Effect of Composition on the Crystallization, Water Absorption, and Biodegradation of Poly(ε-caprolactam-co-ε-caprolactone) Copolymers
Abstract
1. Introduction
2. Experiments
2.1. Materials
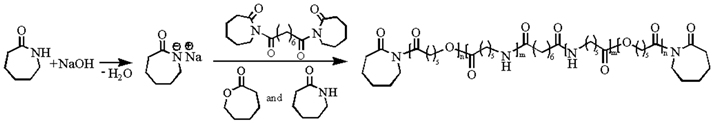
2.2. Synthesis of P(CLA-co-CLO) Copolymers
2.3. Water Absorption Test
2.4. Enzymatic Degradation
2.5. Characterizations
3. Results and Discussion
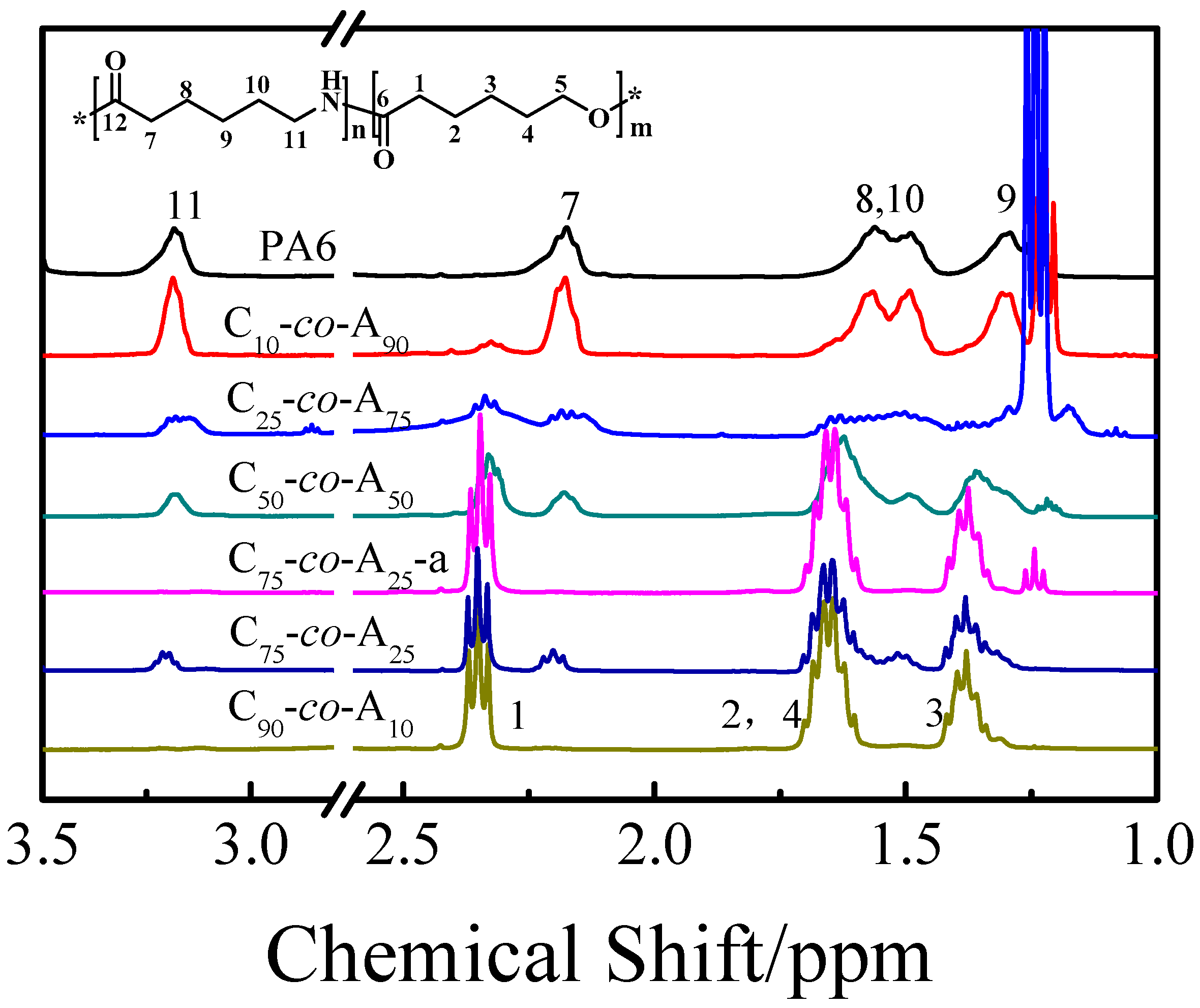
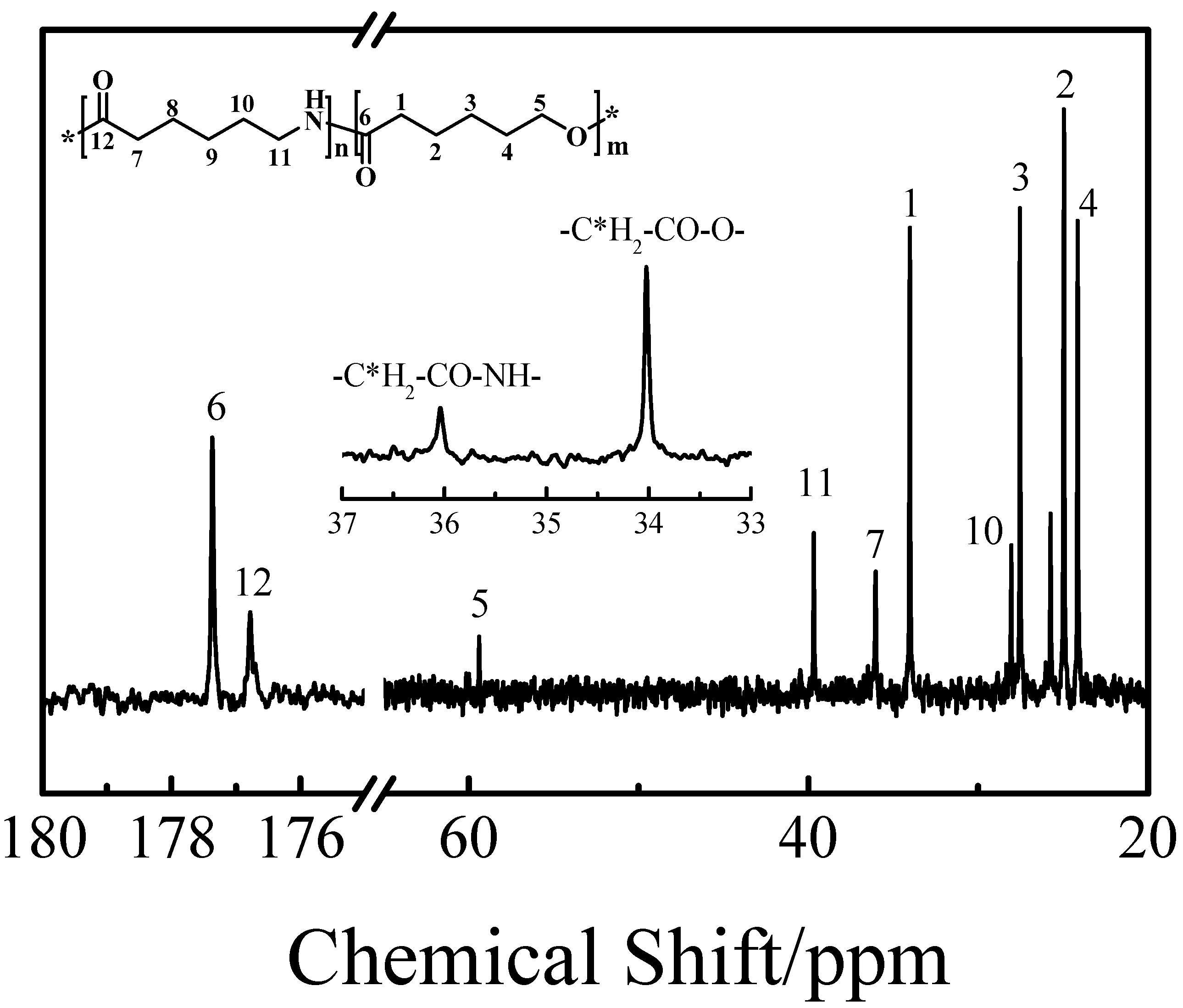
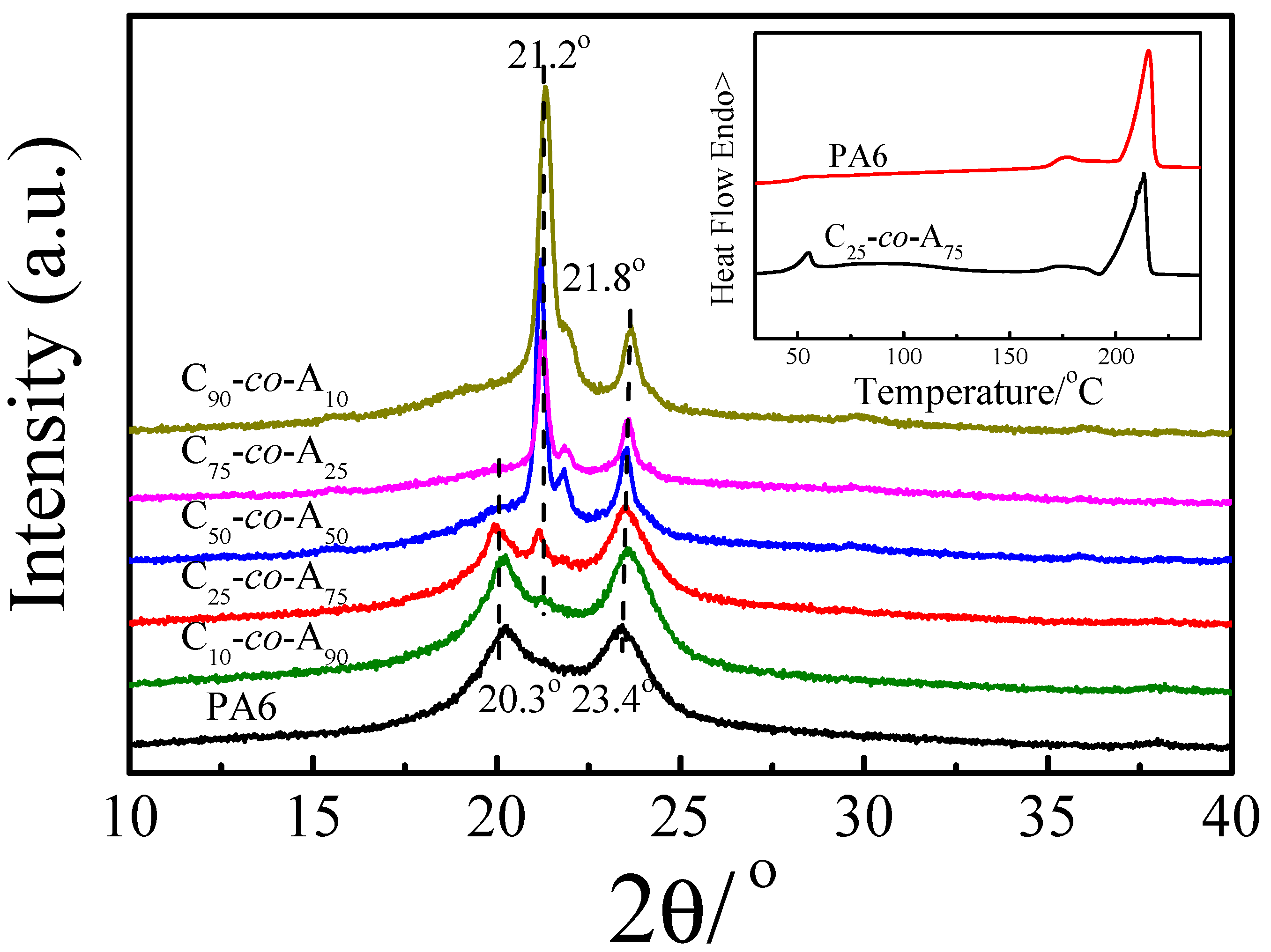
3.1. Structure Characterization
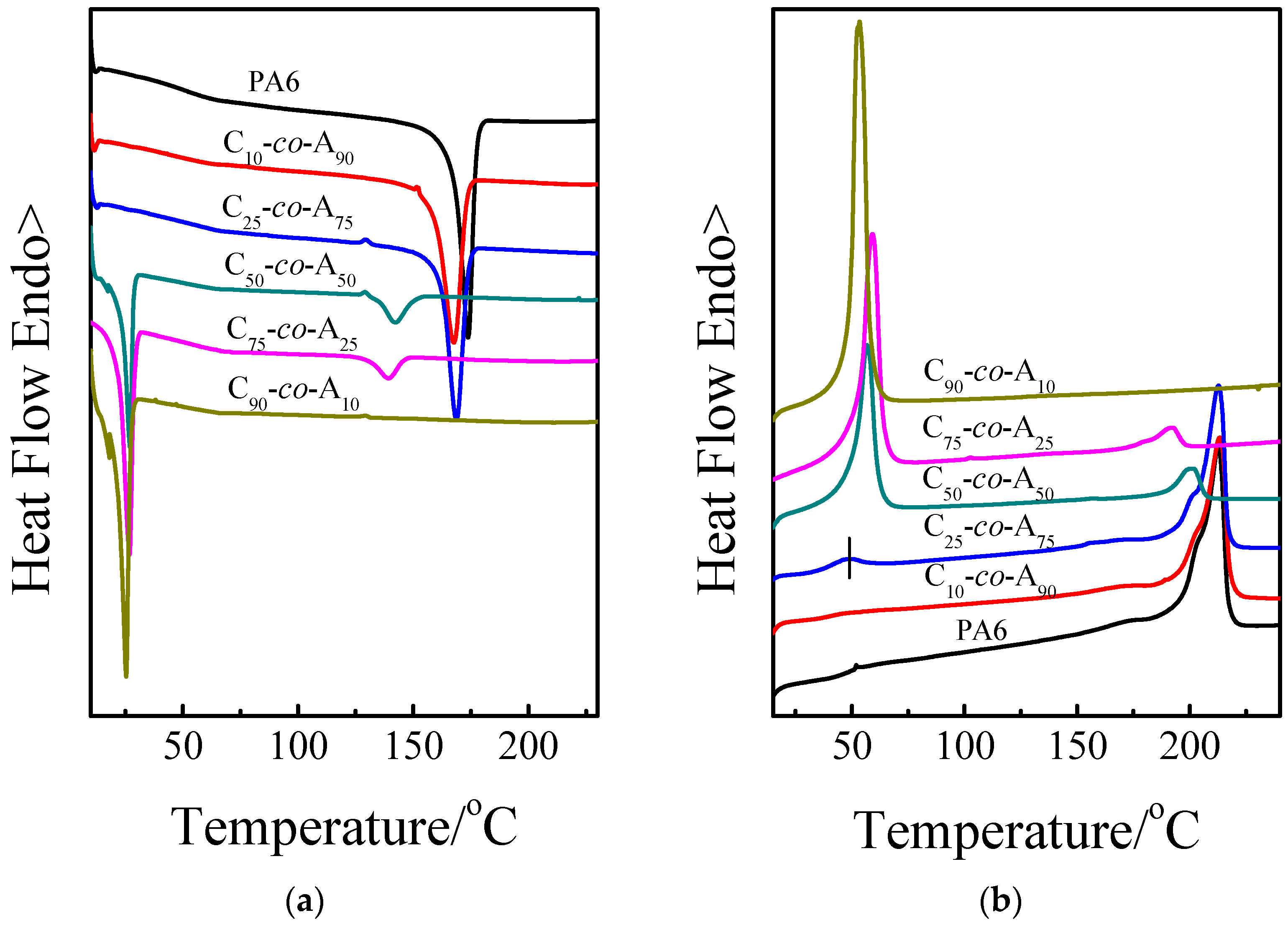
3.2. Crystallization Behavior
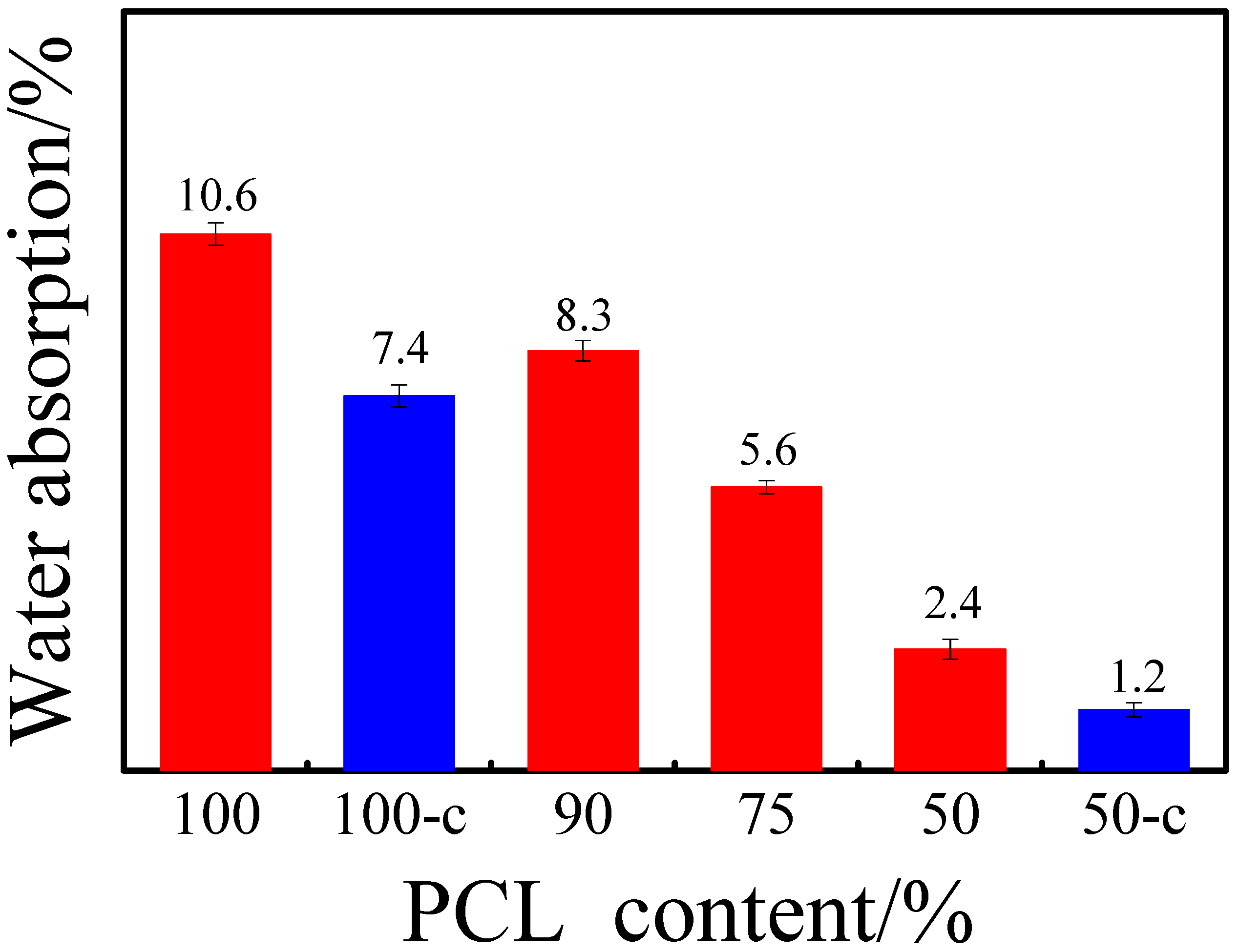
3.3. Water Absorption
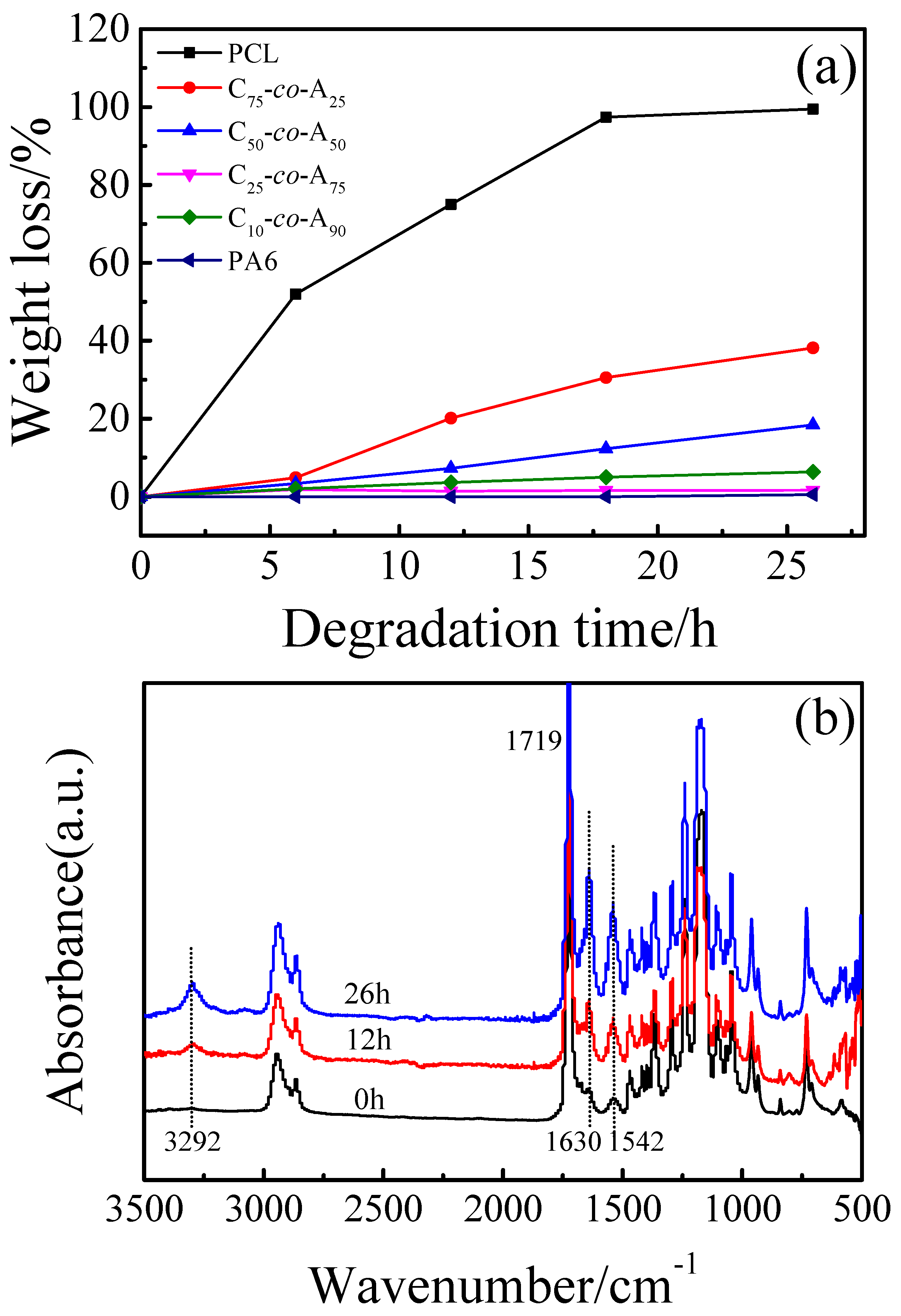
3.4. Biodegradation Behavior
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Becker, G.; Wurm, F.R. Functional biodegradable polymers via ring-opening polymerization of monomers without protective groups. Chem. Soc. Rev. 2018, 47, 7739–7782. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Dominguez-Candela, I.; Castello-Palacios, S.; Vallés-Lluch, A.; Fombuena, V. Development and characterization of polyester and acrylate-based composites with hydroxyapatite and halloysite nanotubes for medical applications. Polymers 2020, 12, 1703. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wu, Y.; Chen, Z.; Yang, T.; Cheng, Y.; Yu, H.; Huang, T. In-situ polymerization of high-molecular weight nylon 66 modified clay nanocomposites with low apparent viscosity. Polymers 2019, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.N.; Wu, C.M.; Lo, H.W.; Lai, C.C.; Teng, W.F.; Liu, L.C.; Chen, C.M. Synthesis and physical properties of non-crystalline nylon 6 containing dimer acid. Polymers 2019, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Michell, R.M.; Müller, A.J.; Castelletto, V.; Hamley, I.; Deshayes, G.L.; Dubois, P. Effect of sequence distribution on the morphology, crystallization, melting, and biodegradation of poly(ε-caprolactone-co-ε-caprolactam) copolymers. Macromolecules 2009, 42, 6671–6681. [Google Scholar] [CrossRef]
- Walha, F.; Lamnawar, K.; Maazouz, A.; Jaziri, M. Rheological, morphological and mechanical studies of sustainably sourced polymer blends based on poly(lactic acid) and polyamide 11. Polymers 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.N.V. Copolyesteramides—II anionic copolymers of ε-caprolactam with ε-caprolactone. Macromolecules 1984, 20, 529–537. [Google Scholar]
- Gonsalves, K.E.; Chen, X.; Cameron, J.A. Degradation of nonalternating poly(ester-amides). Macromolecules 1992, 25, 3309–3312. [Google Scholar] [CrossRef]
- Deshayes, G.; Delcourt, C.; Verbruggen, I.; Trouillet-Fonti, L.; Touraud, F.; Fleury, E.; Degée, P.; Destarac, M.; Willem, R.; Dubois, P. Novel polyesteramide-based diblock copolymers: Synthesis by ring-opening copolymerization and characterization. Macromol. Chem. Phys. 2009, 210, 1033–1043. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, C.G.; Zhao, J.B.; Zhang, Z.Y.; Yang, W.T. Synthesis and characterization of polyesteramides having short nylon-6 segments. Polymer 2011, 52, 6046–6055. [Google Scholar] [CrossRef]
