Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering
Abstract
1. Introduction
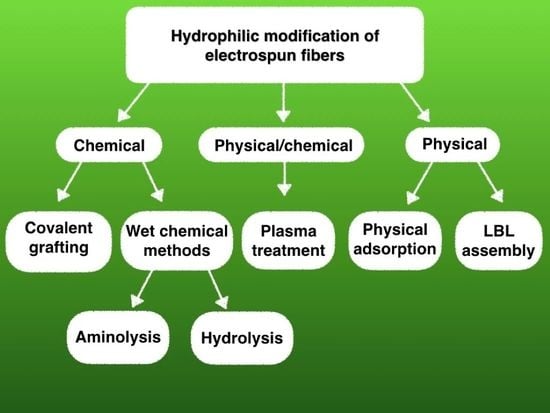
2. Surface Functionalization Methods
2.1. Chemical Methods
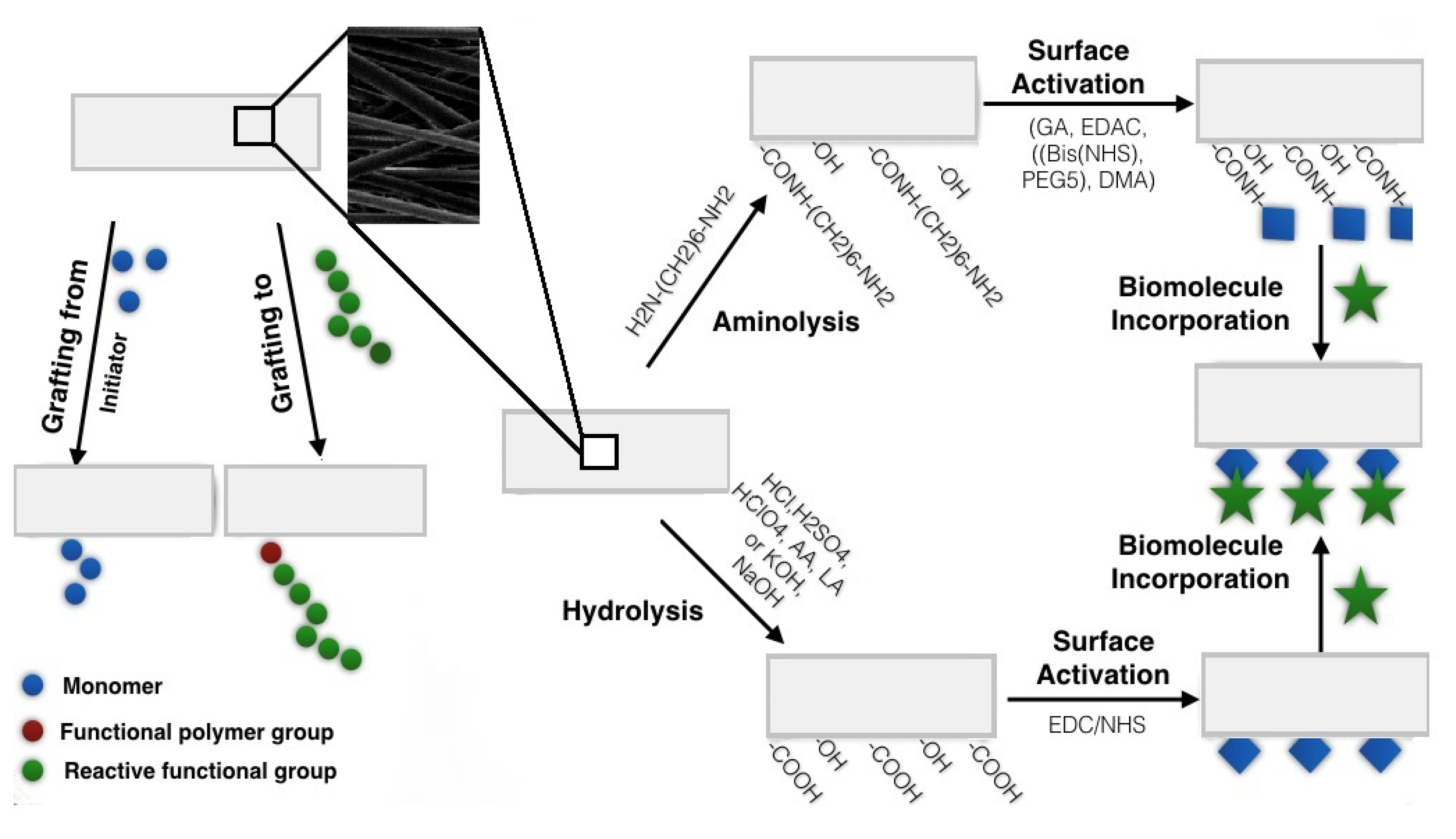
2.1.1. Wet Chemical Functionalization
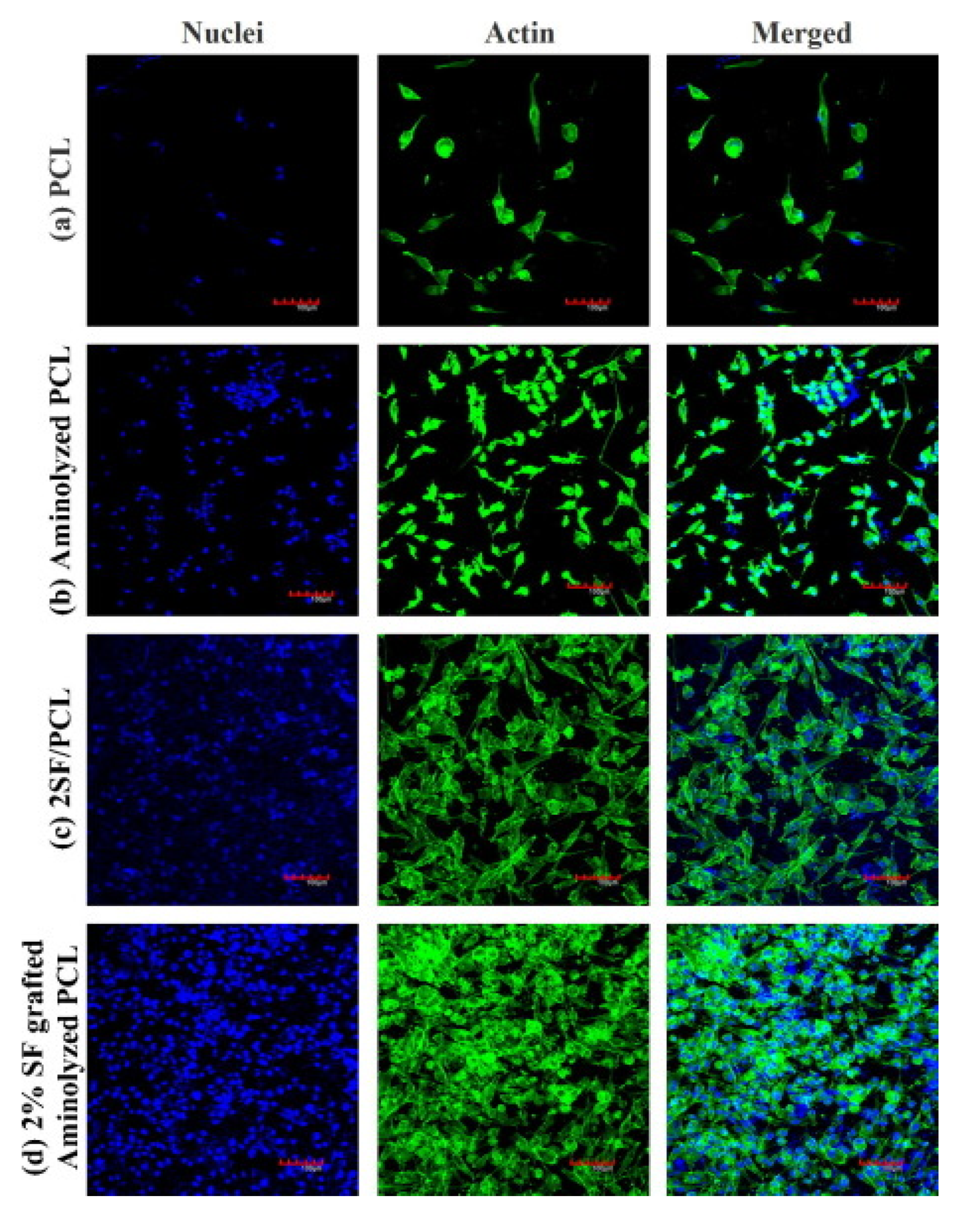
Aminolysis
Hydrolysis
2.1.2. Covalent Grafting
| Chemical Method | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Aminolysis | Splitting of polymer chains by reacting with-NH2 groups and resulting introduction of active -NH2 and -OH on the surface, which may further be explored in secondary reactions to incorporate other functional groups [6,31] |
| |
| Hydrolysis | Cleavage of chemical bonds in polymeric chains by water molecules resulting in OH and COOH formation on the modified surface |
|
|
| Covalent grafting | Chemical functionalization of the polymer backbone to introduce reactive functional groups on the surface [47,48] |
|
|
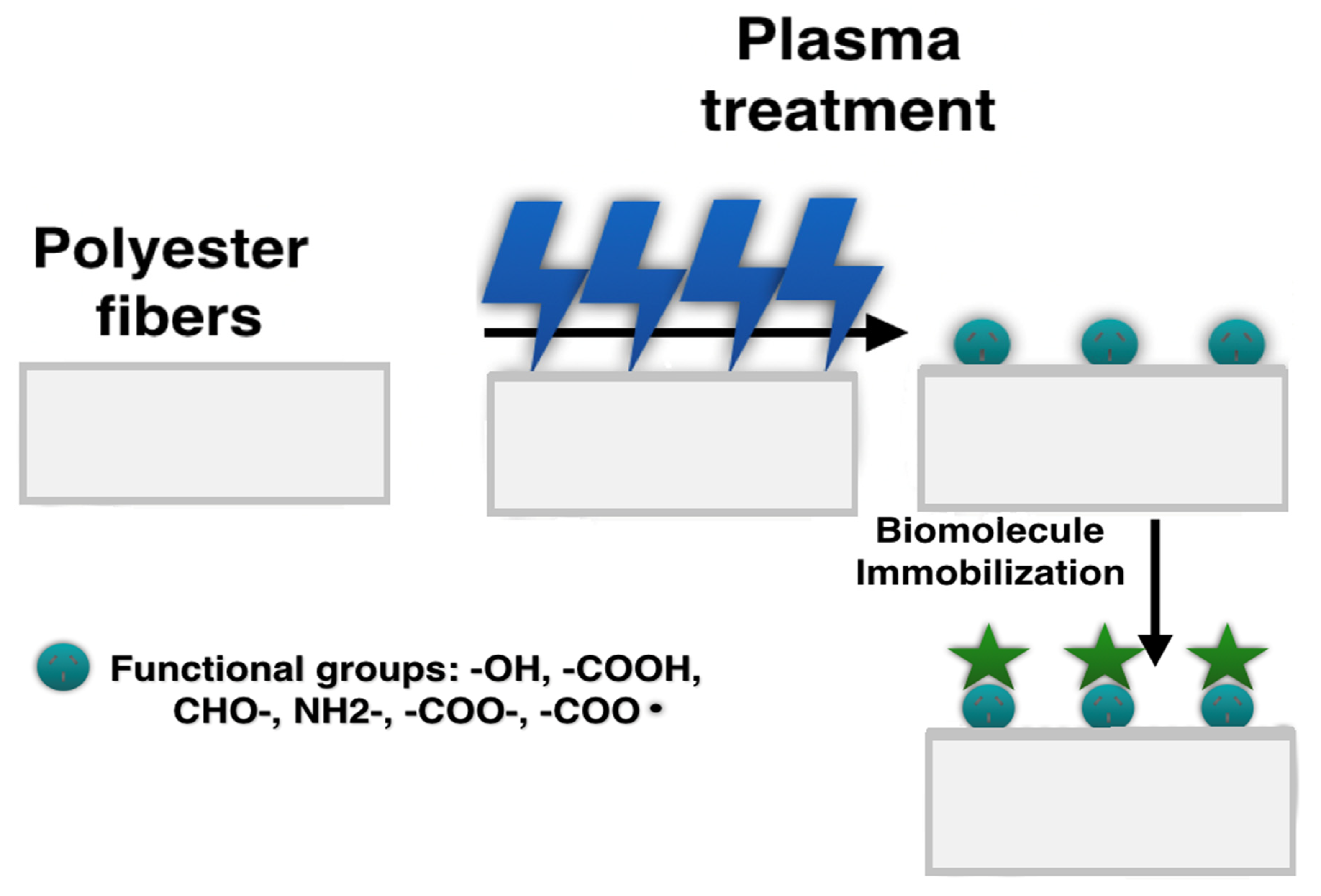
2.2. Physically/Chemically Functionalized Fibers
Plasma Treatment
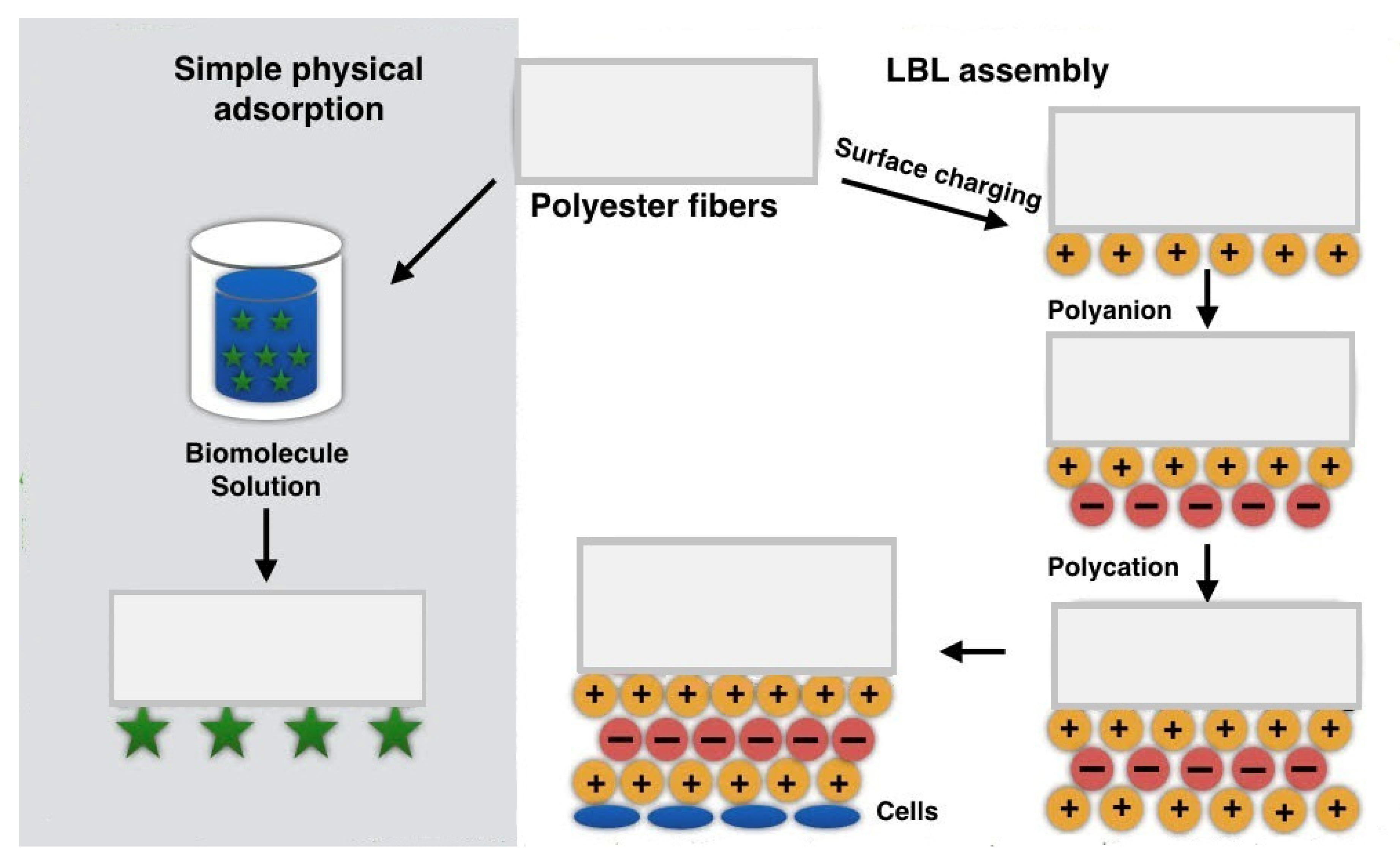
2.3. Physically Functionalized Fibers by Physical Adsorption
2.3.1. Simple Physical Adsorption
2.3.2. LBL Assembly
| Physical Method | Mechanism | Advantages | Disadvantages |
|---|---|---|---|
| Simple physical adsorption | Weak physical interactions such as hydrophobic interactions, hydrogen bonds, van der Waals interactions [24,26] |
| |
| LBL | Electrostatic interactions as an effect of alternate embedding of oppositely charged substances [26] |
|
3. Tissue Engineering Applications of Functionalized Polymer Nanofibers
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Carvalho, J.; Carvalho, P.; Gomes, D.; Goes, A. Chapter 11—Innovative Strategies for Tissue Engineering. In Advances in Biomaterials Science and Biomedical Application; Pignatello, R., Ed.; IntechOpen: London, UK, 2013; pp. 295–313. [Google Scholar]
- Niemczyk, B.; Sajkiewicz, P.; Kołbuk, D. Injectable hydrogels as novel materials for central nervous system regeneration. J. Neural Eng. 2018, 15, 051002. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, H.; Wang, Y.; Li, G.; Zheng, Z.; Kaplan, D.L.; Wang, X. Flexible Water-Absorbing Silk-Fibroin Biomaterial Sponges with Unique Pore Structure for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 1641–1649. [Google Scholar] [CrossRef]
- Kołbuk, D.; Heljak, M.; Choińska, E.; Urbanek, O. Novel 3D hybrid nanofiber scaffolds for bone regeneration. Polymers 2020, 12, 544. [Google Scholar] [CrossRef] [PubMed]
- Kołbuk, D.; Urbanek, O.; Denis, P.; Choińska, E. Sonochemical coating as an effective method of polymeric nonwovens functionalization. J. Biomed. Mater. Res. A 2019, 107, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Jeznach, O.; Kołbuk, D.; Sajkiewicz, P. Aminolysis of various aliphatic polyesters in a form of nanofibers and films. Polymers 2019, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Arbade, G.K.; Srivastava, J.; Tripathi, V.; Lenka, N.; Patro, T.U. Enhancement of hydrophilicity, biocompatibility and biodegradability of poly(ε-caprolactone) electrospun nanofiber scaffolds using poly(ethylene glycol) and poly(L-lactide-co-ε-caprolactone-co-glycolide) as additives for soft tissue engineering. J. Biomater. Sci. 2020, 1–17, just-accepted. [Google Scholar]
- Wang, D.; Xu, Y.; Li, Q.; Turng, L.-S. Artificial Small-Diameter Blood Vessels: Materials, Fabrication, Surface Modification, Mechanical Properties, and Bioactive Functionalities. J. Mater. Chem. B. 2020, 8, 1801–1822. [Google Scholar] [CrossRef]
