Influence of Temperature on Mechanical Properties of P(BAMO-r-THF) Elastomer
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Elastomer Preparation
2.3. Characterization
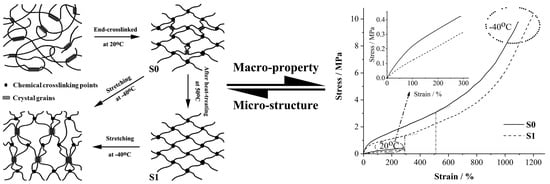
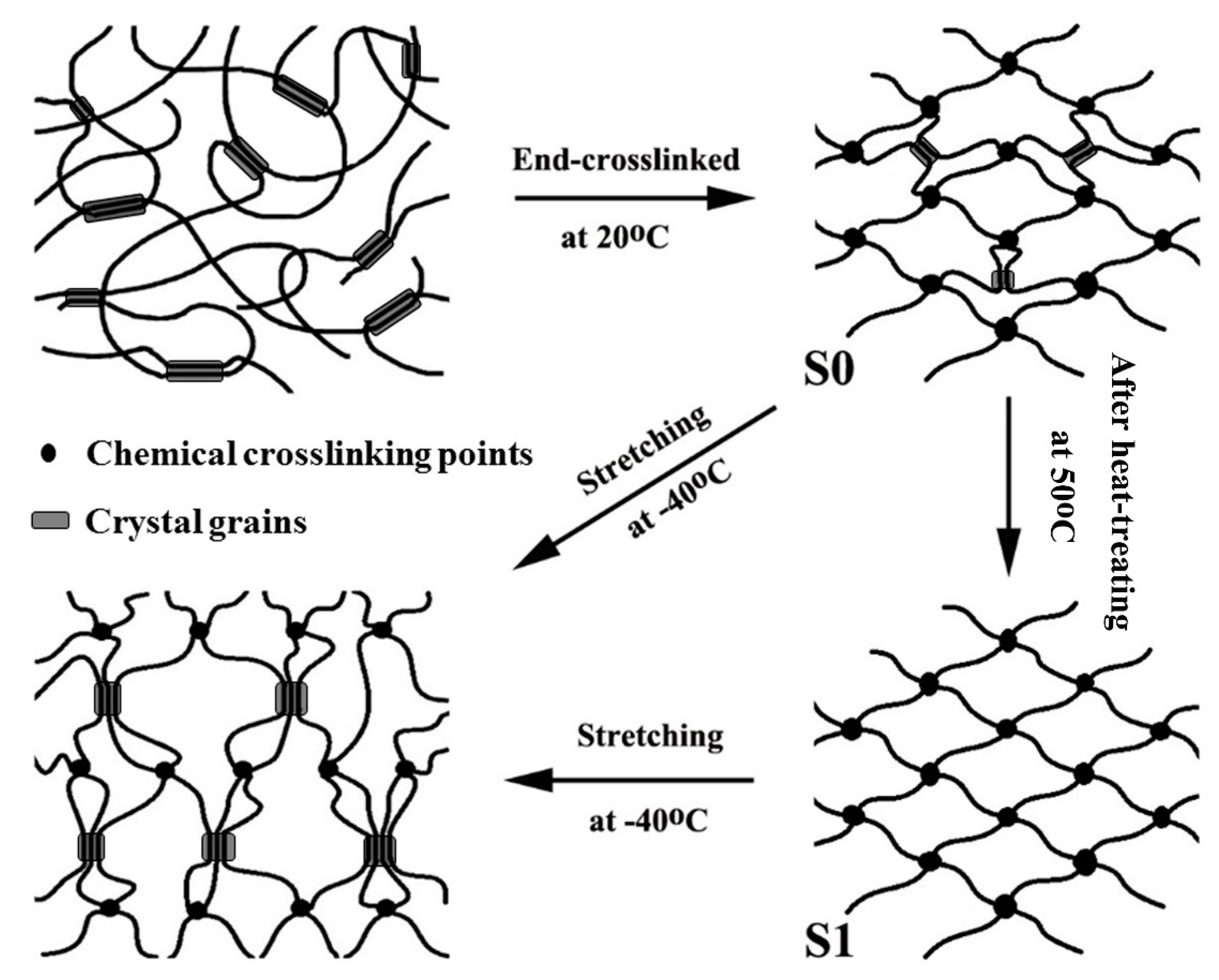
3. Results and Discussion
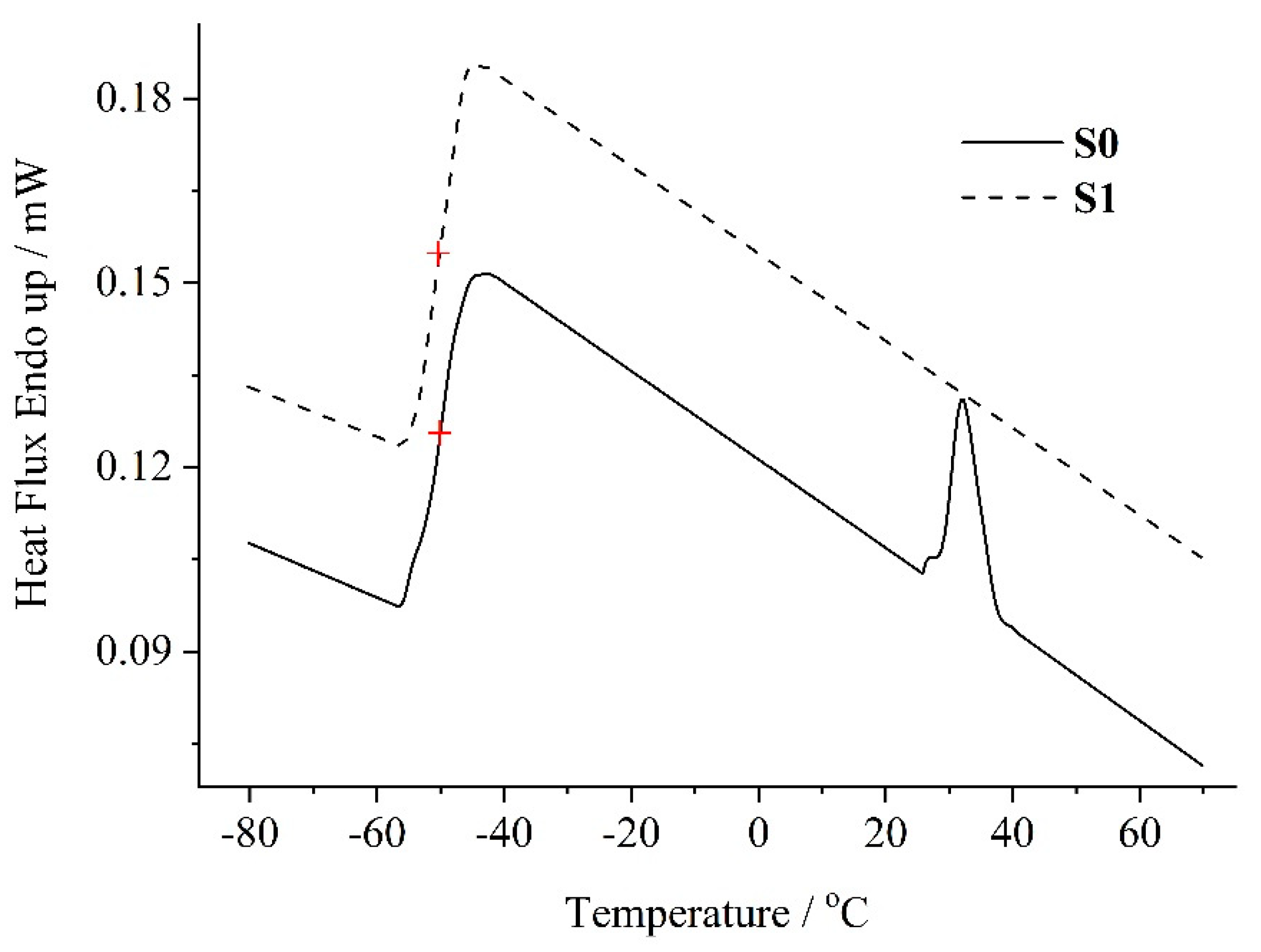
3.1. Thermal Properties
3.2. Aggregation Morphologies
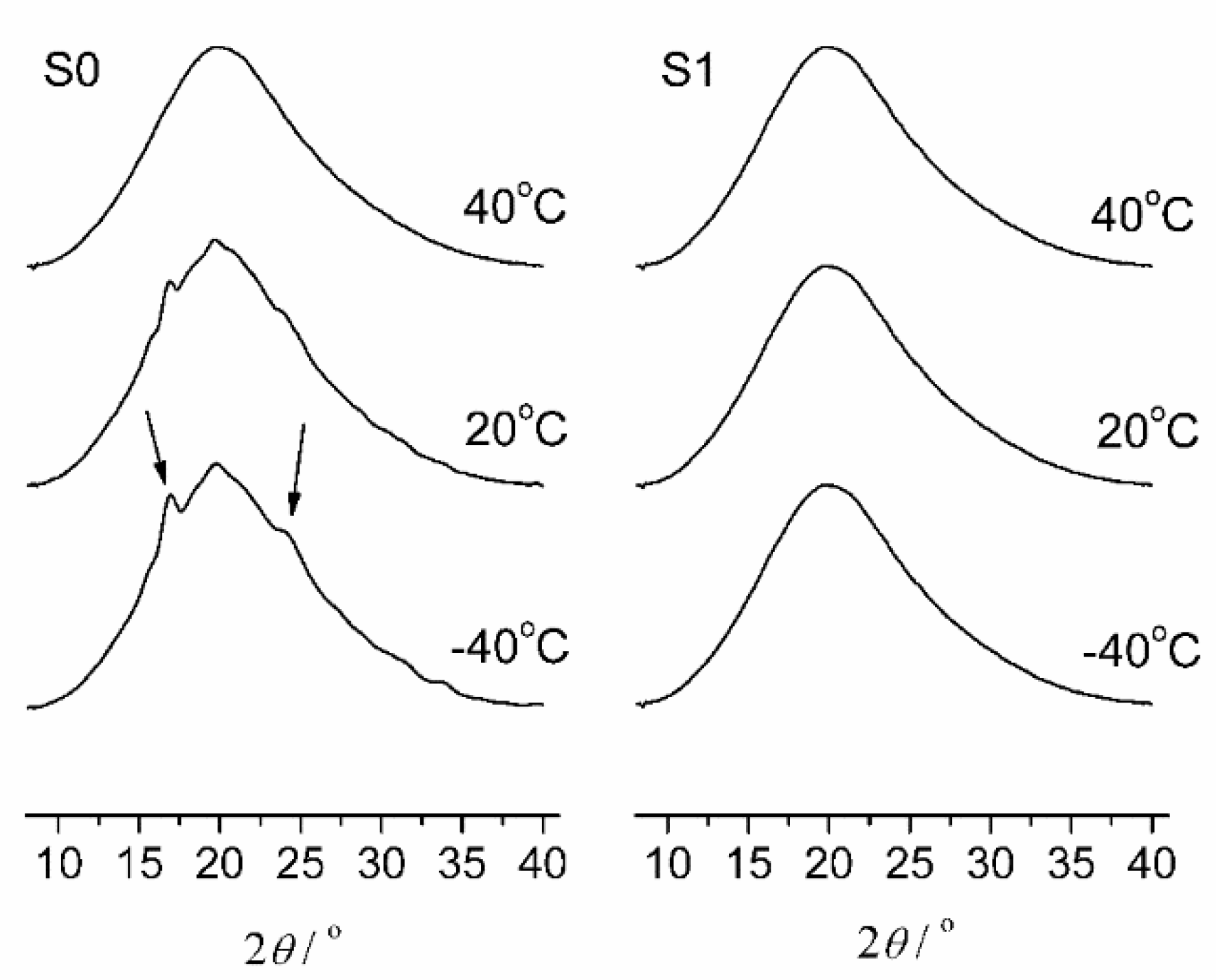
3.3. Wide-Angle X-ray Diffraction
3.4. Mechanical Properties
3.5. Aggregation Evolution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mason, B.P.; Roland, C.M. Solid Propellants. Rubber Chem. Technol. 2019, 92, 1–24. [Google Scholar] [CrossRef]
- Sikder, A.K.; Reddy, S. Review on Energetic Thermoplastic Elastomers (ETPEs) for Military Science. Propellants Explos. Pyrotech. 2012, 38, 14–28. [Google Scholar] [CrossRef]
- Badgujar, D.M.; Talawar, M.B.; Zarko, V.E.; Mahulikar, P.P. New directions in the area of modern energetic polymers: An overview. Combust. Explos. Shock. Waves 2017, 53, 371–387. [Google Scholar] [CrossRef]
- Ma, M.; Kwon, Y. Reactive Energetic Plasticizers Utilizing Cu-Free Azide-Alkyne 1,3-Dipolar Cycloaddition for In-Situ Preparation of Poly(THF-co-GAP)-Based Polyurethane Energetic Binders. Polymers 2018, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhou, J.; Dai, S.; Ma, Z. Influence of Combustion Modifiers on the Cure Kinetics of Glycidyl Azide Polymer Based Propellant-Evaluated through Rheo-Kinetic Approach. Polymers 2019, 11, 637. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, J.; Li, G.; Li, X.; Luo, Y.; Zheng, J. Influence of diisocyanate types on properties of chain-extended poly(3,3-bis(azidomethyl)oxetane). Soft Mater. 2017, 15, 205–213. [Google Scholar] [CrossRef]
