Utility of Zinc Oxide Nanoparticles Catalytic Activity in the Electrochemical Determination of Minocycline Hydrochloride
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Instruments
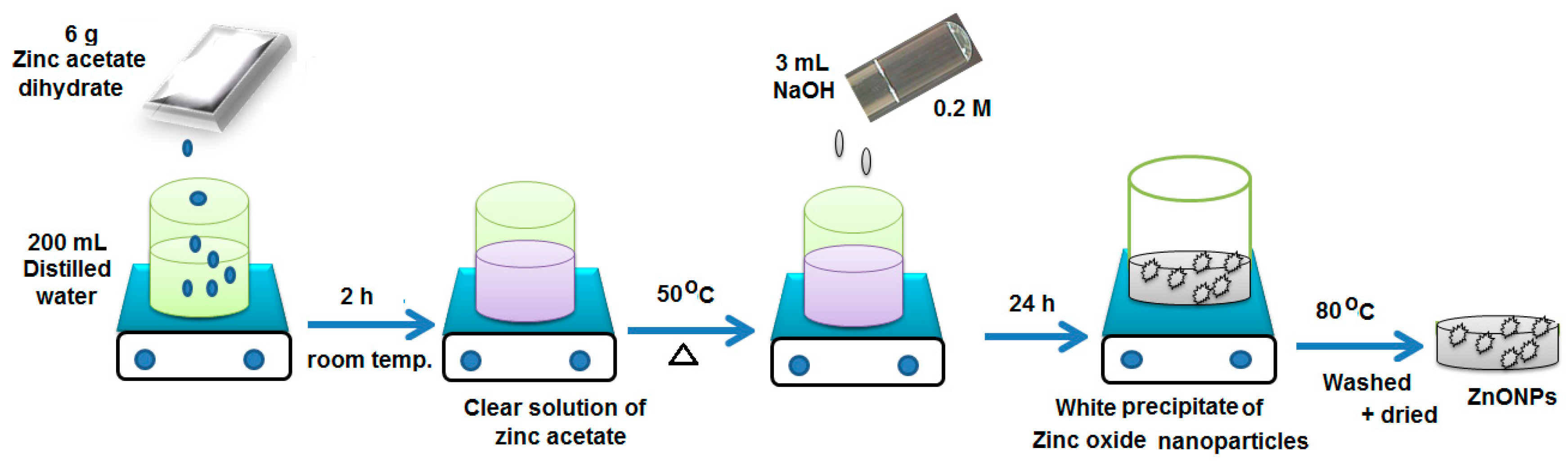
2.2. Synthesis of Zinc Oxide Nanoparticles
2.3. Characterization of Nanoparticles
2.4. Preparation of Stock Minocycline Hydrochloride Solution
2.5. Preparation of Ion Pair
2.6. Sensor Construction
2.7. Calibration Graphs
2.8. Factors Affecting the Potential Readings
2.9. Analytical Applications
3. Results and Discussion
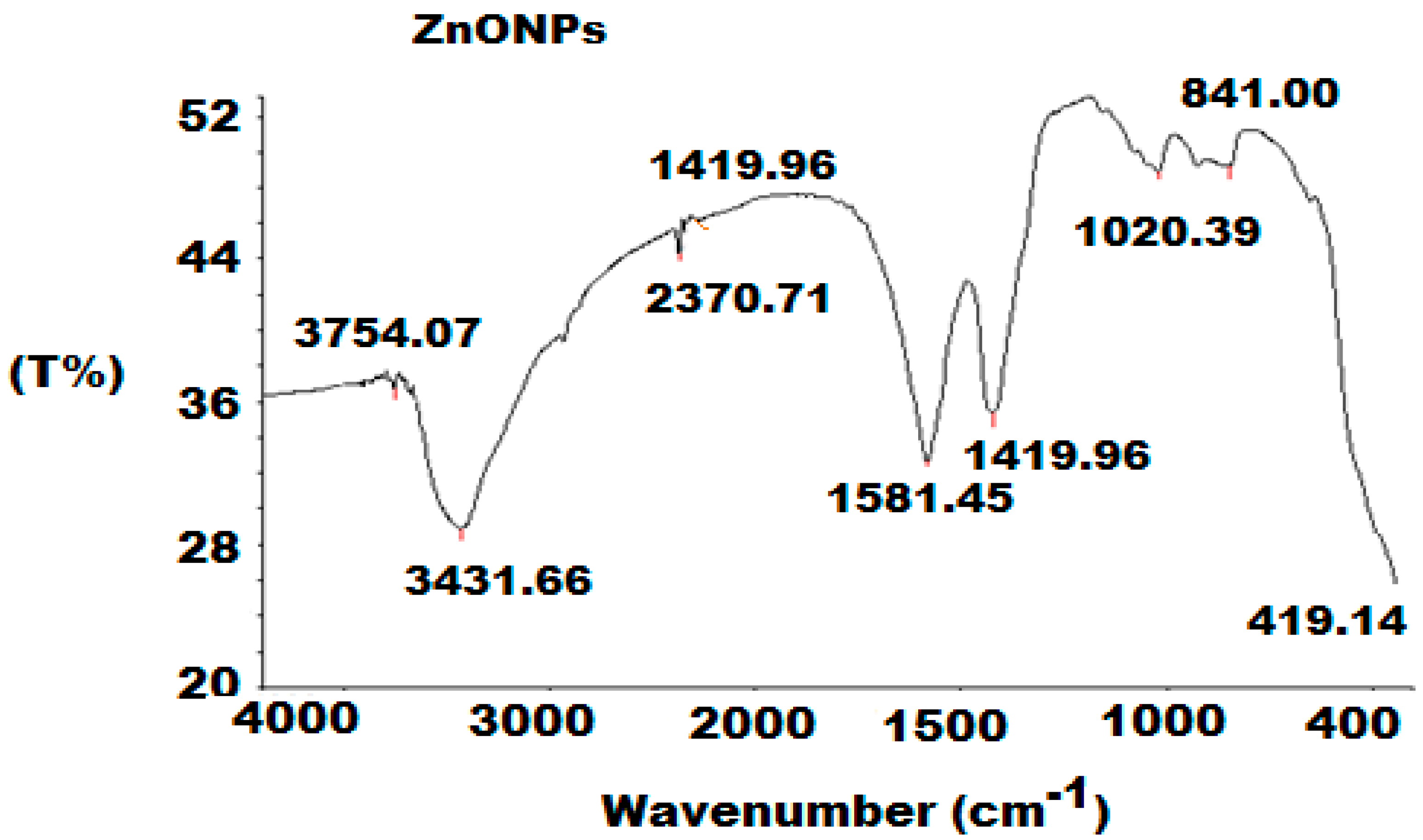
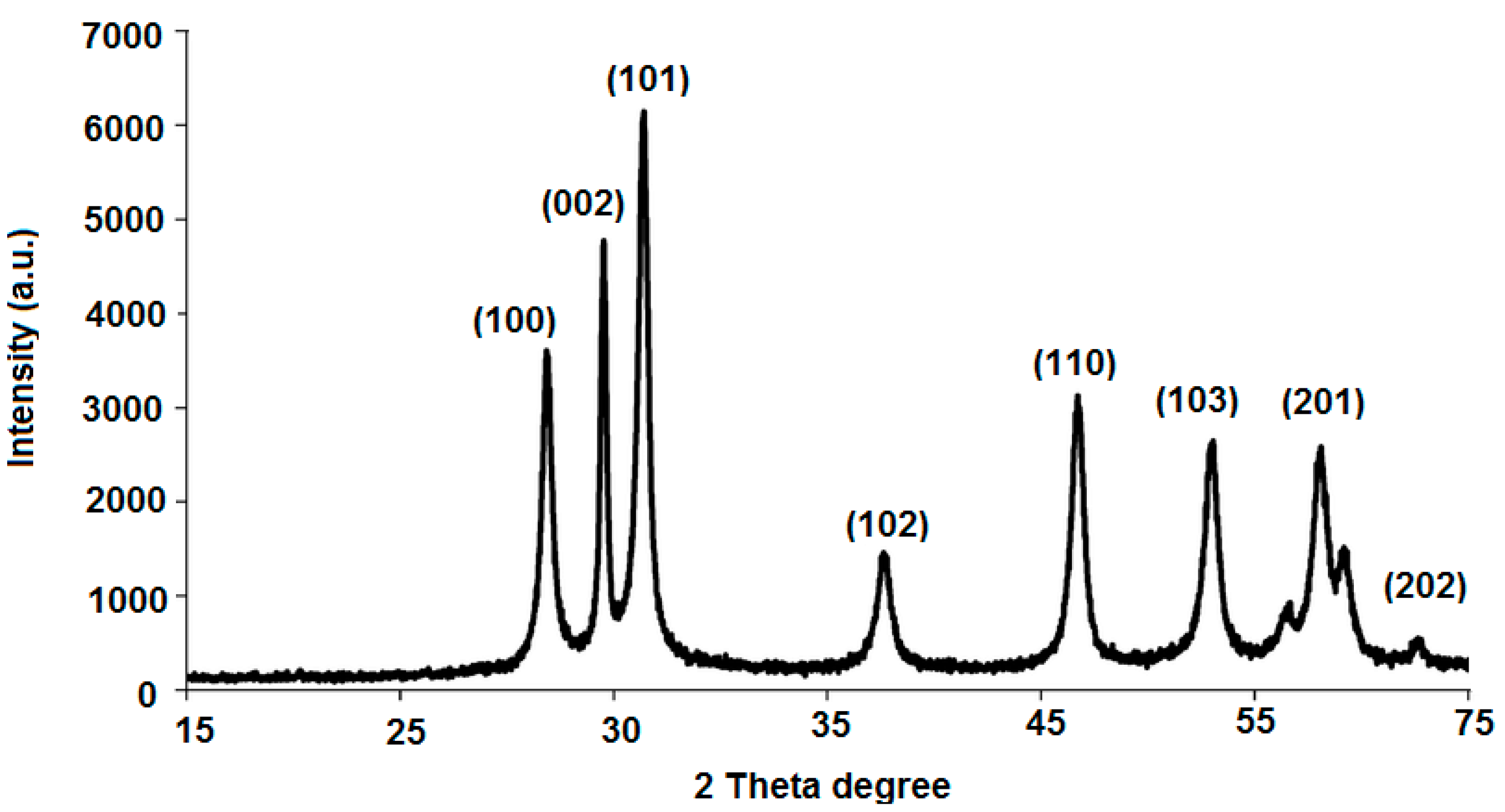
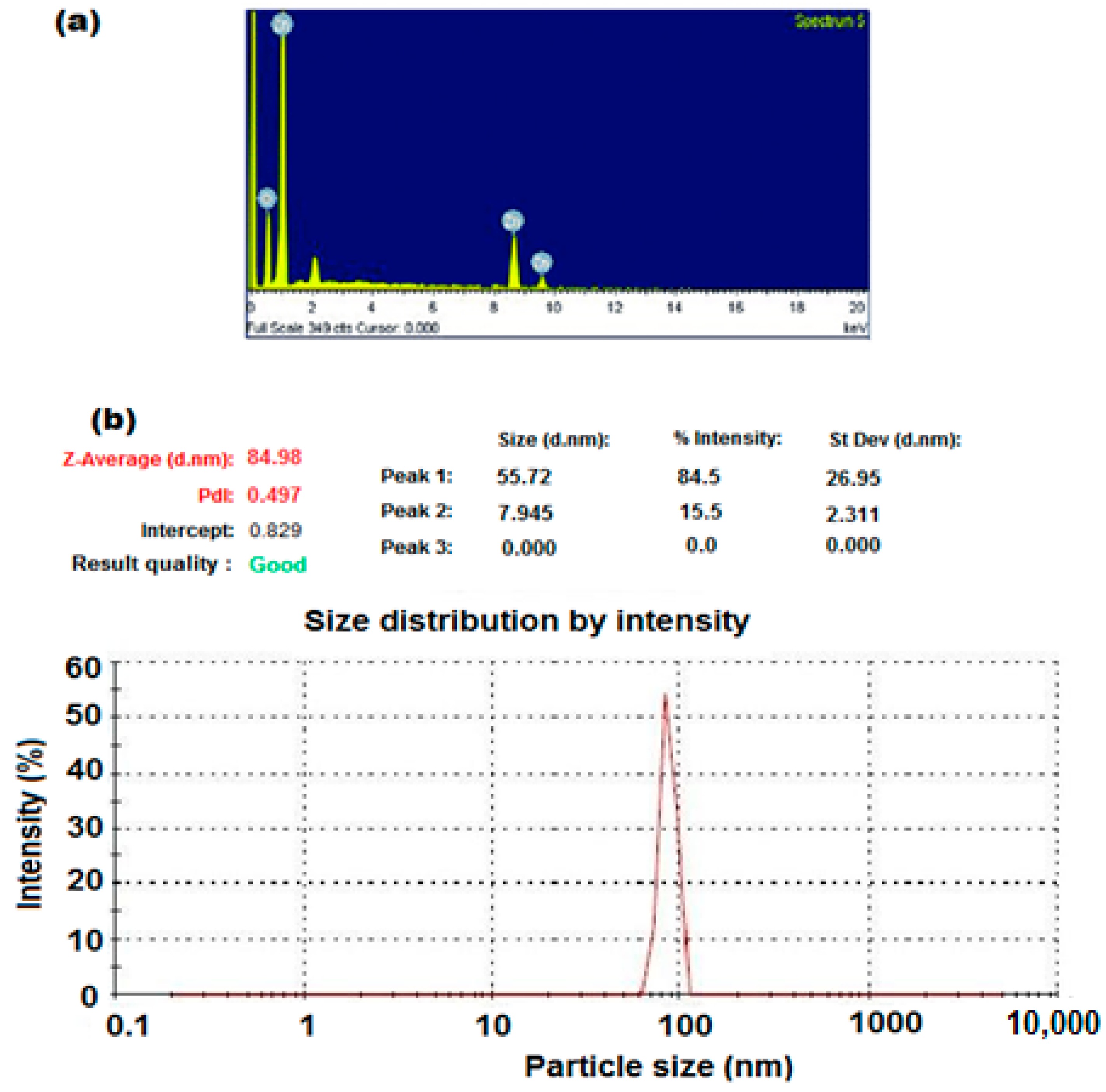
3.1. Characterization of ZnONPs
3.2. The Nature of the Suggested Sensors
3.3. Quantification of Minocycline Hydrochloride
3.4. Method Validation
3.5. Quantification of the Drug in Tablets
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reddy, C.V.; Reddy, K.R.; Shetti, N.P.; Shim, J.; Aminabhavi, T.M.; Dionysiou, D.D. Hetero-nanostructured metal oxide-based hybrid photocatalysts for enhanced photoelectrochemical water splitting—A review. Int. J. Hydrogen Energy 2020, 45, 18331–18347. [Google Scholar] [CrossRef]
- Bukkitgar, S.D.; Kumar, S.; Singh, S.; Singh, V.; Reddy, K.R.; Sadhu, V.; Bagihalli, G.B.; Shetti, N.P.; Reddy, C.V.; Ravindranadh, K.; et al. Functional nanostructured metal oxides and its hybrid electrodes–Recent advancements in electrochemical biosensing applications. Microchem. J. 2020, 159, 105522. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, W.; Nan, H.; Wang, G. Ultrathin zinc oxide nanofilm on zinc substrate for high performance electrochemical sensors. Electrochim. Acta 2014, 144, 186–193. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Sakhraoui, H.E.E.Y.; Fourati, N.; Zerrouki, C.; Maouche, N.; Yaakoubi, N.; Touzani, R.; Chehimi, M.M. High performance zinc oxide nanorod-doped ion imprinted polypyrrole for the selective electrosensing of mercury II ions. Appl. Sci. 2020, 10, 7010. [Google Scholar] [CrossRef]
- Ossai, A.N.; Ezike, S.C.; Dikko, A.B. Bio-synthesis of zinc oxide nanoparticles from bitter leaf (vernonia amygdalina) extract for dye-sensitized solar cell fabrication. J. Mater. Environ. Sci. 2020, 11, 421–428. [Google Scholar]
- Liu, S.; Zhang, Y.; Gao, S.; Fei, T.; Zhang, Y.; Zheng, X.; Zhang, T. An organometallic chemistry-assisted strategy for modification of zinc oxide nanoparticles by tin oxide nanoparticles: Formation of n-n heterojunction and boosting NO2 sensing properties. J. Colloid Inter. Sci. 2020, 567, 328–338. [Google Scholar] [CrossRef]
- Nandanapalli, K.R.; Mudusu, D.; Yu, J.S.; Lee, S. Stable and sustainable photoanodes using zinc oxide and cobalt oxide chemically gradient nanostructures for water-splitting applications. J. Colloid Interf. Sci. 2020, 558, 9–20. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S. A Review on Green Synthesis of Zinc Oxide Nanoparticles Using Plant Extracts and Its Biomedical Applications. BioNanoScience 2020, 1–16. [Google Scholar] [CrossRef]
- Santiago, K.; Mundle, R.; Samantaray, C.B.; Bahoura, M.; Pradhan, A.K. Nanopatterning of atomic layer deposited Al:ZnO films using electron beam lithography for waveguide applications in the NIR region. Opt. Mater. Express 2012, 2, 1743–1750. [Google Scholar] [CrossRef]