- Cegielska, O.; Sajkiewicz, P. Targeted drug delivery systems for the treatment of glaucoma: Most advanced systems review. Polymers 2019, 11, 1742. [Google Scholar] [CrossRef]
- Zaszczynska, A.; Sajkiewicz, P.; Gradys, A. Piezoelectric Scaffolds as Smart Materials for Neural Tissue Engineering. Polymers 2020, 12, 161. [Google Scholar] [CrossRef]
- Denis, P.; Wrzecionek, M.; Gadomska-Gajadhur, A.; Sajkiewicz, P. Poly(glycerol sebacate)–poly(l-lactide) nonwovens. Towards attractive electrospun material for tissue engineering. Polymers 2019, 11, 2113. [Google Scholar] [CrossRef]
- Guo, X.; Xia, B.; Lu, X.B.; Zhang, Z.J.; Li, Z.; Li, W.L.; Huang, Y.C. Grafting of mesenchymal stem cell-seeded small intestinal submucosa to repair the deep partial-thickness burns. Connect. Tissue Res. 2016, 57, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Dulnik, J.; Denis, P.; Sajkiewicz, P.; Kołbuk, D.; Choińska, E. Biodegradation of bicomponent PCL/gelatin and PCL/collagen nanofibers electrospun from alternative solvent system. Polym. Degrad. Stab. 2016, 130, 10–21. [Google Scholar] [CrossRef]
- Wei, Z.; Gu, J.; Ye, Y.; Fang, M.; Lang, J.; Yang, D.; Pan, Z. Biodegradable poly (butylene succinate) nanofibrous membrane treated with oxygen plasma for superhydrophilicity. Surf. Coat. Technol. 2020, 381, 125147. [Google Scholar] [CrossRef]
- Webb, K.; Hlady, V.; Tresco, P.A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and cytoskeletal organization. J. Biomed. Mater. Res. 1998, 41, 422–430. [Google Scholar] [CrossRef]
- Ikada, Y. Surface modification of polymers for medical applications. Biomaterials 1994, 15, 725–736. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–771. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef]
- Asadian, M.; Dhaenens, M.; Onyshchenko, I.; De Waele, S.; Declercq, H.; Cools, P.; De Geyter, N. Plasma functionalization of PCL nanofibers changes protein interactions with cells resulting in increased cell viability. ACS Appl. Mater. Interfaces 2018, 10, 41962–41977. [Google Scholar] [CrossRef]
- Dulnik, J.; Kołbuk, D.; Denis, P.; Sajkiewicz, P. The effect of a solvent on cellular response to PCL/gelatin and PCL/collagen electrospun nanofibres. Eur. Polym. J. 2018, 104, 147–156. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, C.; Liu, X.; Shen, J. Surface modification of polycaprolactone membrane via aminolysis and biomacromolecule immobilization for promoting cytocompatibility of human endothelial cells. Biomacromolecules 2002, 3, 1312–1319. [Google Scholar] [CrossRef]
- You, X.; Piao, C.; Chen, L. Preparation of a magnetic molecularly imprinted polymer by atom-transfer radical polymerization for the extraction of parabens from fruit juices. J. Sep. Sci. 2016, 39, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.; Pinto, S.; de Sousa, H.C.; Gil, M.H. Surface modification of a thermoplastic polyurethane by low-pressure plasma treatment to improve hydrophilicity. J. Appl. Polym. Sci. 2011, 122, 2302–2308. [Google Scholar] [CrossRef]
- Yang, J.; Bei, J.; Wang, S. Enhanced cell affinity of poly (d,l-lactide) by combining plasma treatment with collagen anchorage. Biomaterials 2002, 23, 2607–2614. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Li, D.; Qin, J.; Zhang, M.; Wang, K.; Zhao, J.; Zhang, L. LBL deposition of chitosan/heparin bilayers for improving biological ability and reducing infection of nanofibers. Int. J. Biol. Macromol. 2020, 154, 999–1006. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Holmes, C.; Tabrizian, M. Surface functionalization of biomaterials. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2015; pp. 187–206. [Google Scholar]
