Poly(N,N′-Diethylacrylamide)-Based Thermoresponsive Hydrogels with Double Network Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogels Synthesis
2.2. Differential Scanning Calorimentry
2.3. 1H NMR Spectroscopy
2.4. UV-Vis Experiments
2.5. Swelling Behaviour
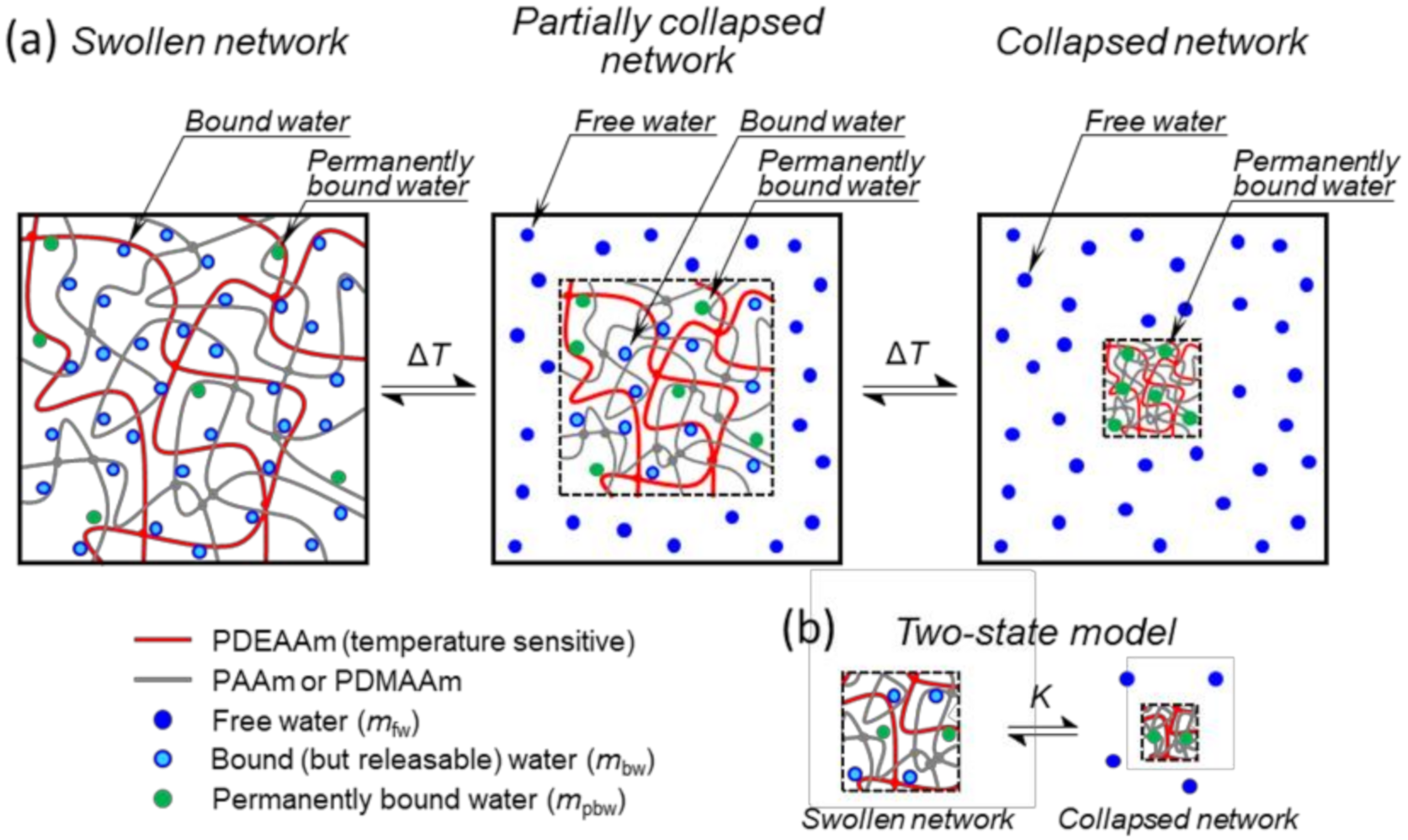
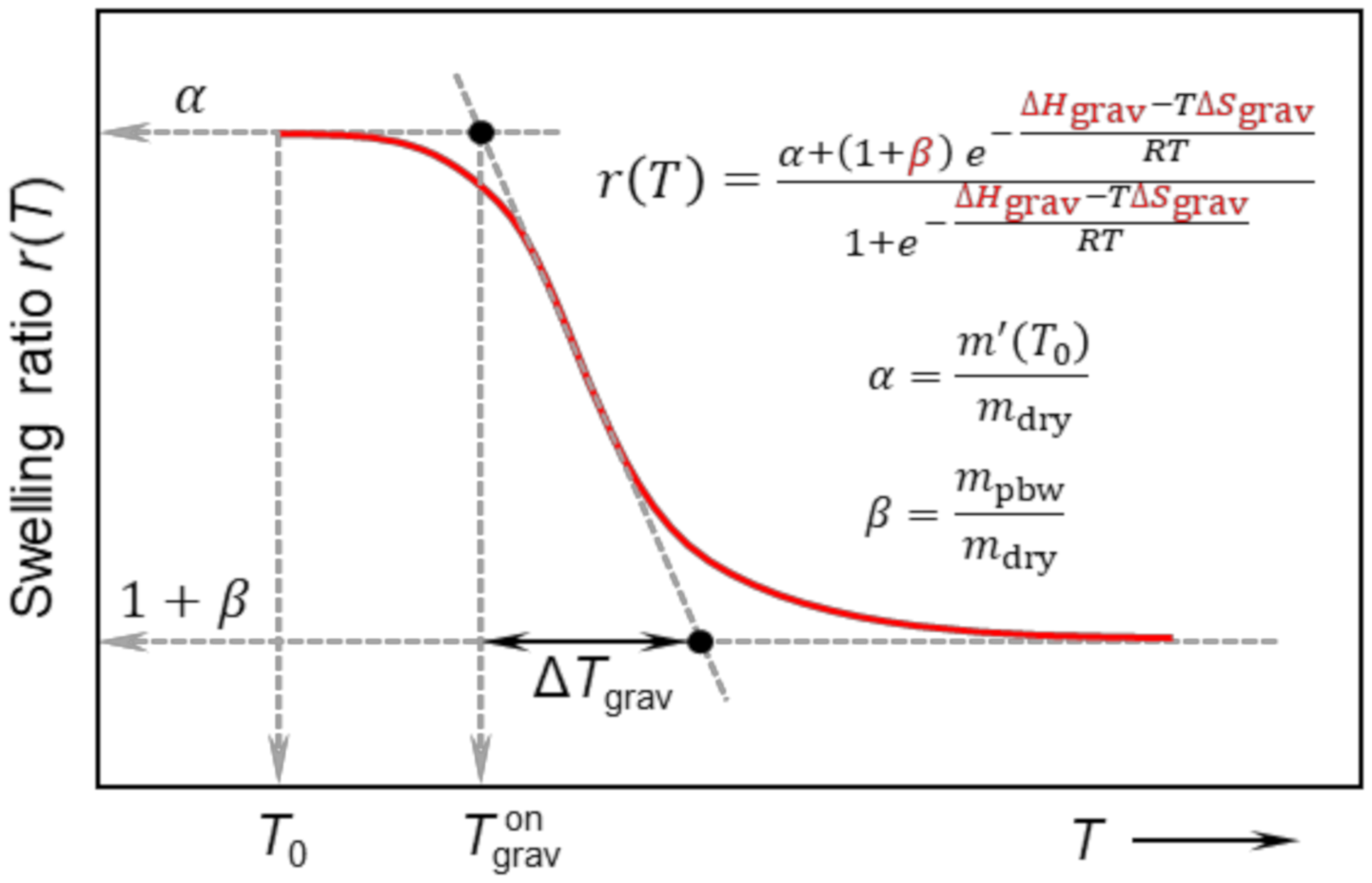
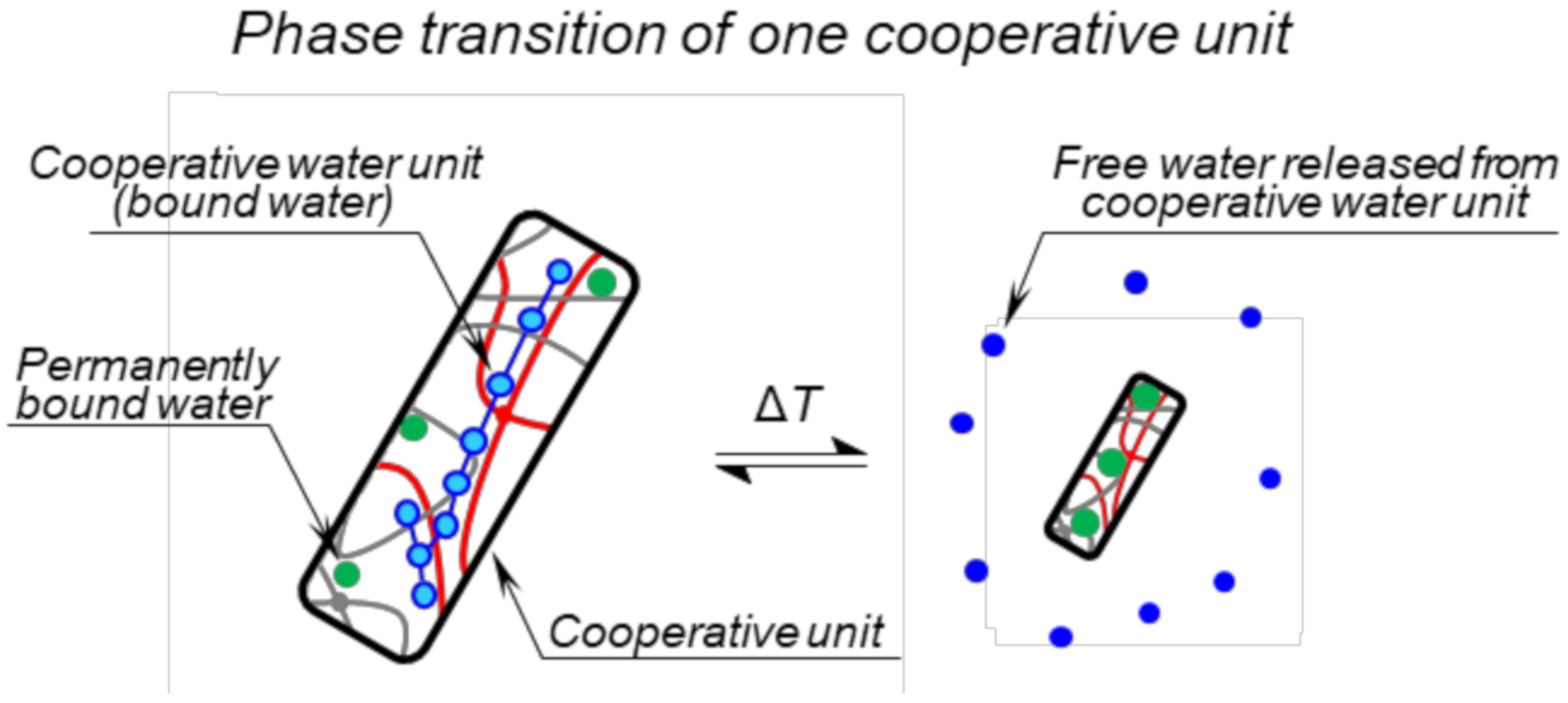
2.6. Thermodynamic Model of Deswelling
3. Results and Discussion
3.1. Swelling Behaviour
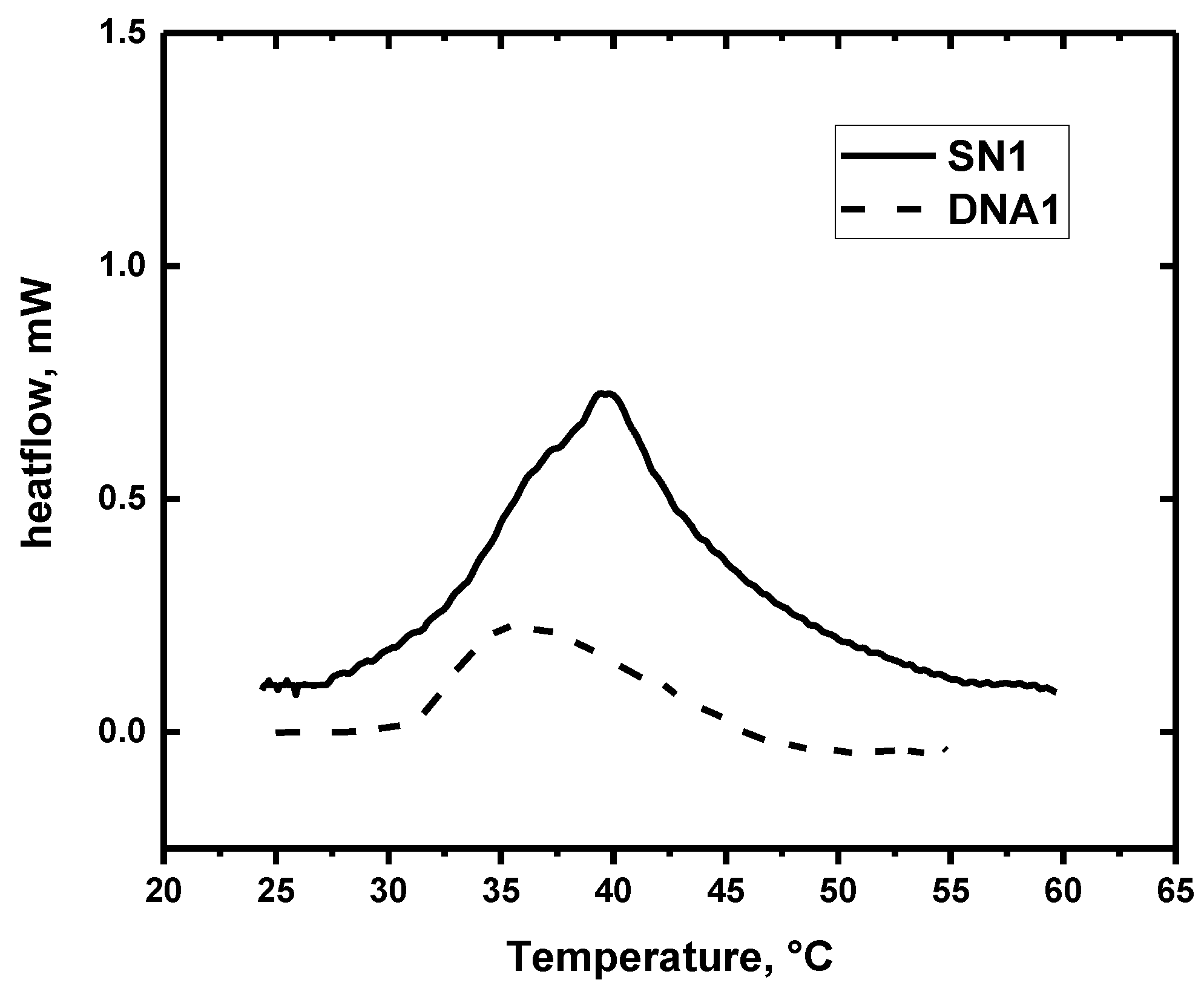
3.2. Differential Scanning Calorimetry
3.3. NMR
3.4. NMR Relaxation
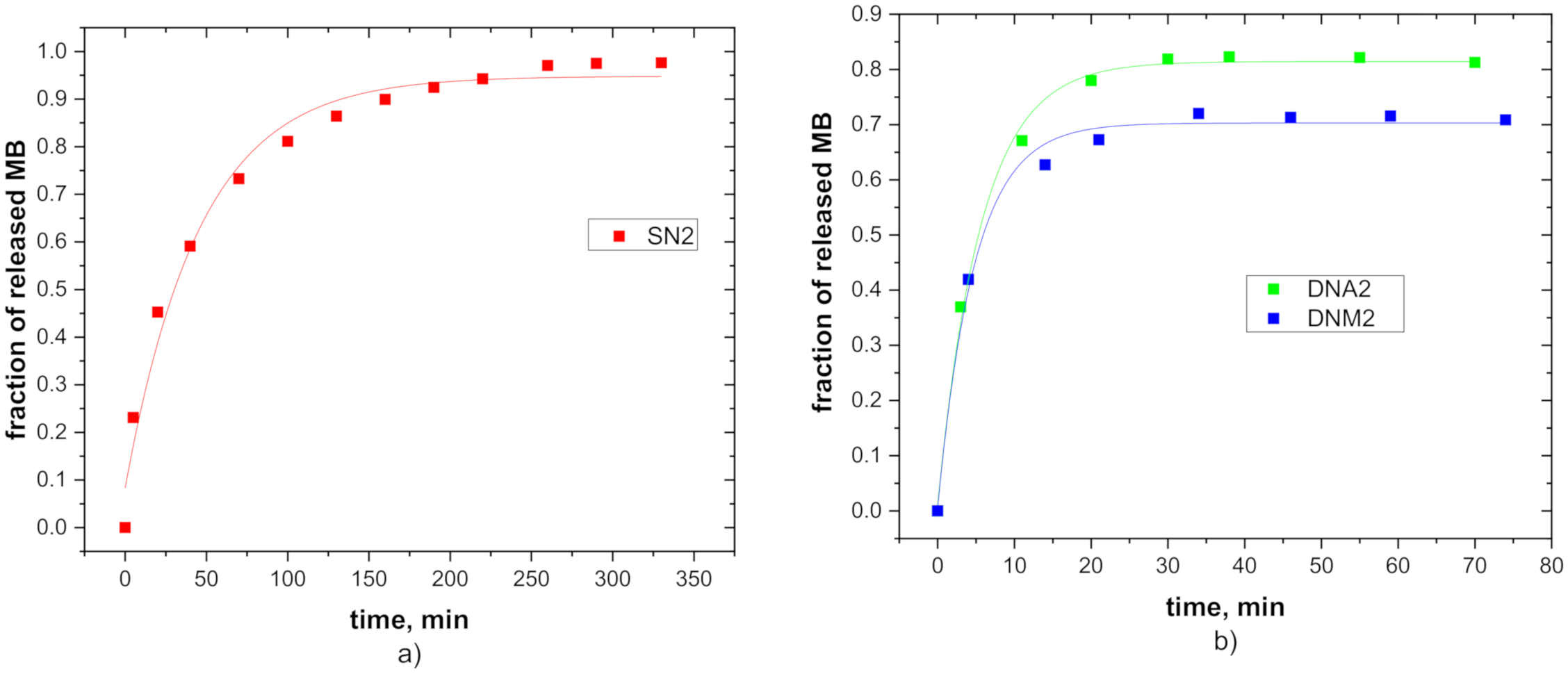
3.5. Release Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, N.; Zinchenko, A.A.; Kidoaki, S.; Murata, S.; Yoshikawa, K. Thermo-Switching of the Conformation of Genomic DNA in Solutions of Poly(N-isopropylacrylamide). Langmuir 2010, 26, 2995–2998. [Google Scholar] [CrossRef]
- Liu, L.; Wang, W.; Ju, X.J.; Xie, R.; Chu, L.Y. Smart thermo-triggered squirting capsules for nanoparticle delivery. Soft Matter 2010, 6, 3759–3763. [Google Scholar] [CrossRef]
- Dong, L.; Jiang, H. Autonomous microfluidics with stimuli-responsive hydrogels. Soft Matter 2007, 3, 1223–1230. [Google Scholar] [CrossRef]
- Asoh, T.A.; Matsusaki, M.; Kaneko, T.; Akashi, M. Fabrication of temperature-responsive bending hydrogels with a nanostructured gradient. Adv. Mater. 2008, 20, 2080–2083. [Google Scholar] [CrossRef]