- Hardenstine, K.E.; Henderson, G.V.S.; Sperling, L.H.; Murphy, C.J.; Manser, G.E. Crystallization behavior of poly(3,3-bisethoxymethyl oxetane) and poly(3,3-bisazidomethyl oxetane). J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 1597–1609. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kubota, N. Energetics of BAMO. Propellants Explos. Pyrotech. 1992, 17, 5–9. [Google Scholar] [CrossRef]
- Nie, Y.; Gao, H.; Wu, Y.; Hu, W. Effect of comonomer sizes on the strain-induced crystal nucleation of random copolymers. Eur. Polym. J. 2016, 81, 34–42. [Google Scholar] [CrossRef]
- Petrov, V.A.; Kuznetsova, N.V.; Muhametshin, T.I.; Gubaydullin, A.T. Structure of Urethane Copolymers of 3,3-Bis(azidomethyl)oxetane and 3-Azidomethyl-3-methyloxetane. Propellants Explos. Pyrotech. 2014, 39, 545–549. [Google Scholar] [CrossRef]
- Sarangapani, R.; Reddy, S.T.; Sikder, A.K. Molecular dynamics simulations to calculate glass transition temperature and elastic constants of novel polyethers. J. Mol. Graph. Model. 2015, 57, 114–121. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Luo, Y.; Yan, S. Synthesis and thermal decomposition of 3,3′-bis-azidomethyl oxetane-3-azidomethyl-3′-methyl oxetane random copolymer. Soft Mater. 2016, 14, 9–14. [Google Scholar] [CrossRef]
- Kawasaki, H.; Anan, T.; Kimura, E.; Oyumi, Y. BAMO/NMMO Copolymer with Polyester Initiation. Propellants Explos. Pyrotech. 1997, 22, 87–92. [Google Scholar] [CrossRef]