- Huang, L.; Arena, J.T.; McCutcheon, J.R. Surface modified PVDF nanofiber supported thin film composite membranes for forward osmosis. J. Membr. Sci. 2016, 499, 352–360. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Waghmare, P.R. Eco-friendly preparation of superhydrophobic copper surfaces for oil/water separation. Environ. Chem. Lett. 2020, 18, 505–510. [Google Scholar] [CrossRef]
- Haddad, T.; Noel, S.; Liberelle, B.; El Ayoubi, R.; Ajji, A.; De Crescenzo, G. Fabrication and surface modification of poly lactic acid (PLA) scaffolds with epidermal growth factor for neural tissue engineering. Biomatter 2016, 6, e1231276. [Google Scholar] [CrossRef]
- Croll, T.I.; O’Connor, A.J.; Stevens, G.W.; Cooper-White, J.J. Controllable Surface Modification of Poly(lactic-co-glycolic acid) (PLGA) by Hydrolysis or Aminolysis I: Physical, Chemical, and Theoretical Aspects. Biomacromolecules 2004, 5, 463–473. [Google Scholar] [CrossRef]
- Morillo, M.D.; Magdi, A.M.; Rodríguez, M.; García, M.; Faccini, M. Aminated Polyethylene Terephthalate (PET) Nanofibers for the Selective Removal of Pb(II) from Polluted Water. Materials 2017, 10, 1352. [Google Scholar] [CrossRef]
- Zhu, Y.; Leong, M.; Ong, W.; Chanpark, M.; Chian, K. Esophageal epithelium regeneration on fibronectin grafted poly(l-lactide-co-caprolactone) (PLLC) nanofiber scaffold. Biomaterials 2007, 28, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Tan, K.; Zhou, Y.; Ye, Z.; Tan, W.-S. A combinatorial variation in surface chemistry and pore size of three-dimensional porous poly(ε-caprolactone) scaffolds modulates the behaviors of mesenchymal stem cells. Mater. Sci. Eng. C 2016, 59, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Chastain, S.R.; Kundu, A.K.; Dhar, S.; Calvert, J.W.; Putnam, A.J. Adhesion of mesenchymal stem cells to polymer scaffolds occurs via distinct ECM ligands and controls their osteogenic differentiation. J. Biomed. Mater. Res. A 2006, 78, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, C.; Liu, X.; He, T.; Shen, J. Immobilization of biomacromolecules onto aminolyzed poly (L-lactic acid) toward acceleration of endothelium regeneration. Tissue Eng. 2004, 10, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zheng, H.; Liang, S.; Gao, C. Aligned PLLA nanofibrous scaffolds coated with graphene oxide for promoting neural cell growth. Acta Biomater. 2016, 37, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mao, Z.; Gao, C. Aminolysis-based surface modification of polyesters for biomedical applications. RSC Adv. 2013, 3, 2509–2519. [Google Scholar] [CrossRef]
- Boland, E.D.; Telemeco, T.A.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly (glycolic acid) for tissue engineering. J. Biomed. Mater. Res. B Biomater. 2004, 71, 144–152. [Google Scholar] [CrossRef]
- Lee, S.J.; Khang, G.; Lee, Y.M.; Lee, H.B. Interaction of human chondrocytes and NIH/3T3 fibroblasts on chloric acid-treated biodegradable polymer surfaces. J. Biomater. Sci. 2002, 13, 197–212. [Google Scholar] [CrossRef]
- Kongdee, A.; Okubayashi, S.; Tabata, I.; Hori, T. Impregnation of silk sericin into polyester fibers using supercritical carbon dioxide. J. Appl. Polym. Sci. 2007, 105, 2091–2097. [Google Scholar] [CrossRef]