- Takashima, Y.; Hatanaka, S.; Otsubo, M.; Nakahata, M.; Kakuta, T.; Hashidzume, A.; Yamaguchi, H.; Harada, A. Expansion-contraction of photoresponsive artificial muscle regulated by host-guest interactions. Nat. Commun. 2012, 3, 1270. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Seuring, J.; Agarwal, S. Polymers with Upper Critical Solution Temperature in Aqueous Solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Vancoillie, G.; Frank, D.; Hoogenboom, R. Thermoresponsive poly(oligo ethylene glycol acrylates). Prog. Polym. Sci. 2014, 39, 1074–1095. [Google Scholar] [CrossRef]
- Halperin, A.; Kroger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) Phase Diagrams: Fifty Years of Research. Angew. Chem. Int. Ed. 2015, 54, 15342–15367. [Google Scholar] [CrossRef]
- Fujishige, S.; Kubota, K.; Ando, I. Phase transition of aqueous solutions of poly(N-isopropylacrylamide) and poly(N-isopropylmethacrylamide). J. Phys. Chem. 1989, 93, 3311–3313. [Google Scholar] [CrossRef]
- Gong, J.P.; Kurokawa, T.; Narita, T.; Kagata, G.; Osada, Y.; Nishimura, G.; Kinjo, M. Synthesis of hydrogels with extremely low surface friction. J. Am. Chem. Soc. 2001, 123, 5582–5583. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Haque, M.A.; Kurokawa, T.; Gong, J.P. Super tough double network hydrogels and their application as biomaterials. Polymer 2012, 53, 1805–1822. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zhu, L.; Qin, G.; Chen, Q. Double network hydrogels with controlled shape deformation: A mini review. J. Polym. Sci. Part B Polym. Phys. 2018, 56, 1351–1362. [Google Scholar] [CrossRef]