- Singh, S.P.; Arya, S.K.; Pandey, P.; Malhotra, B.D.; Saha, S.; Sreenivas, K.; Gupta, V. Cholesterol biosensor based on rf sputtered zinc oxide nanoporous thin film. Appl. Phys. Lett. 2007, 91, 063901. [Google Scholar] [CrossRef]
- Lioyd, J.S.; Fung, C.M.; Deganello, D.; Wang, R.J.; Maffeis, T.G.G.; Lau, S.P.; Teng, K.S. Flexographic printing-assisted fabrication of ZnO nanowire devices. Nanotechnology 2013, 24, 195602. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Alloisio, M.; Vicini, S.; Castellano, M. Preparation of composite alginate-based electrospun membranes loaded with ZnO nanoparticles. Carbohydr. Polym. 2020, 227, 115371. [Google Scholar] [CrossRef] [PubMed]
- Israr-Qadir, M.; Jamil-Rana, S.; Nur, O.; Willander, M.; Lu, J.; Hultman, L. Cathodo luminescence characterization of ZnO nanorods synthesized by chemical solution and of its conversion to ellipsoidal morphology. J. Mater. Res. 2014, 29, 2425–2431. [Google Scholar] [CrossRef]
- Israr-Qadir, M.; Jamil-Rana, S.; Nur, O.; Willander, M. Zinc oxide-based self-powered potentiometric chemical sensors for biomolecules and metal ions. Sensors 2017, 17, 1645. [Google Scholar] [CrossRef]
- Prkic, A.; Vukusic, T.; Giljanovic, J.; Sokol, V.; Boskovic, P.; Lavcevic, M.L.; Mitar, I.; Jakic, M. Development of a New Potentiometric Sensor based on homemade Iodide ISE Enriched with ZnO Nanoparticles and its Application for Determination of Penicillamine. Int. J. Electrochem. Sci. 2018, 13, 10894–10903. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Fahimmunisha, B.A.; Ishwarya, R.; AlSalhi, M.S.; Devanesan, S.; Govindarajan, M.; Vaseeharan, B. Green fabrication, characterization and antibacterial potential of zinc oxide nanoparticles using Aloe socotrina leaf extract: A novel drug delivery approach. J. Drug Deliv. Sci. Tech. 2020, 55, 101465. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.Y.; Sebastian, N. A novel electrochemical sensor based on flower shaped zinc oxide nanoparticles for the efficient detection of dopamine. Int. J. Electrochem. Sci. 2018, 13, 1542–1555. [Google Scholar] [CrossRef]
- Pechenkina, I.A.; Mikhelson, K.N. Materials for the ionophore-based membranes for ion-selective electrodes: Problems and achievements. Russ. J. Electrochem. 2015, 51, 93–102. [Google Scholar] [CrossRef]
- Tumur, M.; Kanberoglu, G.S.; Coldur, F. A novel potentiometric PVC-membrane cysteamine-selective electrode based on cysteamine-phosphomolybdate ion-pair. Curr. Pharm. Anal. 2020, 16, 168–175. [Google Scholar] [CrossRef]
- Soleymanpour, A.; Shafaatian, B.; Kor, K.; Hasaninejad, A.R. Coated wire lead (II)-selective electrode based on a Schiff base ionophore for low concentration measurements. Mon. Für Chem.-Chem. Mon. 2012, 143, 181–188. [Google Scholar] [CrossRef]
- Elashery, S.E.; Frag, E.Y.; Mousa, M.G. A comparative study of tetra-n-butylammonium bromide potentiometric selective screen printed, carbon paste and carbon nanotube modified graphite sensors. J. Iran. Chem. Soc. 2020, 17, 911–921. [Google Scholar] [CrossRef]
- Minocycline Hydrochloride monograph for professionals. Drugs. Am. Soc. Health-Syst. Pharm. 2019.