- Spinella, S.; Re, G.L.; Liu, B.; Dorgan, J.; Habibi, Y.; Leclere, P.; Raquez, J.M.; Dubois, P.; Gross, R.A. Polylactide/cellulose nanocrystal nanocomposites: Efficient routes for nanofiber modification and effects of nanofiber chemistry on PLA reinforcement. Polymer 2015, 65, 9–17. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Cai, J.Y. Enhanced cell affinity of poly (L-lactic acid) modified by base hydrolysis: Wettability and surface roughness at nanometer scale. Curr. Appl. Phy. 2007, 7, e108–e111. [Google Scholar] [CrossRef]
- Getnet, M.; Chavan, R. Catalyzation of alkaline hydrolysis of polyester by oxidizing agents for surface modification. Int. J. Sci. Basic Appl. Res. 2015, 22, 232. [Google Scholar]
- Sadeghi, A.R.; Nokhasteh, S.; Molavi, A.M.; Khorsand-Ghayeni, M.; Naderi-Meshkin, H.; Mahdizadeh, A. Surface modification of electrospun PLGA scaffold with collagen for bioengineered skin substitutes. Mater. Sci. Eng. 2016, 66, 130–137. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.C.; Terenghi, G.; Downes, S. Chemical surface modification of poly-ε-caprolactone improves Schwann cell proliferation for peripheral nerve repair. J. Tissue Eng. Regen. M 2012, 8, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.D.; Lei, J.Y.; Yao, C.; Li, X.S.; Yao, F.; Nie, S.Z.; Kang, T.; Neoh, K.G. Core−Sheath Nanofibers from Combined Atom Transfer Radical Polymerization and Electrospinning. Macromolecules 2008, 41, 6854–6858. [Google Scholar] [CrossRef]
- Fu, G.D.; Xu, L.Q.; Yao, F.; Zhang, K.; Wang, X.F.; Zhu, M.F.; Nie, S.Z. Smart Nanofibers from Combined Living Radical Polymerization, “Click Chemistry”, and Electrospinning. ACS Appl. Mater. Interfaces 2009, 1, 239–243. [Google Scholar] [CrossRef]
- Maffei, A.; Michieli, N.; Brun, P.; Zamuner, A.; Zaggia, A.; Roso, M.; Kalinic, B.; Falzacappa, E.; Scopece, P.; Gross, S.; et al. An atmospheric pressure plasma jet to tune the bioactive peptide coupling to polycaprolactone electrospun layers. Appl. Surf. Sci. 2020, 507, 144713. [Google Scholar] [CrossRef]
- Minko, S. Grafting on Solid Surfaces: “Grafting to” and “Grafting from” Methods. In Polymer Surfaces and Interfaces; Springer: Berlin/Heidelberg, Germany, 2008; pp. 215–234. [Google Scholar]
- Hesari, S.M.; Ghorbani, F.; Ghorbani, F.; Zamanian, A.; Khavandi, A. Plasma surface modification technique–induced gelatin grafting on bio-originated polyurethane porous matrix: Physicochemical and in vitro study. Polym. Compos. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Sun, Y.; Du, H.; Deng, Y.; Lan, Y.; Feng, C. Preparation of polyacrylamide via surface-initiated electrochemical-mediated atom transfer radical polymerization (SI-eATRP) for Pb2+ sensing. J. Solid State Electr. 2016, 20, 105–113. [Google Scholar] [CrossRef]
- Mushtaq, M.; Jindani, R.; Farooq, A.; Li, X.; Saba, H.; Wasim, M.; Siddiqui, Q. Characterization of electrospun polylactide nanofibers modified via atom transfer radical polymerization. J. Ind. Text. 2020, 1–11. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Macromolecular engineering by atom transfer radical polymerization. J. Am. Chem. Soc. 2014, 136, 6513–6533. [Google Scholar] [CrossRef]
- Ma, Z.; Kotaki, M.; Ramakrishna, S. Surface modified nonwoven polysulphone (PSU) fiber mesh by electrospinning: A novel affinity membrane. J. Membr. Sci. 2006, 272, 179–187. [Google Scholar] [CrossRef]