- Song, X.F.; Chu, Y.Y. Preparation and characterization of poly(sodium acrylate/cement clinker) DN hydrogel composites. Polym. Comp. 2019, 40, 2462–2472. [Google Scholar] [CrossRef]
- Xu, Y.W.; Chen, J.; Zhang, H.; Wei, H.; Zhou, L.J.; Wang, Z.W.; Pan, Y.X.; Su, X.Y.; Zhang, A.; Fu, J. White-light-emitting flexible display devices based on double network hydrogels crosslinked by YAG:Ce phosphors. J. Mater. Chem. C 2020, 8, 247–252. [Google Scholar] [CrossRef]
- Fei, R.; George, J.T.; Park, J.; Grunlan, M.A. Thermoresponsive nanocomposite double network hydrogels. Soft Matter 2012, 8, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Fei, R.; George, J.T.; Park, J.; Means, A.K.; Grunlan, M.A. Ultra-strong thermoresponsive double network hydrogels. Soft Matter 2013, 9, 2912–2919. [Google Scholar] [CrossRef]
- Fei, R.; Hou, H.; Munoz-Pinto, D.; Han, A.; Hahn, M.S.; Grunlan, M.A. Thermoresponsive double network micropillared hydrogels for controlled cell release. Macromol. Biosci. 2014, 14, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Fei, R.; Means, A.K.; Abraham, A.A.; Locke, A.K.; Coté, G.L.; Grunlan, M.A. Self-cleaning, thermoresponsive P(NIPAAm-co-AMPS) double network membranes for implanted glucose biosensors. Macromol. Mater. Eng. 2016, 301, 935–943. [Google Scholar] [CrossRef]