- Bednarek, M.; Kubisa, P. Cationic copolymerization of tetrahydrofuran with ethylene oxide in the presence of diols: Composition, microstructure, and properties of copolymers. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3455–3463. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Han, J.; Xie, Z.; Xu, J.; Guo, B. Copolymerization with Polyether Segments Improves the Mechanical Properties of Biodegradable Polyesters. ACS Omega 2017, 2, 2639–2648. [Google Scholar] [CrossRef]
- Manser, G.E.; Guimont, J.; Ross, D.L. A new polymerization technique for preparing low molecular weight polyether glycols. In Proceedings of the JANNAF Propulsion Meeting, New Orleans, LA, USA, 26–28 May 1981; pp. 29–38. [Google Scholar]
- Chen, T.; Li, W.; Jiang, W.; Hao, G.; Xiao, L.; Ke, X.; Liu, J.; Gao, H. Preparation and characterization of RDX/BAMO-THF energetic nanocomposites. J. Energetic Mater. 2018, 36, 424–434. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Guo, S.F.; Zhao, L.M.; Chen, W.; Hao, G.Z.; Xiao, L.; Ke, X.; Jiang, W. Preparation and property of CL-20/BAMO-THF energetic nanocomposites. Def. Technol. 2019, 15, 306–312. [Google Scholar] [CrossRef]
- Zhai, J.; Yang, R.; Li, J. Catalytic thermal decomposition and combustion of composite BAMO–THF propellants. Combust. Flame 2008, 154, 473–477. [Google Scholar] [CrossRef]
- Eroğlu, M.S.; Güven, O. Characterization of network structure of poly(glycidyl azide) elastomers by swelling, solubility and mechanical measurements. Polymer 1998, 39, 1173–1176. [Google Scholar] [CrossRef]
