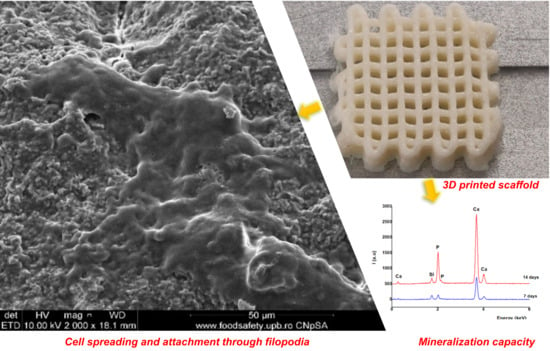
Development of 3D Bioactive Scaffolds through 3D Printing Using Wollastonite–Gelatin Inks
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. CS Obtaining
2.2.2. Ink Preparation
2.2.3. 3D Printing
2.2.4. Morpho-Structural Characterization
2.2.5. Mechanical Properties Evaluation
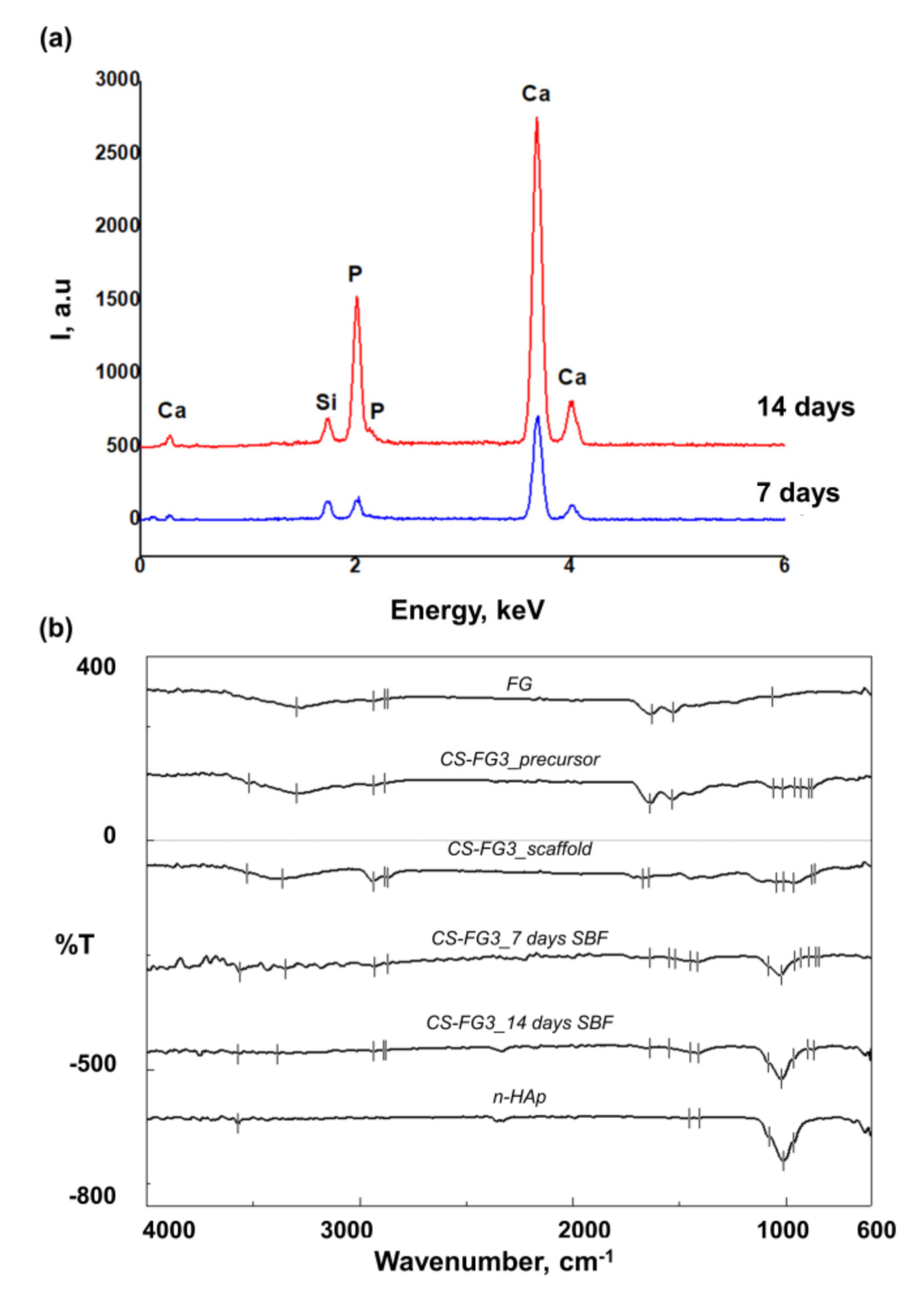
2.2.6. Evaluation of the Mineralization Capacity
2.2.7. In Vitro Evaluation of Cell Behavior
MTT Assay for Cell Viability
GSH-Glo Test for Toxicological Responses and Fluorescence Microscopy
3. Results and Discussion
3.1. Characterization of CS Powder
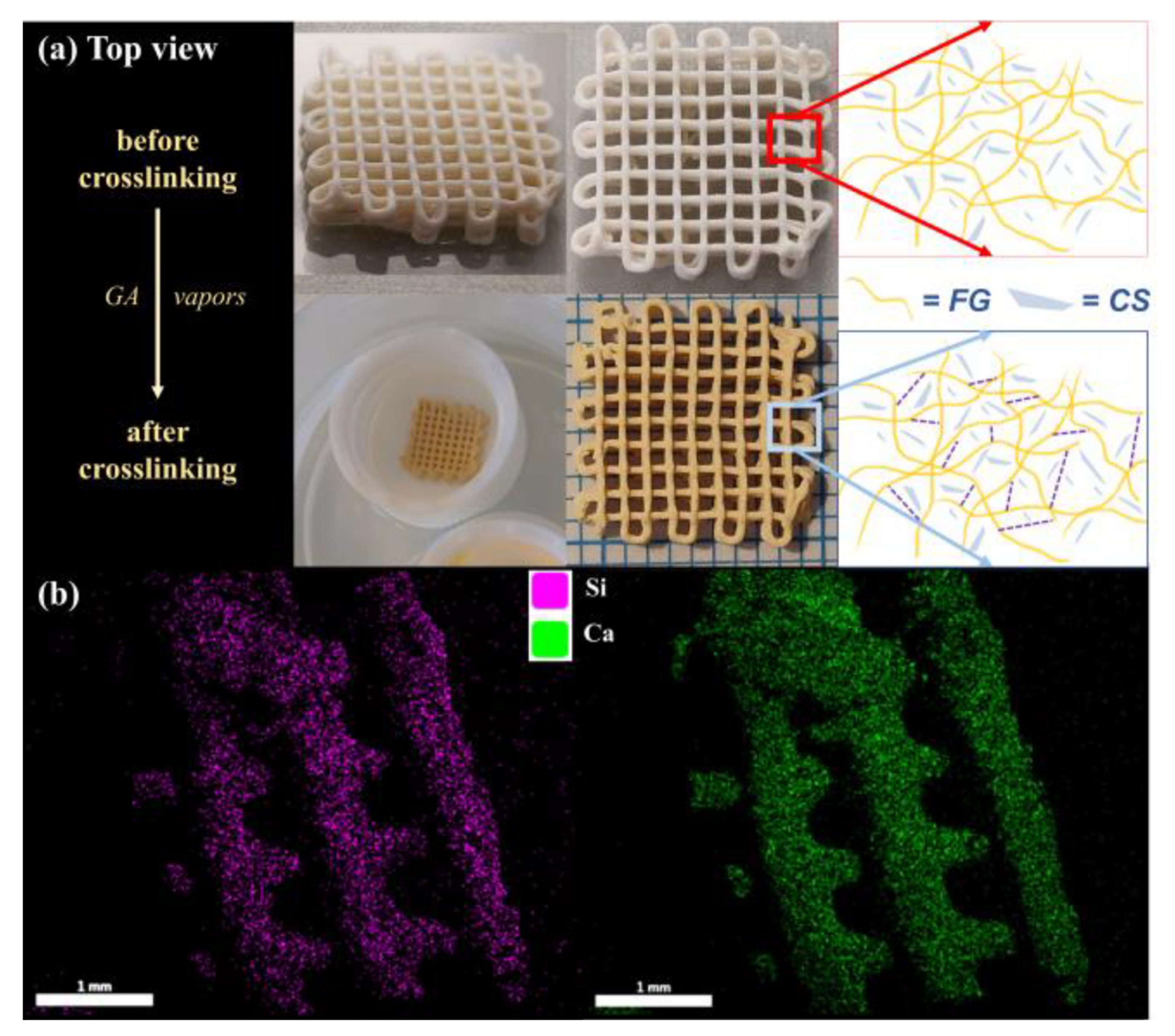
3.2. Fabrication of CS–FG Scaffolds
3.3. Morpho-Structural Characterization
3.4. Mechanical Characterization
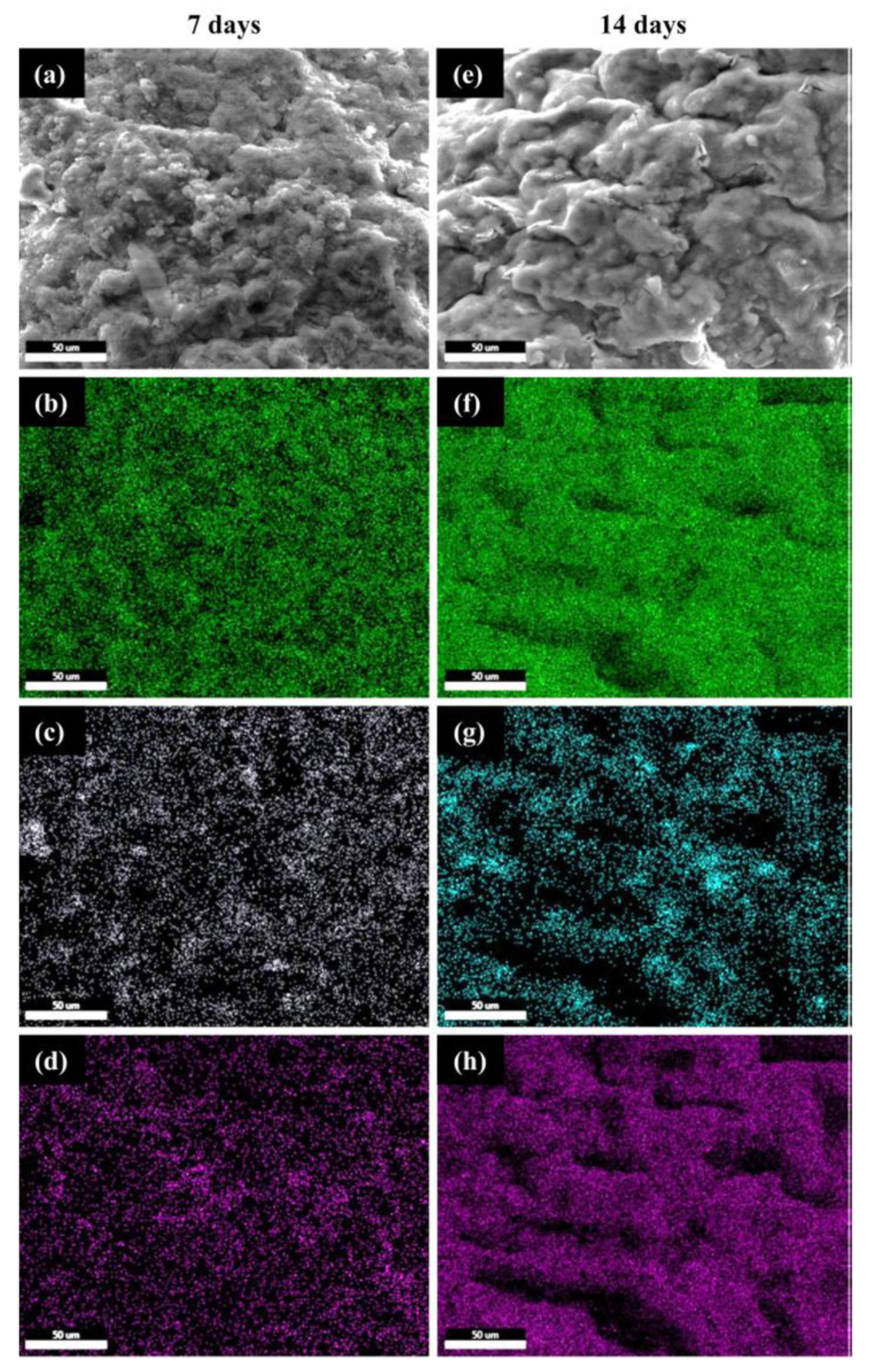
3.5. Effect of CS–FG3 Scaffold on Bioactivity
3.6. Effect of CS–FG3 Scaffold on In Vitro Biocompatibility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D printing of bioceramic scaffolds—Barriers to the clinical translation: From promise to reality, and future perspectives. Materials 2019, 12, 2660. [Google Scholar] [CrossRef]
- Li, X.; Yuan, Y.; Liu, L.; Leung, Y.S.; Chen, Y.; Guo, Y.; Chai, Y.; Chen, Y. 3D printing of hydroxyapatite/tricalcium phosphate scaffold with hierarchical porous structure for bone regeneration. Bio-Des. Manuf. 2020, 3, 15–29. [Google Scholar] [CrossRef]
- Nezhurina, E.K.; Karalkin, P.A.; Komlev, V.S.; Sviridova, I.K.; Kirsanova, V.A.; Akhmedova, S.A.; Shanskiy, Y.D.; Fedotov, A.Y.; Barinov, S.M.; Sergeeva, N.S. Physicochemical and osteoplastic characteristics of 3D printed bone grafts based on synthetic calcium phosphates and natural polymers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 347, 012047. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, H.; Lin, T.; Zhang, Y.; He, J.; Wang, L. 3D gel printing of porous calcium silicate scaffold for bone tissue engineering. J. Mater. Sci. 2019, 54, 10430–10436. [Google Scholar] [CrossRef]