- Shen, J.; Li, N.; Ye, M. Preparation and characterization of dual-sensitive double network hydrogels with clay as a physical crosslinker. Appl. Clay Sci. 2015, 103, 40–45. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of pH- and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 2013, 33, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Kostanski, L.K.; Huang, R.; Ghosh, R.; Filipe, C.D.M. Biocompatible poly(N-vinyllactam)-based materials with environmentally-responsive permeability. J. Biomater. Sci. Polym. Ed. 2008, 19, 275–290. [Google Scholar] [CrossRef]
- Panayiotou, M.; Pöhner, C.; Vandevyver, C.; Wandrey, C.; Hilbrig, F.; Freitag, R. Synthesis and characterization of thermo-responsive poly(N,N-diethylacrylamide) microgels. React. Funct. Polym. 2007, 67, 807–819. [Google Scholar] [CrossRef]
- Hanyková, L.; Labuta, J.; Spěváček, J. NMR study of temperature-induced phase separation and polymer–solvent interactions in poly(vinyl methyl ether)/D2O/ethanol solutions. Polymer 2006, 47, 6107–6116. [Google Scholar] [CrossRef]
- Velychkivska, N.; Bogomolova, A.; Filippov, S.K.; Starovoytova, L.; Labuta, J. Thermodynamic and kinetic analysis of phase separation of temperature-sensitive poly(vinyl methyl ether) in the presence of hydrophobic tert-butyl alcohol. Colloid Polym. Sci. 2017, 295, 1419–1428. [Google Scholar] [CrossRef]