- Xiaoxia, J.; Yiwen, H.; Qilong, Z. Effect of thermal processing temperature on the microphase separation and mechanical properties of BAMO/THF polyurethane. J. Polym. Eng. 2017, 37, 169–176. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Luo, Y. Synthesis and characterization of 3,3′-Bisazidomethyl oxetane-3-azidomethyl-3′-methyl oxetane alternative block energetic thermoplastic elastomer. Propellants Explos. Pyrotech. 2012, 37, 235–240. [Google Scholar] [CrossRef]
- Zhai, J.; Jia, H.; Guo, X.; Guo, A. Sequence Structure, Morphology and Viscosity Behavior of 3,3-bis(azidomethyl) Oxetane-tetrahydrofuran Random Copolyether. Propellants Explos. Pyrotech. 2017, 42, 643–648. [Google Scholar] [CrossRef]
- Zhou, Y.; Xin-Ping, L.; Qing-Xuan, Z. Simulation study on the liquid-crystalline ordering and fluidity of energetic diblock copolymers based on poly[3,3-bis(azidomethyl) oxetane]. J. Appl. Polym. Sci. 2013, 129, 2772–2778. [Google Scholar]
- Mark, J.E.; Erman, B.; Eirich, F.R. The Science and Technology of Rubber, 3rd ed.; Elsevier academic press: Burlington, NJ, USA, 2005; pp. 1–27. [Google Scholar]
- Mark, J.E. Rubber elasticity. J. Chem. Educ. 1981, 58, 898. [Google Scholar] [CrossRef]
- Fernandez, J.O.; Swallowe, G.M. Crystallization of PET with strain, strain rate and temperature. J. Mater. Sci. 2000, 35, 4405–4414. [Google Scholar] [CrossRef]
- Mark, J.E. The effect of strain-induced crystallization on the ultimate properties of an elastomeric polymer network. Polym. Eng. Sci. 1979, 19, 409–413. [Google Scholar] [CrossRef]



