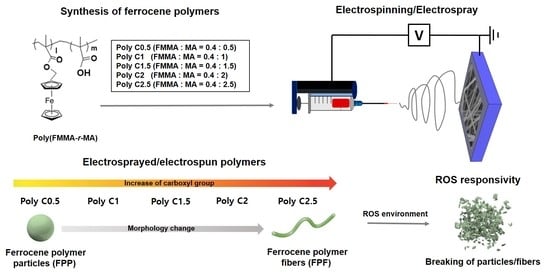
Electrospinning/Electrospray of Ferrocene Containing Copolymers to Fabricate ROS-Responsive Particles and Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
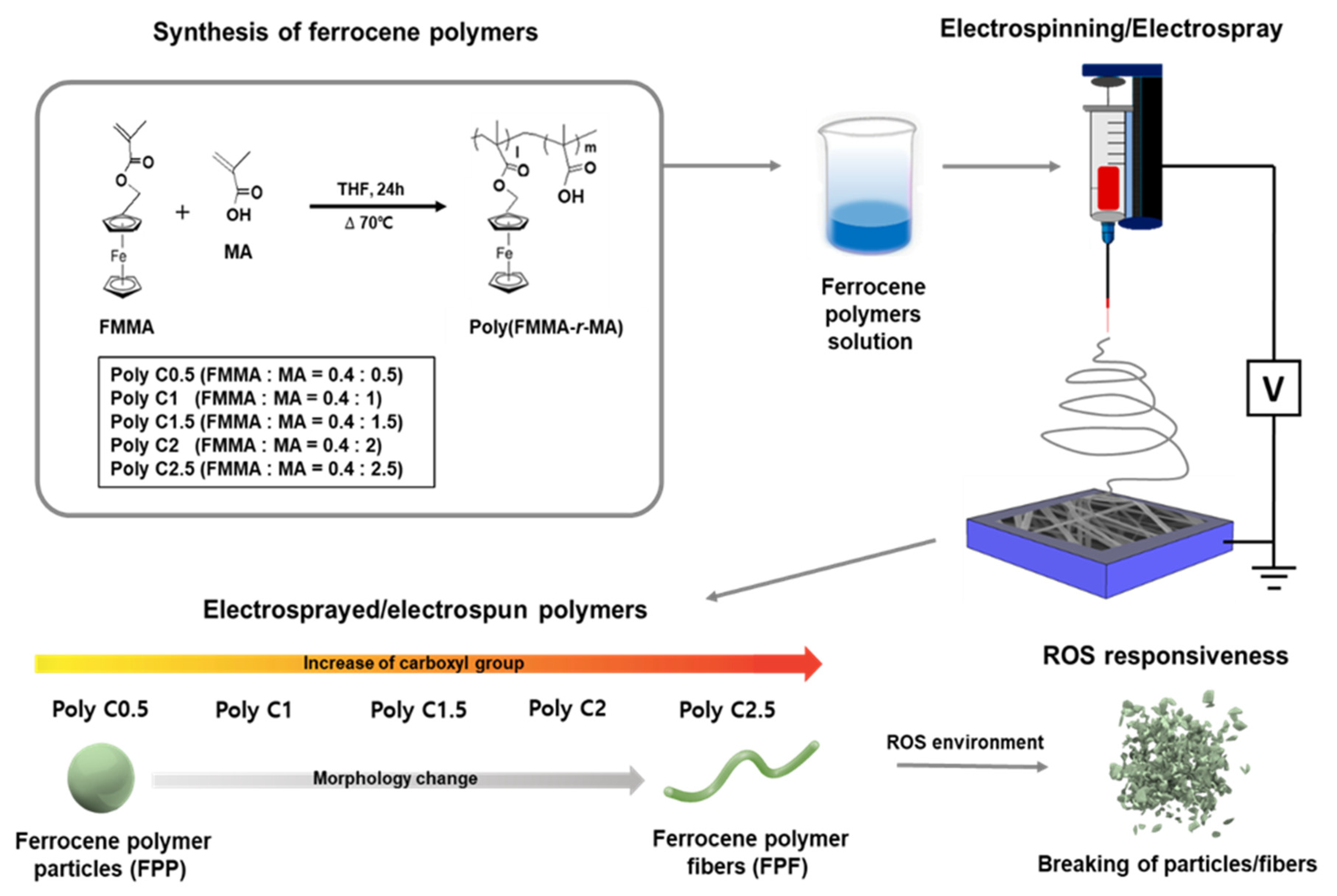
2.3. Synthesis of Poly(FMMA-r-MA)
2.4. Electrospinning of Ferrocene-Containing Polymers
3. Results and Discussion
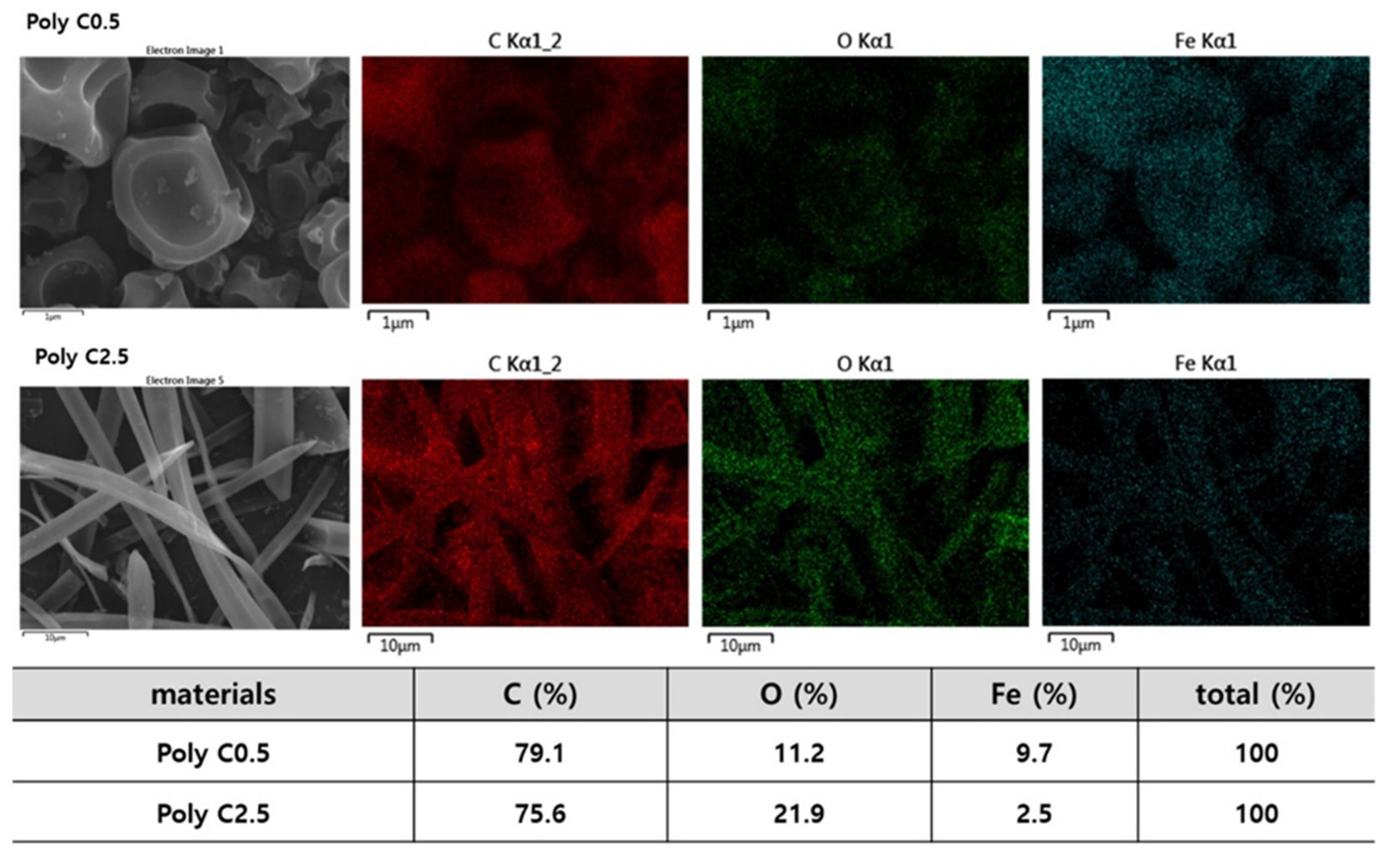
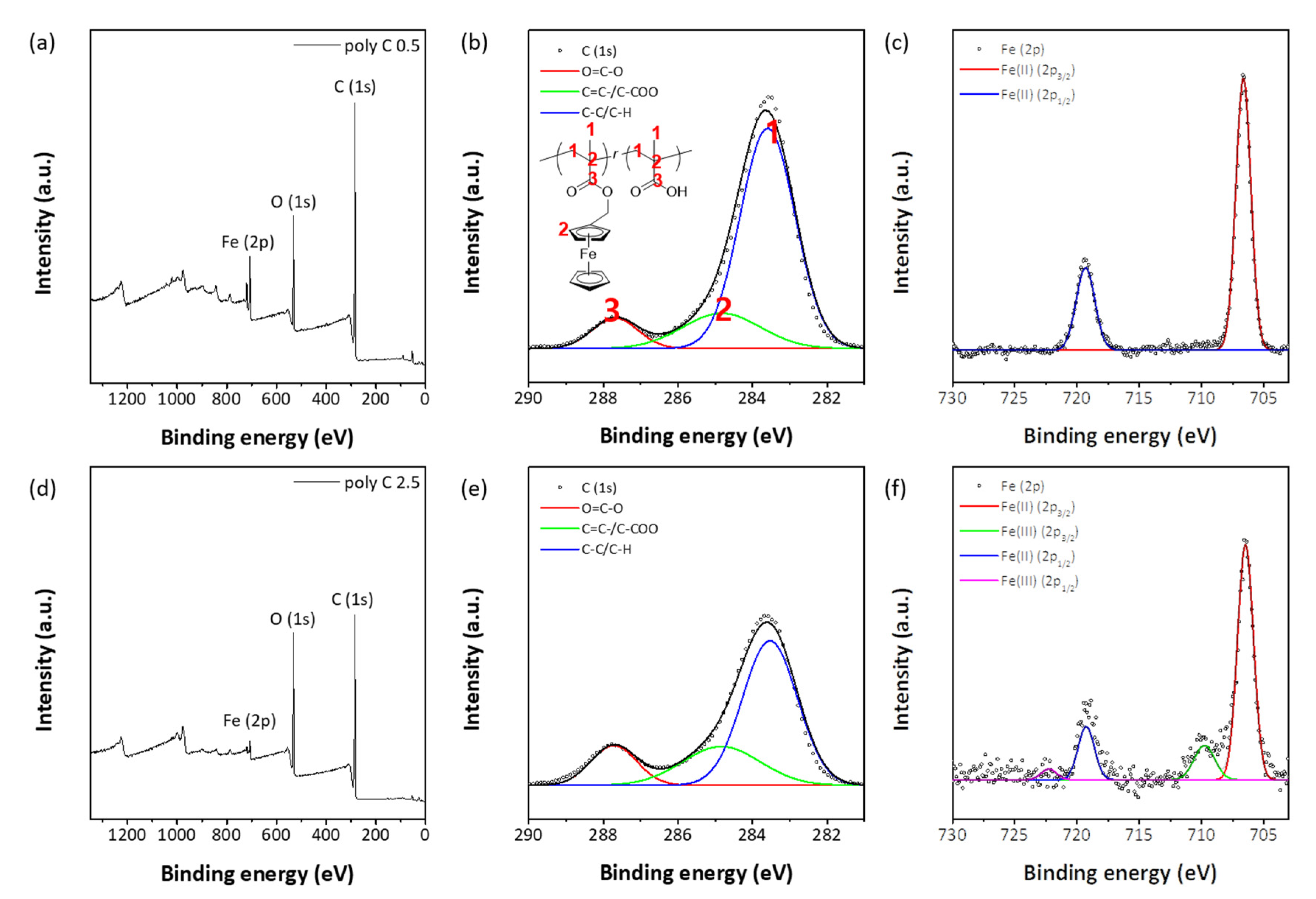
3.1. Morphology Studies
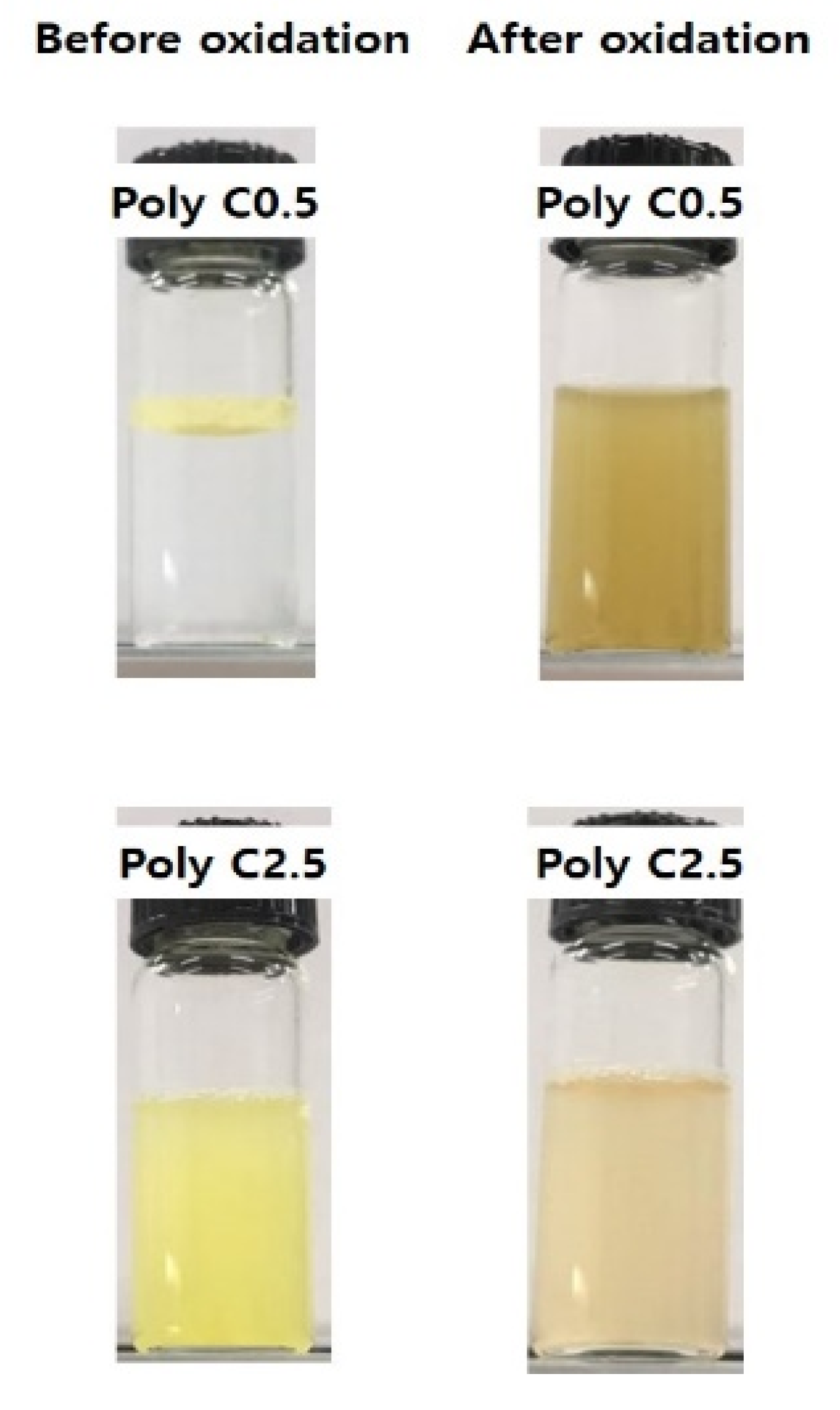
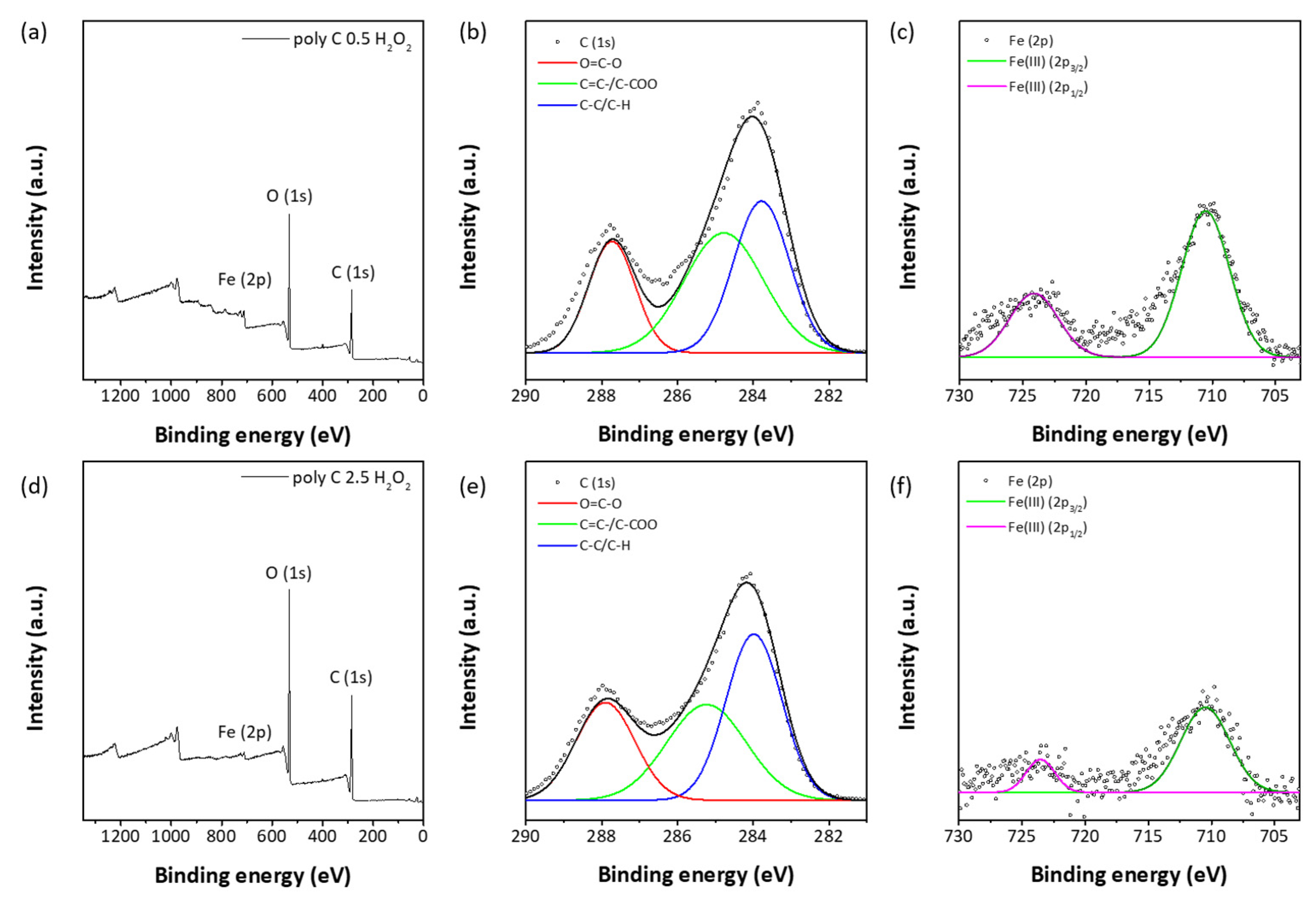
3.2. Responsiveness on Reactive Oxygen Species
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, M.; Li, Y.F.; Besenbacher, F. Electrospun Nanofibers-Mediated On-Demand Drug Release. Adv. Healthc. Mater. 2014, 3, 1721–1732. [Google Scholar] [CrossRef]
- Pirmoradi, F.N.; Jackson, J.K.; Burt, H.M.; Chiao, M. On-demand controlled release of docetaxel from a battery-less MEMS drug delivery device. Lab. Chip 2011, 11, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Caldorera-Moore, M.; Peppas, N.A. Micro-and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv. Drug Deliv. Rev. 2009, 61, 1391–1401. [Google Scholar] [CrossRef]