- Mohite, P.B.; Pandhare, R.B.; Khanage, S.G.; Bhaskar, V.H. Derivative spectrophotometric estimation of minocycline hydrochloride in bulk and pharmaceutical dosage forms. Int. J. PharmTech. Res. 2010, 2, 177–180. [Google Scholar]
- Kondaiah, S.; Surendra Babu, M.S.; Surya Narayana Rao, V. Spectrophotometric determination of demeclocycline and minocycline using molybdenum (VI). Asian J. Res. Chem. 2011, 4, 1587–1590. [Google Scholar]
- Gao, Y.X.; Jiang, J.G.; Du, Z.H. Determination of capsule and tablet of Minocycline hydrochloride and its related substances by HPLC. West China J. Pharm. Sci. 2005, 20, 531. [Google Scholar]
- Zhou, H.; Liu, Y.; Wang, D.K. Determination of minocycline hydrochloride for injection by RP-HPLC. Chinese J. Hosp. Pharm. 2003, 23, 515–516. [Google Scholar]
- Colovic, M.; Caccia, S. Liquid chromatographic determination of minocycline in brain-to-plasma distribution studies in the rat. J. Chromatogr. B 2003, 791, 337–343. [Google Scholar] [CrossRef]
- Jain, N.; Jain, G.K.; Ahmad, F.J.; Khar, R.K. Validated stability-indicating densitometric thin-layer chromatography: Application to stress degradation studies of minocycline. Anal. Chim. Acta 2007, 599, 302–309. [Google Scholar] [CrossRef]
- Tanase, I.G.; David, I.G.; Radu, G.L.; Iorgulescu, E.E.; Litescu, S. Electrochemical determination of minocycline in pharmaceutical preparations. Analusis 1998, 26, 175–178. [Google Scholar] [CrossRef]
- Di, J.; Xu, X.; Luo, J. Determination of minocycline by semi differential cyclic voltammetry at a liquid/liquid interface. Anal. Lett. 1996, 29, 2691–2700. [Google Scholar] [CrossRef]
- Alwan, R.M.; Kadhim, Q.A.; Sahan, K.M.; Ali, R.A.; Mahdi, R.J.; Kassim, N.A.; Jassim, A.N. Synthesis of zinc oxide nanoparticles via sol–gel route and their characterization. Nanosci. Nanotech. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Rajendraprasad, N.; Basavaiah, K. Development of membrane electrodes for selective determination of lisinopril in pharmaceuticals. J. Anal. Sci. Technol. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Rani, S.; Singh, G. Ion selective electrodes for potentiometric determination of cyclizine in its pharmaceutical dosage form. Asian J. Pharm. Sci. Technol. 2016, 6, 120–126. [Google Scholar]
- Bakker, E.; Pretsch, E.; Buhlmann, P. Selectivity of potentiometric ion sensors. Anal. Chem. 2000, 72, 1127–1133. [Google Scholar] [CrossRef]
- Hedayati, K. Fabrication and optical characterization of zinc oxide nanoparticles prepared via a simple sol-gel method. J. Nanostructures 2015, 5, 395–401. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; White, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO nanostructures using sol-gel method. Proced. Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Rana, S.B.; Singh, P.; Sharma, A.K.; Carbonari, A.W.; Dogra, R. Synthesis and characterization of pure and doped ZnO nanoparticles. J. Optoelectron. Adv. Mater. 2010, 12, 257–261. Available online: https://www.ipen.br/biblioteca/2010/16402.pdf (accessed on 15 February 2017).