- Mocioiu, A.M.; Tutuianu, R.; Cursaru, L.M.; Piticescu, R.M.; Stanciu, P.; Vasile, B.S.; Trusca, R.; Sereanu, V.; Meghea, A. 3D structures of hydroxyapatite obtained from Rapana venosa shells using hydrothermal synthesis followed by 3D printing. J. Mater. Sci. 2019, 54, 13901–13913. [Google Scholar] [CrossRef]
- Kankala, R.K.; Xu, X.M.; Liu, C.G.; Chen, A.Z.; Wang, S. Bin 3D-printing of microfibrous porous scaffolds based on hybrid approaches for bone tissue engineering. Polymers 2018, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fan, W.; Zhou, Y.; Luo, Y.; Gelinsky, M.; Chang, J.; Xiao, Y. 3D-printing of highly uniform CaSiO 3 ceramic scaffolds: Preparation, characterization and in vivo osteogenesis. J. Mater. Chem. 2012, 22, 12288–12295. [Google Scholar] [CrossRef]
- Kalkandelen, C.; Ulag, S.; Ozbek, B.; Eroglu, G.O.; Ozerkan, D.; Kuruca, S.E.; Oktar, F.N.; Sengor, M.; Gunduz, O. 3D Printing of Gelatine/Alginate/β-Tricalcium Phosphate Composite Constructs for Bone Tissue Engineering. In ChemistrySelect; Wiley: New York, NY, USA, 2019; Volume 4, pp. 12032–12036. [Google Scholar]
- Li, Q.; Lei, X.; Wang, X.; Cai, Z.; Lyu, P.; Zhang, G. Hydroxyapatite/Collagen Three-Dimensional Printed Scaffolds and Their Osteogenic Effects on Human Bone Marrow-Derived Mesenchymal Stem Cells; Mary Ann Liebert: New Rochelle, NY, USA, 2019; Volume 25, ISBN 0086139107339. [Google Scholar]