- Sanchez-Sanchez, A.; Basterretxea, A.; Mantione, D.; Etxeberria, A.; Elizetxea, C.; Calle, A.; García-Arrieta, S.; Sardon, H.; Mecerreyes, D. Organic-acid mediated bulk polymerization of ε-caprolactam and its copolymerization with ε-caprolactone. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2394–2402. [Google Scholar] [CrossRef]
- Zeng, F.R.; Xu, J.; Sun, L.H.; Ma, J.; Jiang, H.; Li, Z.L. Copolymers of ε-caprolactone and ε-caprolactam via polyesterification: Towards sequence-controlled poly(ester amide)s. Polym. Chem. 2020, 11, 1211–1219. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Tang, L.; Huang, J.; Fang, Y.; Ji, P.; Wang, C.; Wang, H. A novel synthetic strategy for preparing polyamide 6 (PA6)-based polymer with transesterification. Polymers 2019, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Cakir, S.; Kierkels, R.; Koning, C. Polyamide 6-polycaprolactone multiblock copolymers: Synthesis, characterization, and degradation. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2823–2833. [Google Scholar] [CrossRef]
- Michell, R.M.; Müller, A.J.; Deshayes, G.; Dubois, P. Effect of sequence distribution on the isothermal crystallization kinetics and successive self-nucleation and annealing (SSA) behavior of poly(ε-caprolactone-co-ε-caprolactam) copolymers. Eur. Polym. J. 2010, 46, 1334–1344. [Google Scholar] [CrossRef]