- Kukowska-Latallo, F.J.; Patri, K.A.; Baker, J., Jr. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv. Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar]
- Hatakeyama, H.; Akita, H.; Kogure, K.; Oishi, M.; Nagasaki, Y.; Kihira, Y.; Ueno, M.; Kobayashi, H.; Kikuchi, H.; Harashima, H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007, 14, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; Kim, S.W.; Park, T.G. Molecular design of functional polymers for gene therapy. Prog. Polym. Sci. 2007, 32, 1239–1274. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Chen, Y.-Y.; Wang, D.-R.; Wei, C.; Guo, J.; Lu, D.-R.; Chu, C.-C.; Wang, C.-C. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 2012, 33, 6570–6579. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M. Gene delivery using temperature-responsive polymeric carriers. Drug Discov. Today 2002, 7, 426–432. [Google Scholar] [CrossRef]
- Li, F.; Li, T.; Cao, W.; Wang, L.; Xu, H. Near-infrared light stimuli-responsive synergistic therapy nanoplatforms based on the coordination of tellurium-containing block polymer and cisplatin for cancer treatment. Biomaterials 2017, 133, 208–218. [Google Scholar] [CrossRef]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-grafted mesoporous silica nanoparticles as ultrasound-responsive drug carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wei, Y.; Chen, K.; Zhang, X.; Xu, X.; Shi, Q.; Han, S.; Chen, X.; Gong, H.; Li, X. Biocompatible reactive oxygen species (ROS)-responsive nanoparticles as superior drug delivery vehicles. Adv. Healthc. Mater. 2015, 4, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.; Lee, J.S.; Woo, J.; Ahn, S.; Lee, E.; Choi, W.I.; Sung, D. Reactive oxygen species (ROS)-responsive ferrocene-polymer-based nanoparticles for controlled release of drugs. J. Mater. Chem. B 2020, 8, 1906–1913. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, K.; Wei, P.; Huang, S.; Pei, Y.; Zhao, W.; Pei, Z. Cationic vesicles based on amphiphilic pillar [5] arene capped with ferrocenium: A redox-responsive system for drug/siRNA co-delivery. Angew. Chem. Int. Ed. Engl. 2014, 53, 13126–13130. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, X.; Che, X.; Yang, M.; Zhai, G. Biomedical application and controlled drug release of electrospun fibrous materials. Mater. Sci. Eng. C 2018, 90, 750–763. [Google Scholar] [CrossRef]
- Bodnár, E.; Rosell-Llompart, J. Growth dynamics of granular films produced by electrospray. J. Colloid Interface Sci. 2013, 407, 536–545. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Yördem, O.S.; Papila, M.; Menceloğlu, Y.Z. Effects of electrospinning parameters on polyacrylonitrile nanofiber diameter: An investigation by response surface methodology. Mater. Des. 2008, 29, 34–44. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Tungprapa, S.; Puangparn, T.; Weerasombut, M.; Jangchud, I.; Fakum, P.; Semongkhol, S.; Meechaisue, C.; Supaphol, P. Electrospun cellulose acetate fibers: Effect of solvent system on morphology and fiber diameter. Cellulose 2007, 14, 563–575. [Google Scholar] [CrossRef]
- Lee, H.; Nishino, M.; Sohn, D.; Lee, J.S.; Kim, I.S. Control of the morphology of cellulose acetate nanofibers via electrospinning. Cellulose 2018, 25, 2829–2837. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Abingdon, UK, 2007; Volume 2. [Google Scholar]
- Chernyy, S.; Wang, Z.; Kirkensgaard, J.J.K.; Bakke, A.; Mortensen, K.; Ndoni, S.; Almdal, K. Synthesis and characterization of ferrocene containing block copolymers. J. Polym. Sci. A Polym. Chem. 2017, 55, 495–503. [Google Scholar] [CrossRef]
- Gallei, M.; Schmidt, B.V.; Klein, R.; Rehahn, M. Defined Poly [styrene-block-(ferrocenylmethyl methacrylate)] Diblock Copolymers via Living Anionic Polymerization. Macromol. Rapid Commun. 2009, 30, 1463–1469. [Google Scholar] [CrossRef]
- Kim, M.; Choi, Y.-W.; Sim, J.-H.; Choo, J.; Sohn, D. End chain length effect of hydrophobically end-capped poly (ethylene oxide) s on their self-assemblies in solution. J. Phys. Chem. B 2004, 108, 8269–8277. [Google Scholar] [CrossRef]