- Kuttappan, S.; Mathew, D.; Nair, M.B. Biomimetic composite scaffolds containing bioceramics and collagen/gelatin for bone tissue engineering—A mini review. Int. J. Biol. Macromol. 2016, 93, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Maji, K.; Dasgupta, S. Hydroxyapatite-Chitosan and Gelatin Based Scaffold for Bone Tissue Engineering. Trans. Indian Ceram. Soc. 2014, 73, 110–114. [Google Scholar] [CrossRef]
- Tcacencu, I.; Rodrigues, N.; Alharbi, N.; Benning, M.; Toumpaniari, S.; Mancuso, E.; Marshall, M.; Bretcanu, O.; Birch, M.; McCaskie, A.; et al. Osseointegration of porous apatite-wollastonite and poly(lactic acid) composite structures created using 3D printing techniques. Mater. Sci. Eng. C 2018, 90, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Mamoory, R.S.; Le, D.Q.S.; Bünger, C.E. Fabrication of gelatin/hydroxyapatite/3D-graphene scaffolds by a hydrogel 3D-printing method. Mater. Chem. Phys. 2020, 239, 122305. [Google Scholar] [CrossRef]
- Park, S.; Park, K.M. Engineered polymeric hydrogels for 3D tissue models. Polymers 2016, 8, 23. [Google Scholar] [CrossRef]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Wu, Q.; Long, H.; Hu, K.; Li, P.; Wang, C.; Sun, M.; Dong, J.; Wei, X.; Suo, J.; et al. Development of chitosan/gelatin hydrogels incorporation of biphasic calcium phosphate nanoparticles for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 1636–1657. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Lode, A.; Akkineni, A.R.; Gelinsky, M. Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting. RSC Adv. 2015, 5, 43480–43488. [Google Scholar] [CrossRef]
- Cecoltan, S.; Serafim, A.; Dragusin, D.M.; Lungu, A.; Lagazzo, A.; Barberis, F.; Stancu, I.C. The potential of NDPs-loaded fish gelatin fibers as reinforcing agent for fish gelatin hydrogels. Key Eng. Mater. 2016, 695, 278–283. [Google Scholar] [CrossRef]