- Chen, J.P.; Chiang, Y.P. Surface modification of non-woven fabric by DC pulsed plasma treatment and graft polymerization with acrylic acid. J. Membr. Sci. 2006, 270, 212–220. [Google Scholar] [CrossRef]
- Ding, Z. Immobilization of chitosan onto poly-?-lactic acid film surface by plasma graft polymerization to control the morphology of fibroblast and liver cells. Biomaterials 2004, 25, 1059–1067. [Google Scholar] [CrossRef]
- Sadeghzade, N.; Nouri, M.; Nateri, A.S. Evaluating Osteogenic Differentiation of Mesenchymal Stem Cells on Poly (caprolactone) Electrospun Scaffolds by Image Processing Techniques. Bionanoscience 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Toledo, A.L.M.M.; Ramalho, B.S.; Picciani, P.H.S.; Baptista, L.S.; Martinez, A.M.B.; Dias, M.L. Effect of three different amines on the surface properties of electrospun polycaprolactone mats. Int. J. Polym. Mater. 2020, 1–13. [Google Scholar] [CrossRef]
- Wu, C.A. Comparison of the Structure, Thermal Properties, and Biodegradability of Polycaprolactone/Chitosan and Acrylic Acid Grafted Polycaprolactone/Chitosan. Polymer 2005, 46, 147–155. [Google Scholar] [CrossRef]
- Chen, J.-P.; Li, S.-F.; Chiang, Y.-P. Bioactive Collagen-Grafted Poly-L-Lactic Acid Nanofibrous Membrane for Cartilage Tissue Engineering. J. Nanosci. Nanotechnol. 2010, 10, 5393–5398. [Google Scholar] [CrossRef]
- Chen, J.P.; Su, C.H. Surface modification of electrospun PLLA nanofibers by plasma treatment and cationized gelatin immobilization for cartilage tissue engineering. Acta Biomater. 2011, 20117, 234–243. [Google Scholar] [CrossRef]
- Singh, M.; Vajpayee, M.; Ledwani, L. Eco-friendly Surface Modification and Nanofinishing of Textile Polymers to Enhance Functionalisation. In Nanotechnology for Energy and Environmental Engineering; Ledwani, L., Sangwai, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 529–559. [Google Scholar]
- Pashkuleva, I.; Marques, A.P.; Vaz, F.; Reis, R.L. Surface modification of starch based biomaterials by oxygen plasma or UV-irradiation. J. Mater. Sci. Mater. Med. 2010, 21, 21–32. [Google Scholar] [CrossRef]
- Dorai, R.; Kushner, M.J. A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D Appl. Phys. 2003, 36, 666. [Google Scholar] [CrossRef]
- Yang, F.; Wolke, J.G.C.; Jansen, J.A. Biomimetic calcium phosphate coating on electrospun poly(ɛ-caprolactone) scaffolds for bone tissue engineering. Chem. Eng. J. 2008, 137, 154–161. [Google Scholar] [CrossRef]
- Fasano, V.; Laurita, R.; Moffa, M.; Gualandi, C.; Colombo, V.; Gherardi, M.; Focarete, M.L. Enhanced electrospinning of active organic fibers by plasma treatment on conjugated polymer solutions. ACS Appl. Mater. Interfaces 2020, 12, 26320–26329. [Google Scholar] [CrossRef] [PubMed]
- Kupka, V.; Dvořáková, E.; Manakhov, A.; Michlíček, M.; Petruš, J.; Vojtová, L.; Zajíčková, L. Well-blended PCL/PEO electrospun nanofibers with functional properties enhanced by plasma processing. Polymers 2020, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Zamanian, A.; Aidun, A. Conductive electrospun polyurethane-polyaniline scaffolds coated with poly (vinyl alcohol)-GPTMS under oxygen plasma surface modification. Mater. Today Commun. 2020, 22, 100752. [Google Scholar] [CrossRef]
- Min, B.M.; Jeong, L.; Yeo, I.S.; Kim, H.N.; Yoon, Y.I.; Jang, D.H.; Park, W.H. Plasma-treated silk fibroin nanofibers for skin regeneration. Int. J. Biol. Macromol. 2009, 44, 222–228. [Google Scholar]