- Shashanka, R.; Esgin, H.; Yilmaz, V.M.; Caglar, Y. Fabrication and characterization of green synthesized ZnO nanoparticle based dye-sensitized solar cell. J. Sci. Adv. Mater. Dev. 2020, 5, 185–191. [Google Scholar] [CrossRef]
- El-Tohamy, M.; Razeq, S.; Shalaby, A.A. Novel-Selective Bromazepam Membrane Sensors: Application Pharmaceutical Preparations and Biological Fluids. Asian J. Chem. 2012, 24, 1341–1347. [Google Scholar]
- Alarfaj, N.A.; El-Tohamy, M.F. Ultrasensitve Modified Carbon Paste Inclusion β-Cyclodextrin and Carbon Nanotubes Sensors for Electrochemical Detection of Anticancer Nimustine Hydrochloride. Int. J. Electrochem. Sci. 2016, 11, 1184–1198. [Google Scholar]
- Asefa, T.; Duncan, C.T.; Sharma, K.K. Recent advances in nanostructured chemosensors and biosensors. Analyst 2009, 134, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Sanjay, S.S.; Pandey, A.C.; Ankit, P.; Chattopadhyaya, M.C. Fabrication of surfactant sensing membrane with ZnO Nano-composite. Proc. Natl. Acad. Sci. India Sec. A 2013, 83, 279–285. [Google Scholar] [CrossRef]
- Isa, I.M.; Sohaimi, N.M.; Hashim, N.; Kamari, A.; Mohamed, A.; Ahmad, M.; Ghani, S.A. Determination of salicylate ion by potentiometric membrane electrode based on inc aluminium layered double hydroxides-4 (2,4-dichlorophenoxy) butyrate nanocomposites. Int. J. Electrochem. Sci. 2013, 8, 2112–2121. [Google Scholar]
- ICH Expert Working Group. ICH-Q2 (R1) Validation and Analytical Procedures: Text and Methodology. In Proceedings of the International Conference on Harmonization Guidelines, Geneva, Switzerland, November 2005. [Google Scholar]
- Miller, J.C.; Miller, J.N. Statistics for Analytical Chemistry, 3rd ed.; Ellis Horwood PTR Prentice Hall: New York, NY, USA, 1993. [Google Scholar]



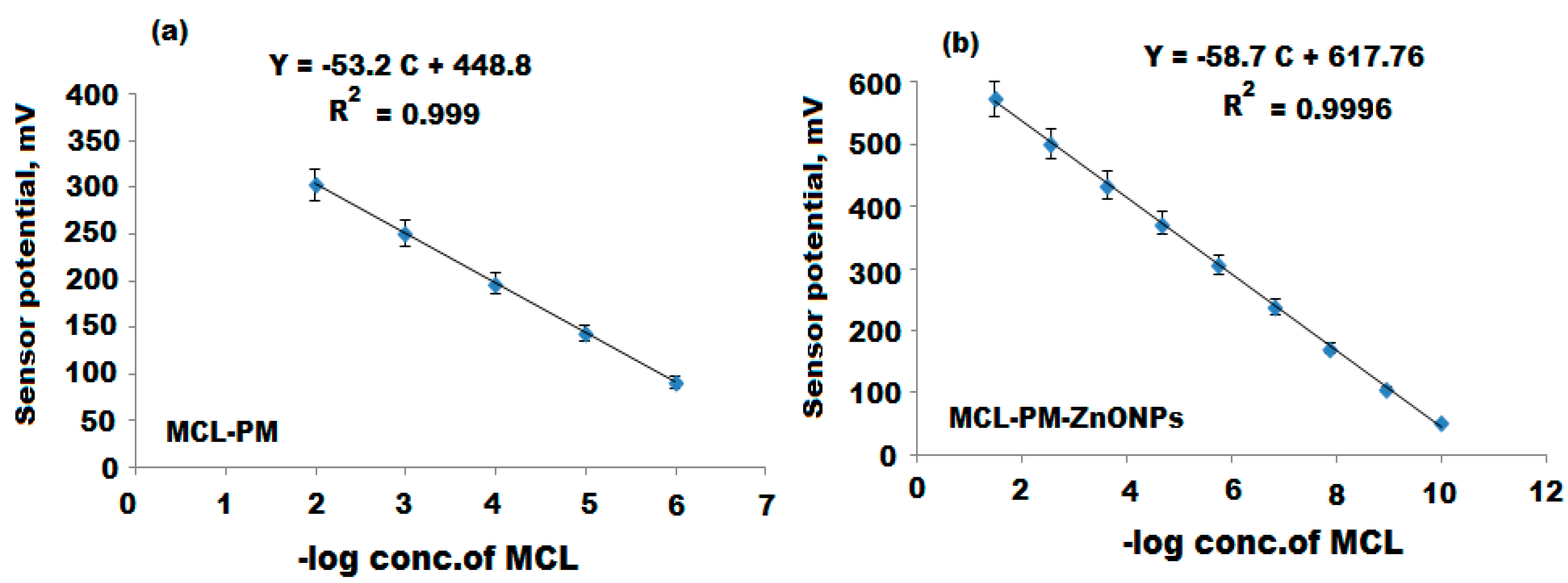
| Parameter | Conventional MCL-PM Coated Wire Sensor | Modified MCL-PM-ZnONPs Coated Wire Sensor |
|---|---|---|