- Velychkivska, N.; Starovoytova, L.; Březina, V.; Hanyková, L.; Hill, J.P.; Labuta, J. Improving the colloidal stability of temperature-sensitive poly(N-isopropylacrylamide) solutions using low molecular weight hydrophobic additives. ACS Omega 2018, 3, 11865–11873. [Google Scholar] [CrossRef]
- Farrar, T.C.; Becker, E.D. Pulse and Fourier Transform NMR; Academic Press: New York, NY, USA, 1971. [Google Scholar]
- Sturtevant, J.M. Some Applications of Calorimetry in Biochemistry and Biology. Annu. Rev. Biophys. Bioeng 1974, 3, 35–51. [Google Scholar] [CrossRef]
- Sugar, I.P. Cooperativity and classification of phase transitions. Application to one- and two-component phospholipid membranes. J. Phys. Chem. 1987, 91, 95–101. [Google Scholar] [CrossRef]
- Stankowski, S.; Gruenewald, B. Evaluation of cooperativity for phase transitions in two- and three-dimensional systems. Biophys. Chem. 1980, 12, 167–176. [Google Scholar] [CrossRef]
- Krakovský, I.; Kouřilová, H.; Hrubovský, M.; Labuta, J.; Hanyková, L. Thermoresponsive double network hydrogels composed of poly(N-isopropylacrylamide) and polyacrylamide. Eur. Polym. J. 2019, 116, 415–424. [Google Scholar] [CrossRef]
- Gan, L.H.; Wensheng, C.; Tam, K.C. Studies of phase transition of aqueous solution of poly(N,N-diethylacrylamide-co-acrylic acid) by differential scanning calorimetry and spectrophotometry. Eur. Polym. J. 2001, 37, 1773–1778. [Google Scholar] [CrossRef]
- Hanyková, L.; Spěváček, J.; Radecki, M.; Zhigunov, A.; Kouřilová, H.; Sedláková, Z. Phase transition in hydrogels of thermoresponsive semi-interpenetrating and interpenetrating networks of poly(N,N-diethylacrylamide) and polyacrylamide. Eur. Polym. J. 2016, 85, 1–13. [Google Scholar] [CrossRef]