- Occhiello, E.; Morra, M.; Morini, G.; Garbassi, F.; Humphrey, P. Oxygen-plasma-treated polypropylene interfaces with air, water, and epoxy resins: Part I. Air and water. J. Appl. Polym. Sci. 1991, 42, 551–559. [Google Scholar] [CrossRef]
- Park, K.; Ju, Y.M.; Son, J.S.; Ahn, K.D.; Han, D.K. Surface modification of biodegradable electrospun nanofiber scaffolds and their interaction with fibroblasts. J. Biomater. Sci. 2007, 18, 369–382. [Google Scholar] [CrossRef]
- Nisticò, R.; Magnacca, G.; Faga, M.G.; Gautier, G.; D’Angelo, D.; Ciancio, E.; Martorana, S. Effect of atmospheric oxidative plasma treatments on polypropylenic fibers surface: Characterization and reaction mechanisms. Appl. Surf. Sci. 2013, 279, 285–292. [Google Scholar] [CrossRef]
- Kooshki, H.; Ghollasi, M.; Halabian, R.; Kazemi, N.M. Osteogenic differentiation of preconditioned bone marrow mesenchymal stem cells with lipopolysaccharide on modified poly-l -lactic-acid nanofibers. J. Cell. Physiol. 2018, 234, 5343–5353. [Google Scholar] [CrossRef]
- Cheng, Q.; Lee, B.L.-P.; Komvopoulos, K.; Yan, Z.; Li, S. Plasma surface chemical treatment of electrospun poly (l -lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration. Tissue Eng. Part. A 2013, 19, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, K.Y.; Lee, S.J.; Park, K.E.; Park, W.H. Plasma-treated poly (lactic-co-glycolic acid) nanofibers for tissue engineering. Macromol. Res. 2007, 15, 238–243. [Google Scholar] [CrossRef]
- Dolci, L.S.; Quiroga, S.D.; Gherardi, M.; Laurita, R.; Liguori, A.; Sanibondi, P.; Focarete, M.L. Carboxyl Surface Functionalization of Poly (l-lactic acid) Electrospun Nanofibers through Atmospheric Non-T hermal Plasma Affects Fibroblast Morphology. Plasma Process. Polym. 2014, 11, 203–213. [Google Scholar] [CrossRef]
- Morra, M.; Occhiello, E.; Garbassi, F. Contact angle hysteresis on oxygen plasma treated polypropylene surfaces. J. Colloid Interface Sci. 1989, 132, 504–508. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Leys, C. Influence of ambient conditions on the ageing behaviour of plasma-treated PET surfaces. Nucl. Instrum. Methods Phys. Res. B Beam Interact. Mater. At. 2008, 266, 3086–3090. [Google Scholar] [CrossRef]
- Mrad, O.; Saunier, J.; Aymes-Chodur, C.; Mazel, V.; Rosilio, V.; Agnely, F.; Yagoubi, N. Aging of a medical device surface following cold plasma treatment: Influence of low molecular weight compounds on surface recovery. Eur. Polym. J. 2011, 47, 2403–2413. [Google Scholar] [CrossRef]
- Pascual, M.; Balart, R.; Sanchez, L.; Fenollar, O.; Calvo, O. Study of the aging process of corona discharge plasma effects on low density polyethylene film surface. J. Mater. Sci. 2008, 43, 4901–4909. [Google Scholar] [CrossRef]
- Vandenbossche, M.; Hegemann, D. Recent approaches to reduce aging phenomena in oxygen-and nitrogen-containing plasma polymer films: An overview. Curr. Opin. Solid State Mater. Sci. 2018, 22, 26–38. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic modification of polyethersulfone membranes by low temperature plasma-induced graft polymerization. J. Membr. Sci. 2002, 209, 255–269. [Google Scholar] [CrossRef]
- Garbassi, F.; Morra, M.; Occhiello, E. Chemical modifications. In Polymer Surfaces from Physics to Technology; Garbassi, F., Morra, M., Occhiello, E., Eds.; Willey: Chichester, UK, 1994; pp. 242–274. [Google Scholar]
- Esfahani, H.; Ghiyasi, Y. Effect of HA Nanoparticles on Adsorption of Vitamin D3 on Super-Hydrophobic PA6 Nanofibrous Scaffold. Matéria 2020, 25. [Google Scholar] [CrossRef]