| Slope (mV. Decade−1) | 53.2 ± 0.5 | 58.7 ± 0.2 |
| Intercept | 448.8 | 617.7 |
| Regression equation | EmV = (53.2 ± 0.5) log [MCL]+448.8 | EmV = (58.7 ± 0.2) log [MCL]+617.7 |
| Correlation coefficient, r | 0.999 | 0.9996 |
| Linear range (mol L−1) | 10 × 10−6–1.0 × 10−2 | 1.0 × 10−10–1.0 × 10−2 |
| LOD (mol L−1) | 4.9 × 10−7 | 5.0 × 10−11 |
| Response time/s | 60 | 30 |
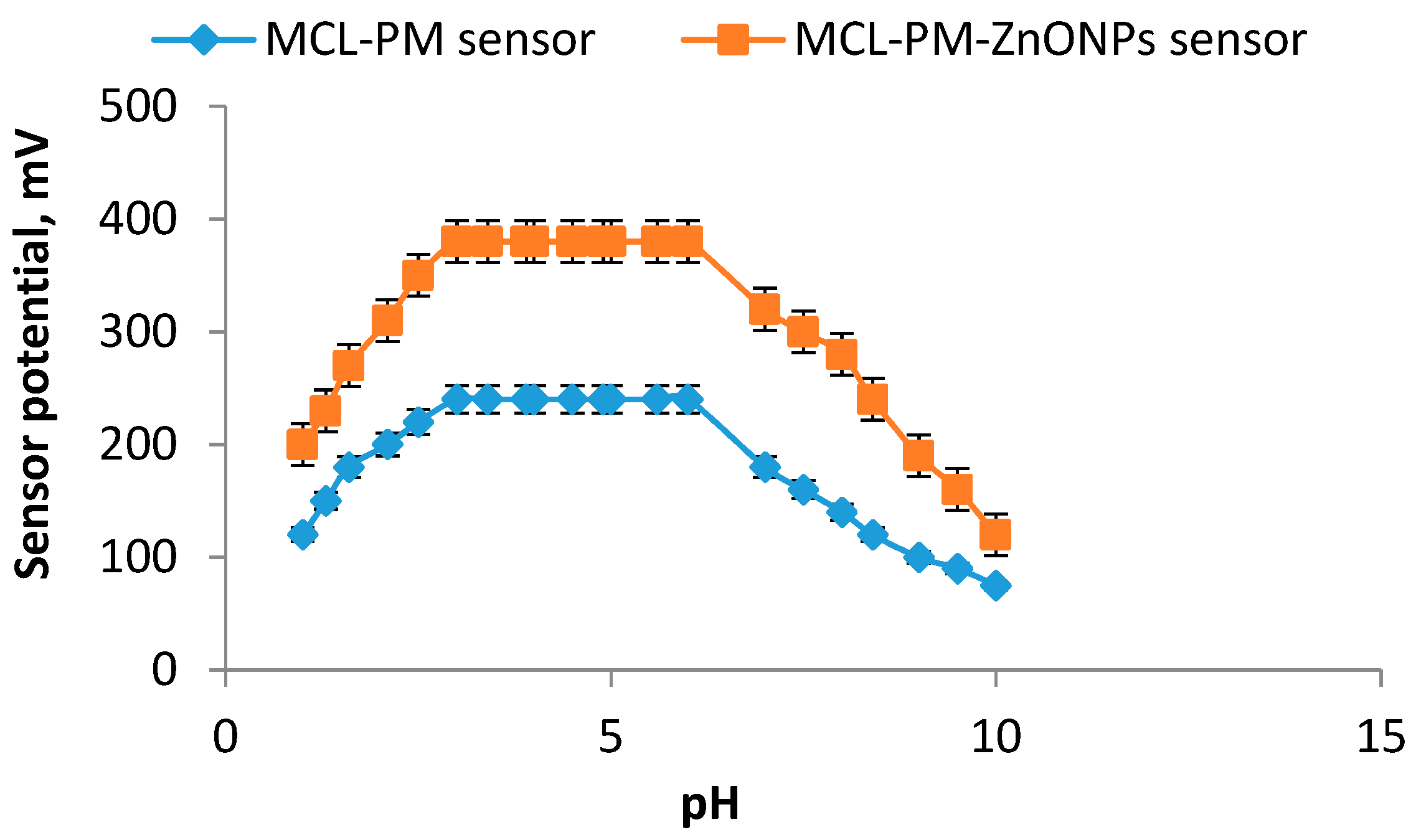
| Working pH range | 3–5 | 3–5 |
| Lifetime/day | 20 | 50 |
| Temperature, °C | 25 | 25 |
| Accuracy (%) | 99.22 ± 0.6 | 99.57 ± 0.4 |
| Interferences | Conventional Coated Wire MCL-PM Sensor (Kpot MCL+) | Modified MCL-PM-ZnONPs Sensor (Kpot MCL+) |
|---|---|---|
| Na+ | 6.2 × 10−3 | 4.7 × 10−6 |
| K+ | 2.4 × 10−3 | 3.1 × 10−4 |
| Ag+ | 7.7 × 10−3 | 6.1 × 10−5 |
| Ni2+ | 1.2 × 10−3 | 9.4 × 10−6 |
| Cu2+ | 5.0 × 10−3 | 2.9 × 10−4 |
| Zn2+ | 6.9 × 10−3 | 6.2 × 10−3 |
| Mg2+ | 3.3 × 10−3 | 9.1 × 10−4 |
| Fe3+ | 5.7 × 10−3 | 4.2 × 10−4 |
| L-histidine | 1.3 × 10−3 | 5.2 × 10−5 |
| Ornithine | 4.4 × 10−3 | 8.4 × 10−5 |
| Glycine | 5.6 × 10−3 | 3.3 × 10−5 |
| Maize starch | 5.1 × 10−3 | 8.7 × 10−4 |
| Lactose | 1.2 × 10−3 | 2.6 × 10−5 |
| Glucose | 2.8 × 10−3 | 8.7 × 10−6 |
| Talc | 3.5 × 10−3 | 6.8 × 10−4 |
| SiO2 | 7.1 × 10−3 | 1.8 × 10−6 |
| TiO2 | 4.6 × 10−3 | 7.4 × 10−4 |
| Magnesium stearate | 2.9 × 10−3 | 9.0 × 10−5 |
| Microcrystalline cellulose | 8.4 × 10−3 | 2.7 × 10−4 |
| Conventional MCL-PM Coated Wire Sensor | Modified MCL-PM ZnONPs Coated Wire Sensor | ||
|---|---|---|---|
| * Test Solution | % Recovery ± SD | * Test solution | % Recovery ± SD |
| 6.0 | 100.0 ± 0.1 | 10.0 | 99.9 ± 0.4 |
| 5.3 | 99.6 ± 0.3 | 9.0 | 99.7 ± 0.2 |
| 5.0 | 99.8 ± 0.3 | 8.0 | 100.0 ± 0.6 |
| 4.3 | 98.1 ± 0.7 | 7.0 | 99.3 ± 0.7 |
| 4.0 | 98.8 ± 0.5 | 6.0 | 99.7 ± 0.9 |
| 3.3 | 98.8 ± 0.2 | 5.0 | 100.0 ± 0.4 |
| 3.0 | 99.7 ± 0.9 | 4.0 | 99.3 ± 0.1 |
| 2.3 | 98.7 ± 0.5 | 3.0 | 98.7 ± 0.8 |
| 2.0 | 99.5 ± 0.3 | 2.0 | 99.5 ± 0.3 |