- Hanyková, L.; Spěváček, J.; Radecki, M.; Zhigunov, A.; Šťastná, J.; Valentová, H.; Sedláková, Z. Structures and interactions in collapsed hydrogels of thermoresponsive interpenetrating polymer networks. Colloid Polym. Sci. 2015, 293, 709–720. [Google Scholar] [CrossRef]
- Spěváček, J.; Hanyková, L. 1H NMR study on the hydration during temperature-induced phase separation in concentrated poly(vinyl methyl ether)/D2O solutions. Macromolecules 2005, 38, 9187–9191. [Google Scholar] [CrossRef]
- Hanyková, L.; Spěváček, J.; Ilavský, M. 1H NMR study of thermotropic phase transition of linear and crosslinked poly(vinyl methyl ether) in D2O. Polymer 2001, 42, 8607–8612. [Google Scholar] [CrossRef]
- Díez-Peña, E.; Quijada-Garrido, I.; Barrales-Rienda, J.M.; Wilhelm, M.; Spiess, H.W. NMR studies of the structure and dynamics of polymer gels based on N-isopropylacrylamide (N-iPAAm) and methacrylic acid (MAA). Macromol. Chem. Phys. 2002, 203, 491–502. [Google Scholar] [CrossRef]
- Wang, N.; Ru, G.; Wang, L.; Feng, J. 1H MAS NMR studies of the phase separation of poly(N-isopropylacrylamide) gel in binary solvents. Langmuir 2009, 25, 5898–5902. [Google Scholar] [CrossRef] [PubMed]