| P(BAMO-r-THF) | N100 | Dibutyltin Dilaurate |
|---|---|---|
| 100 | 6.86 | 0.13 |
| Temperature | Sample | Mechanical Properties | 1 | 2 | 3 | 4 | Averages |
|---|---|---|---|---|---|---|---|
| 20 °C | S0 | εb/% | 342 | 318 | 290 | 310 | 315 ± 22 |
| σb/MPa | 0.47 | 0.45 | 0.44 | 0.46 | 0.46 ± 0.01 | ||
| S1 | εb/% | 298 | 291 | 286 | 300 | 294 ± 6 | |
| σb/MPa | 0.33 | 0.30 | 0.31 | 0.33 | 0.32 ± 0.02 | ||
| −40 °C | S0 | εb/% | 1090 | 1054 | 1096 | 1100 | 1085 ± 21 |
| σb/MPa | 8.85 | 8.03 | 9.78 | 8.94 | 8.90 ± 0.72 | ||
| S1 | εb/% | 1179 | 1204 | 1192 | 1147 | 1181 ± 25 | |
| σb/MPa | 9.98 | 10.26 | 10.84 | 9.84 | 10.23 ± 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, J.; Zhao, H.; Guo, X.; Li, X.; Song, T. Influence of Temperature on Mechanical Properties of P(BAMO-r-THF) Elastomer. Polymers 2020, 12, 2507. https://doi.org/10.3390/polym12112507
Zhai J, Zhao H, Guo X, Li X, Song T. Influence of Temperature on Mechanical Properties of P(BAMO-r-THF) Elastomer. Polymers. 2020; 12(11):2507. https://doi.org/10.3390/polym12112507
Chicago/Turabian StyleZhai, Jinxian, Hanpeng Zhao, Xiaoyan Guo, Xiaodong Li, and Tinglu Song. 2020. "Influence of Temperature on Mechanical Properties of P(BAMO-r-THF) Elastomer" Polymers 12, no. 11: 2507. https://doi.org/10.3390/polym12112507
APA StyleZhai, J., Zhao, H., Guo, X., Li, X., & Song, T. (2020). Influence of Temperature on Mechanical Properties of P(BAMO-r-THF) Elastomer. Polymers, 12(11), 2507. https://doi.org/10.3390/polym12112507