP; PCL; PA; PLA; PEK; PVA/HA; PC; Ti alloys; cobalt-chromium; stainless steel; Ni-Ti alloy |
|
|
| [1,43,50,52,53,54,55,56,57,58,74,85] |
| SLA | PPF; PEG; PEGDA; GelMa hydrogel; PCL resin; PCL |
|
|
| [1,43,67,69,72,73,86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okolie, O.; Stachurek, I.; Kandasubramanian, B.; Njuguna, J. 3D Printing for Hip Implant Applications: A Review. Polymers 2020, 12, 2682. https://doi.org/10.3390/polym12112682
Okolie O, Stachurek I, Kandasubramanian B, Njuguna J. 3D Printing for Hip Implant Applications: A Review. Polymers. 2020; 12(11):2682. https://doi.org/10.3390/polym12112682
Chicago/Turabian StyleOkolie, Obinna, Iwona Stachurek, Balasubramanian Kandasubramanian, and James Njuguna. 2020. "3D Printing for Hip Implant Applications: A Review" Polymers 12, no. 11: 2682. https://doi.org/10.3390/polym12112682
APA StyleOkolie, O., Stachurek, I., Kandasubramanian, B., & Njuguna, J. (2020). 3D Printing for Hip Implant Applications: A Review. Polymers, 12(11), 2682. https://doi.org/10.3390/polym12112682