- Alam, T.M.; Childress, K.K.; Pastoor, K.; Rice, C.V. Characterization of free, restricted, and entrapped water environments in poly(N-isopropyl acrylamide) hydrogels via 1H HRMAS PFG NMR spectroscopy. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1521–1527. [Google Scholar] [CrossRef]



| Sample | cDEAAm (g.L−1) | cMBAAm (g.L−1) | cAPS (g.L−1) | cTEMED (g.L−1) | DEAAm/MBAAm |
|---|---|---|---|---|---|
| SN1 | 127.2 | 2.0 | 1 | 15 | 77 |
| SN2 SN3 | 127.2 127.2 | 1.2 0.4 | 1 1 | 15 15 | 128 385 |
| Sample | ΔHgrav (kJ.mol−1) | ΔSgrav (J.mol−1. K−1) | α | β | γ | ||
|---|---|---|---|---|---|---|---|
| SN1 | 246 | 832 | 11.1 | 2.93 | 0.150 | 21 | 10 |
| SN2 SN3 DNA1 DNA2 DNA3 DNM1 DNM2 DNM3 | 284 289 211 199 152 141 115 90 | 954 971 694 652 505 460 374 295 | 14.6 21.5 6.98 8.81 14.5 17.6 20.5 33.7 | 4.41 4.28 2.70 4.37 9.12 7.93 10.3 18.7 | 0.162 0.098 0.453 0.539 0.672 0.450 0.429 0.572 | 23 24 25 25 24 28 26 24 | 8 8 14 15 19 16 21 28 |
| Sample | ΔHDSC-ms (J.g−1) | ΔHDSC-pn (J.g−1) | Nwcu | ||
|---|---|---|---|---|---|
| SN1 SN2 SN3 DNA1 DNA2 DNA3 DNM1 DNM2 DNM3 | 2.10 1.86 0.85 0.53 0.29 0.20 0.24 0.27 0.15 | 19 23 23 3.2 2.5 2.6 0.2 0.3 0.03 | 30 31 31 32 34 32 32 33 32 | 39 40 35 36 39 37 42 37 45 | 5300 5350 16,600 10,400 14,900 12,900 16,100 10,500 13,900 |
| Sample | ΔHNMR (kJ.mol−1) | ΔSNMR (J.mol−1.K−1) | ΔTNMR (°C) | NA/NDEAAm | NM/NDEAAm | |
|---|---|---|---|---|---|---|
| SN1 SN2 SN3 DNA1 DNA2 DNA3 DNM1 DNM2 DNM3 | 398 422 387 324 320 333 289 276 245 | 1289 1374 1251 1043 1028 1078 926 884 786 | 32 32 33 33 33 32 34 34 32 | 8 7 8 10 10 10 11 12 13 | --- --- --- 2.4 2.7 4.9 --- --- --- | --- --- --- --- --- --- 2.0 2.2 3.6 |