- Guan, T.; Du, Z.; Peng, J.; Zhao, D.; Sun, N.; Ren, B. Polymerizable Hydrophobically Modified Ethoxylated Urethane Acrylate Polymer: Synthesis and Viscoelastic Behavior in Aqueous Systems. Macromolecules 2020, 53, 7420–7429. [Google Scholar] [CrossRef]
- Serero, Y.; Aznar, R.; Porte, G.; Berret, J.-F.; Calvet, D.; Collet, A.; Viguier, M. Associating polymers: From “flowers” to transient networks. Phys. Rev. Lett. 1998, 81, 5584. [Google Scholar] [CrossRef]
- Noviandri, I.; Brown, K.N.; Fleming, D.S.; Gulyas, P.T.; Lay, P.A.; Masters, A.F.; Phillips, L. The decamethylferrocenium/decamethylferrocene redox couple: A superior redox standard to the ferrocenium/ferrocene redox couple for studying solvent effects on the thermodynamics of electron transfer. J. Phys. Chem. B 1999, 103, 6713–6722. [Google Scholar] [CrossRef]


| Sample | fFMMA | FFMMAa | Mn (g/mol) b | Đb |
|---|---|---|---|---|
| Poly C0.5 | 0.444 | 0.484 | 11,100 | 1.75 |
| Poly C1 | 0.286 | 0.308 | 9800 | 2.15 |
| Poly C1.5 | 0.211 | 0.236 | 11,100 | 2.03 |
| Poly C2 | 0.167 | 0.184 | 9400 | 1.81 |