| Modified MCL-PM-ZnONPs Coated Wire Sensor | |||
|---|---|---|---|
| Intra-Day Assay | Inter-Day Assay | ||
| * Test Solution | % Recovery ± SD | * Test Solution | % Recovery ± SD |
| 9.0 | 99.7 ± 0.3 | 9.0 | 99.7 ± 0.8 |
| 6.0 | 99.3 ± 0.4 | 6.0 | 99.3 ± 1.0 |
| 3.0 | 100.0 ± 0.2 | 3.0 | 100.0 ± 0.6 |
| Conventional MCL-PM Coated Wire Sensor | Modified MCL-PM ZnONPs Coated Wire Sensor | |||
|---|---|---|---|---|
| * Test solution | % Recovery | * Test solution | % Recovery | |
| 6.0 | 99.7 ± 0.3 | 7.0 | 99.4 ± 0.1 | Reported Method [24] |
| 5.3 | 99.2 ± 0.6 | 6.0 | 99.7 ± 0.4 | |
| 5.0 | 99.4 ± 0.2 | 5.0 | 99.8 ± 0.3 | |
| 4.0 | 100.0 ± 0.1 | 4.0 | 100.0 ± 0.1 | |
| 3.0 | 99.7 ± 0.6 | 3.0 | 100.0 ± 0.2 | |
| 2.0 | 99.0 ± 0.4 | 2.0 | 100.0 ± 0.5 | |
| 99.50 ± 0.4 0.700 (2.228) *** 1.78 (5.05) *** | 99.82 ± 0.2 1.248 (2.228) *** 2.25 (5.05) *** | 99.64 ± 0.36 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mohaimeed, A.M.; A. Al-Onazi, W.; El-Tohamy, M.F. Utility of Zinc Oxide Nanoparticles Catalytic Activity in the Electrochemical Determination of Minocycline Hydrochloride. Polymers 2020, 12, 2505. https://doi.org/10.3390/polym12112505
Al-Mohaimeed AM, A. Al-Onazi W, El-Tohamy MF. Utility of Zinc Oxide Nanoparticles Catalytic Activity in the Electrochemical Determination of Minocycline Hydrochloride. Polymers. 2020; 12(11):2505. https://doi.org/10.3390/polym12112505
Chicago/Turabian StyleAl-Mohaimeed, Amal M., Wedad A. Al-Onazi, and Maha F. El-Tohamy. 2020. "Utility of Zinc Oxide Nanoparticles Catalytic Activity in the Electrochemical Determination of Minocycline Hydrochloride" Polymers 12, no. 11: 2505. https://doi.org/10.3390/polym12112505
APA StyleAl-Mohaimeed, A. M., A. Al-Onazi, W., & El-Tohamy, M. F. (2020). Utility of Zinc Oxide Nanoparticles Catalytic Activity in the Electrochemical Determination of Minocycline Hydrochloride. Polymers, 12(11), 2505. https://doi.org/10.3390/polym12112505