| Sample | T2 (s) | |
|---|---|---|
| 17 °C | 45 °C | |
| SN1 SN2 SN3 DNA1 DNA2 DNA3 DNM1 DNM2 DNM3 | 2.8 4.4 4.1 4.3 3.9 3.0 3.8 3.1 3.7 | 2.2 * 0.1 * 4.8 * 0.03 * 5.1 * 0.04 * 3.9 * 0.9 * 3.3 2.7 4.1 3.8 4.3 |
| Sample | fMB | τ |
|---|---|---|
| (min) | ||
| SN1 SN2 SN3 DNA1 DNA2 DNA3 DNM1 DNM2 DNM3 | 0.93 0.96 0.91 0.85 0.81 0.89 0.69 0.71 0.76 | 64 59 55 9.5 7.2 9.1 8.3 8.4 12.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanyková, L.; Krakovský, I.; Šestáková, E.; Šťastná, J.; Labuta, J. Poly(N,N′-Diethylacrylamide)-Based Thermoresponsive Hydrogels with Double Network Structure. Polymers 2020, 12, 2502. https://doi.org/10.3390/polym12112502
Hanyková L, Krakovský I, Šestáková E, Šťastná J, Labuta J. Poly(N,N′-Diethylacrylamide)-Based Thermoresponsive Hydrogels with Double Network Structure. Polymers. 2020; 12(11):2502. https://doi.org/10.3390/polym12112502
Chicago/Turabian StyleHanyková, Lenka, Ivan Krakovský, Eliška Šestáková, Julie Šťastná, and Jan Labuta. 2020. "Poly(N,N′-Diethylacrylamide)-Based Thermoresponsive Hydrogels with Double Network Structure" Polymers 12, no. 11: 2502. https://doi.org/10.3390/polym12112502
APA StyleHanyková, L., Krakovský, I., Šestáková, E., Šťastná, J., & Labuta, J. (2020). Poly(N,N′-Diethylacrylamide)-Based Thermoresponsive Hydrogels with Double Network Structure. Polymers, 12(11), 2502. https://doi.org/10.3390/polym12112502