- Yoshida, M.; Langer, R.; Lendlein, A.; Lahann, J. From advanced biomedical coatings to multi-functionalized biomaterials. J. Macromol. Sci. Polym. Rev. 2006, 46, 347–375. [Google Scholar] [CrossRef]
- Casper, C.L.; Yamaguchi, N.; Kiick, K.L.; Rabolt, J.F. Functionalizing electrospun fibers with biologically relevant macromolecules. Biomacromolecules 2005, 6, 1998–2007. [Google Scholar] [CrossRef]
- Patel, S.; Kurpinski, K.; Quigley, R.; Gao, H.; Hsiao, B.S.; Poo, M.M.; Li, S. Bioactive nanofibers: Synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007, 7, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Shitole, A.A.; Raut, P.; Giram, P.; Rade, P.; Khandwekar, A.; Garnaik, B.; Sharma, N. Poly (vinylpyrrolidone)-iodine engineered poly (ε-caprolactone) nanofibers as potential wound dressing materials. Mater. Sci. Eng. C 2020, 110, 110731. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Mi, Y.; Gao, Z. Green approaches for the fabrication of electrospun poly (vinyl alcohol) nanofibers loaded epidermal growth factor derivative. Mater. Lett. 2020, 276, 128237. [Google Scholar] [CrossRef]
- Shelke, N.B.; James, R.; Laurencin, C.T.; Kumbar, S.G. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym. Adv. Technol. 2014, 25, 448–460. [Google Scholar] [CrossRef]
- Sakai, S.; Liu, Y.; Yamaguchi, T.; Watanabe, R.; Kawabe, M.; Kawakami, K. Immobilization of Pseudomonas cepacia lipase onto electrospun polyacrylonitrile fibers through physical adsorption and application to transesterification in nonaqueous solvent. Biotechnol. Lett. 2010, 32, 1059–1062. [Google Scholar] [CrossRef]
- Kowalczyk, T. Functional Micro-and Nanofibers Obtained by Nonwoven Post-Modification. Polymers 2020, 12, 1087. [Google Scholar] [CrossRef]
- Rao, G.K.; Kurakula, M.; Yadav, K.S. Application of Electrospun Materials in Gene Delivery. In Electrospun Materials and Their Allied Applications; Inamuddin, R.B., Mohd, I.A., Abdullah, M.A., Eds.; Scrivener publishing: Austin, TX, USA, 2020; pp. 265–306. [Google Scholar]
- Bu, Y.; Ma, J.; Bei, J.; Wang, S. Surface modification of aliphatic polyester to enhance biocompatibility. Front. Bioeng. Biotechnol. 2019, 7, 98. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Kijeńska-Gawrońska, E.; Biadasz, A.; Jesionowski, T. Electrospun poly (methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation. Environ. Res. 2020, 184, 109332. [Google Scholar] [CrossRef]
- Müller, K.J.F.; Quinn, A.P.R.; Johnston, M.; Becker, A.; Greiner, F. Caruso Polyelectrolyte functionalization of electrospunfibers. Chem. Mater. 2006, 18, 2397–2403. [Google Scholar] [CrossRef]
- Truong, Y.B.; Glattauer, V.; Briggs, K.L.; Zappe, S.; Ramshaw, J.A. Collagen-based layer-by-layer coating on electrospun polymer scaffolds. Biomaterials 2012, 33, 9198–9204. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.J.; Soenen, E.; van Gulck, J.; Rejman, G.; Vanham, B.; Lucas, B.; Geers, K.; Braeckmans, V.; Shahin, P.; Spanoghe, J.; et al. Electrospun polystyrene fibers for HIV entrapment. Polym. Adv. Technol. 2014, 25, 827–834. [Google Scholar] [CrossRef][Green Version]
- Chunder, A.; Sarkar, S.; Yu, Y.; Zhai, L. Fabrication of ultrathin polyelectrolyte fibers and their controlled release properties. Colloids Surf. B 2007, 58, 172–179. [Google Scholar] [CrossRef]
- Wu, Z.R.; Ma, J.; Liu, B.F.; Xu, Q.Y.; Cui, F.Z. Layer-by-layer assembly of polyelectrolyte films improving cytocompatibility to neural cells. J. Biomed. Mater. Res. Part. A 2007, 81, 355–362. [Google Scholar] [CrossRef]