- Deshayes, G.; Delcourt, C.; Verbruggen, I.; Trouillet-Fonti, L.; Touraud, F.; Fleury, E.; Degée, P.; Destarac, M.; Willem, R.; Dubois, P. Activation of the hydrolytic polymerization of ε-caprolactam by ester functions: Straightforward route to aliphatic polyesteramides. React. Funct. Polym. 2008, 68, 1392–1407. [Google Scholar] [CrossRef]
- Deshayes, G.; Delcourt, C.; Verbruggen, I.; Trouillet-Fonti, L.; Touraud, F.; Fleury, E.; Degée, P.; Destarac, M.; Willem, R.; Dubois, P. Novel polyesteramide-based di- and triblock copolymers: From thermo-mechanical properties to hydrolytic degradation. Eur. Polym. J. 2011, 47, 98–110. [Google Scholar] [CrossRef]
- Chen, X.; Gonsalves, K.E.; Cameron, J.A. Further studies on biodegradation of aliphatic poly (ester-amides). J. Appl. Polym. Sci. 1993, 50, 1999–2006. [Google Scholar] [CrossRef]
- Rusu, E.; Rusu, G.; Rusu, D. Effects of temperature and comonomer content on poly(ε-caprolactam-co-ε-caprolactone) copolymers properties: An evaluation of structural changes and dielectric behavior. Polym. Eng. Sci. 2019, 59, 465–477. [Google Scholar] [CrossRef]
- Xue, F.; Jiang, S. Crystallization behaviors and structure transitions of biocompatible and biodegradable diblock copolymers. Polymers 2014, 6, 2116–2145. [Google Scholar] [CrossRef]
- Eastmond, G.C. Poly(ε-caprolactone) blends. In Biomedical Applications Polymer Blends; Eastmond, G.C., Höcker, H., Klee, D., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1999; pp. 59–223. [Google Scholar]
- Sun, Z.; Choi, B.; Feng, A.; Moad, G.; Thang, S.H. Nonmigratory poly(vinyl chloride)-block-polycaprolactone plasticizers and compatibilizers prepared by sequential RAFT and ring-opening polymerization (RAFT-T-ROP). Macromolecules 2019, 52, 1746–1756. [Google Scholar] [CrossRef]
- Newman, D.; Laredo, E.; Bello, A.; Dubois, P. Combined effect of humidity and composition on the molecular mobilities of poly(ε-caprolactone-ran-ε-caprolactam) copolymers. Macromolecules 2014, 47, 2471–2478. [Google Scholar] [CrossRef]