| Poly C2.5 | 0.138 | 0.143 | 13,100 | 1.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Woo, J.; Son, D.; Kim, M.; Choi, W.I.; Sung, D. Electrospinning/Electrospray of Ferrocene Containing Copolymers to Fabricate ROS-Responsive Particles and Fibers. Polymers 2020, 12, 2520. https://doi.org/10.3390/polym12112520
Lee H, Woo J, Son D, Kim M, Choi WI, Sung D. Electrospinning/Electrospray of Ferrocene Containing Copolymers to Fabricate ROS-Responsive Particles and Fibers. Polymers. 2020; 12(11):2520. https://doi.org/10.3390/polym12112520
Chicago/Turabian StyleLee, Hoik, Jiseob Woo, Dongwan Son, Myungwoong Kim, Won Il Choi, and Daekyung Sung. 2020. "Electrospinning/Electrospray of Ferrocene Containing Copolymers to Fabricate ROS-Responsive Particles and Fibers" Polymers 12, no. 11: 2520. https://doi.org/10.3390/polym12112520
APA StyleLee, H., Woo, J., Son, D., Kim, M., Choi, W. I., & Sung, D. (2020). Electrospinning/Electrospray of Ferrocene Containing Copolymers to Fabricate ROS-Responsive Particles and Fibers. Polymers, 12(11), 2520. https://doi.org/10.3390/polym12112520