- Zhang, K.; Chooi, W.H.; Liu, S.; Chin, J.S.; Murray, A.; Nizetic, D.; Chew, S.Y. Localized delivery of CRISPR/dCas9 via layer-by-layer self-assembling peptide coating on nanofibers for neural tissue engineering. Biomaterials 2020, 256, 120225. [Google Scholar] [CrossRef]
- He, L.; Shi, Y.; Han, Q.; Zuo, Q.; Ramakrishna, S.; Xue, W.; Zhou, L. Surface modification of electrospun nanofibrous scaffolds via polysaccharide–protein assembly multilayer for neurite outgrowth. J. Mater. Chem. 2012, 22, 13187–13196. [Google Scholar] [CrossRef]
- Sánchez, L.D.; Brack, N.; Postma, A.; Pigram, P.J.; Meagher, L. Surface modification of electrospunfibres for biomedical applications: A focus on radical polymerization methods. Biomaterials 2016, 106, 24–45. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. Electrospun nanofibers as a platform for multifunctional, hierarchically organized nanocomposite. Compos. Sci. Technol. 2009, 69, 1804–1817. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Li, X.; Jiang, L.; Chang, Z.; Li, Q. Biologically responsive, long-term release nanocoating on an electrospun scaffold for vascular endothelialization and anticoagulation. Mater. Sci. Eng. C 2020, 107, 110212. [Google Scholar] [CrossRef]
- Ghobeira, R.; De Geyter, N.; Morent, R. Plasma surface functionalization of biodegradable electrospun scaffolds for tissue engineering applications. In Biodegradable Polymers: Recent Developments and New Perspectives; Geraldine, R.C., Ed.; IAPC Publishing: Zagreb, Croatia, 2017; pp. 191–236. [Google Scholar]
- Dufay, M.; Jimenez, M.; Degoutin, S. Effect of cold plasma treatment on electrospun nanofibers properties: A review. ACS Appl. Bio. Mater. 2020, 3, 4696–4716. [Google Scholar] [CrossRef]
- Bhattacharjee, P.; Naskar, D.; Kim, H.-W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Non-mulberry silk fibroin grafted PCL nanofibrous scaffold: Promising ECM for bone tissue engineering. Eur. Polym. J. 2015, 71, 490–509. [Google Scholar] [CrossRef]
- Krithica, N.; Natarajan, V.; Madhan, B.; Sehgal, P.K.; Mandal, A.B. Type I collagen immobilized poly (caprolactone) nanofibers: Characterization of surface modification and growth of fibroblasts. Adv. Eng. Mater. 2012, 14, B149–B154. [Google Scholar] [CrossRef]
- de Sousa, A.M.C.; Rodrigues, C.A.; Ferreira, I.A.; Diogo, M.M.; Linhardt, R.J.; Cabral, J.; Ferreira, F.C. Functionalization of Electrospun Nanofibers and Fiber Alignment Enhance Neural Stem Cell Proliferation and Neuronal Differentiation. Front. Bioeng. Biotechnol. 2020, 8, 1215. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemczyk-Soczynska, B.; Gradys, A.; Sajkiewicz, P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers 2020, 12, 2636. https://doi.org/10.3390/polym12112636
Niemczyk-Soczynska B, Gradys A, Sajkiewicz P. Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers. 2020; 12(11):2636. https://doi.org/10.3390/polym12112636
Chicago/Turabian StyleNiemczyk-Soczynska, Beata, Arkadiusz Gradys, and Pawel Sajkiewicz. 2020. "Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering" Polymers 12, no. 11: 2636. https://doi.org/10.3390/polym12112636
APA StyleNiemczyk-Soczynska, B., Gradys, A., & Sajkiewicz, P. (2020). Hydrophilic Surface Functionalization of Electrospun Nanofibrous Scaffolds in Tissue Engineering. Polymers, 12(11), 2636. https://doi.org/10.3390/polym12112636