- Heidarzadeh, N.; Rafizadeh, M.; Taromi, F.A.; Puiggalí, J.; Del Valle, L.J.; Franco, L. Preparation of random poly(butylene alkylate-co-terephthalate)s with different methylene group contents: Crystallization and degradation kinetics. J. Polym. Res. 2017, 24, 163. [Google Scholar] [CrossRef]


| Sample | CLO:CLA | Cooling Scan | Second Heating | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Feed Ratio | MPCLO:MPCLA a | Tcc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | |||||
| PCL | PA6 | PCL | PA6 | PCL | PA6 | PCL | PA6 | |||
| PA6 | 0:100 | - | - | 173.8 | - | 66.4 | - | 212.7 | - | 54.2 |
| C10-co-A90 | 10:90 | 18:82 | - | 167.2 | - | 59.6 | - | 213.4 | - | 46.3 |
| C25-co-A75 | 25:75 | 61:39 | - | 168.7 | - | 52.9 | - | 212.8 | - | 49.5 |
| C50-co-A50 | 50:50 | 76:24 | 27.0 | 142.2 | 36.2 | 9.0 | 56.8 | 201.4 | 39.9 | 10.6 |
| C75-co-A25 | 75:25 | 80:20 | 26.8 | 139.4 | 57.4 | 6.6 | 59.2 | 191.9 | 54.2 | 5.9 |
| C75-co-A25-a | 75:25 | - | 25.3 | - | 64.9 | - | 53.6 | - | 70.9 | - |
| C90-co-A10 | 90:10 | - | 25.3 | - | 70.9 | 53.4 | - | 78.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Mu, X.; Chen, Y.; Ning, Z.; Gan, Z.; Jiang, N. Effect of Composition on the Crystallization, Water Absorption, and Biodegradation of Poly(ε-caprolactam-co-ε-caprolactone) Copolymers. Polymers 2020, 12, 2488. https://doi.org/10.3390/polym12112488
Dou Y, Mu X, Chen Y, Ning Z, Gan Z, Jiang N. Effect of Composition on the Crystallization, Water Absorption, and Biodegradation of Poly(ε-caprolactam-co-ε-caprolactone) Copolymers. Polymers. 2020; 12(11):2488. https://doi.org/10.3390/polym12112488
Chicago/Turabian StyleDou, Yuanyuan, Xinyu Mu, Yuting Chen, Zhenbo Ning, Zhihua Gan, and Ni Jiang. 2020. "Effect of Composition on the Crystallization, Water Absorption, and Biodegradation of Poly(ε-caprolactam-co-ε-caprolactone) Copolymers" Polymers 12, no. 11: 2488. https://doi.org/10.3390/polym12112488
APA StyleDou, Y., Mu, X., Chen, Y., Ning, Z., Gan, Z., & Jiang, N. (2020). Effect of Composition on the Crystallization, Water Absorption, and Biodegradation of Poly(ε-caprolactam-co-ε-caprolactone) Copolymers. Polymers, 12(11), 2488. https://doi.org/10.3390/polym12112488