- Serafim, A.; Cecoltan, S.; Lungu, A.; Vasile, E.; Iovu, H.; Stancu, I.C. Electrospun fish gelatin fibrous scaffolds with improved bio-interactions due to carboxylated nanodiamond loading. RSC Adv. 2015, 5, 95467–95477. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, D.; Chen, J.; Zhang, X.; Li, X.; Zhao, W.; Xu, T. Biomaterials based on marine resources for 3D bioprinting applications. Mar. Drugs 2019, 17, 555. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, T.; Qi, Y.; Luo, J.; Fang, J.; Yang, X.; Liu, X.; Xu, T.; Yang, Q.; Gou, Z.; et al. In vitro Chondrocyte Responses in Mg-doped Wollastonite/Hydrogel Composite Scaffolds for Osteochondral Interface Regeneration. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Xie, J.; Yang, X.; Shao, H.; Ye, J.; He, Y.; Fu, J.; Gao, C.; Gou, Z. Simultaneous mechanical property and biodegradation improvement of wollastonite bioceramic through magnesium dilute doping. J. Mech. Behav. Biomed. Mater. 2016, 54, 60–71. [Google Scholar] [CrossRef]
- Xie, J.; Shao, H.; He, D.; Yang, X.; Yao, C.; Ye, J.; He, Y.; Fu, J.; Gou, Z. Ultrahigh strength of three-dimensional printed diluted magnesium doping wollastonite porous scaffolds. MRS Commun. 2015, 5, 631–639. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.A.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Shen, J.; Yang, X.; Wu, R.; Shen, M.; Lu, F.; Zhang, F.; Chen, Z.; Chen, X.; Xu, S.; Gao, C.; et al. Direct ink writing core-shell Wollastonite@Diopside scaffolds with tailorable shell micropores favorable for optimizing physicochemical and biodegradation properties. J. Eur. Ceram. Soc. 2020, 40, 503–512. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Zhou, X.; Yang, D.; Shi, Z.; Hao, Y. Effect of strontium-containing on the properties of Mg-doped wollastonite bioceramic scaffolds. Biomed. Eng. Online 2019, 18, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Casas-Luna, M.; Torres-Rodríguez, J.A.; Valdés-Martínez, O.U.; Obradović, N.; Slámečka, K.; Maca, K.; Kaiser, J.; Montúfar, E.B.; Čelko, L. Robocasting of controlled porous CaSiO3–SiO2 structures: Architecture—Strength relationship and material catalytic behavior. Ceram. Int. 2019, 46, 8853–8861. [Google Scholar] [CrossRef]
- Fuchs, R.K.; Thompson, W.R.; Warden, S.J. Bone biology. In Bone Repair Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 15–52. ISBN 9780081024515. [Google Scholar]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Zanfir, A.V.; Nenu, N.; Voicu, G.; Badanoiu, A.I.; Ghitulica, C.D.; Iordache, F. Modified calcium silicophosphate cements with improved properties. Mater. Chem. Phys. 2019, 238, 121965. [Google Scholar] [CrossRef]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kepa, K.; Coleman, R.; Grøndahl, L. In vitro mineralization of functional polymers. Biosurf. Biotribol. 2015, 1, 214–227. [Google Scholar] [CrossRef]
- Palakurthy, S.; Samudrala, R.K. In vitro bioactivity and degradation behaviour of β-wollastonite derived from natural waste. Mater. Sci. Eng. C 2019, 98, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Morsy, R.; Abuelkhair, R.; Elnimr, T. Synthesis and in vitro bioactivity mechanism of synthetic α-wollastonite and β-wollastonite bioceramics. J. Ceram. Sci. Technol. 2016, 7, 65–70. [Google Scholar]
- Ismail, H.; Shamsudin, R.; Azmi, M.; Hamid, A. Characteristics of β -wollastonite derived from rice straw ash and limestone. J. Aust. Ceram. Soc. 2016, 52, 163–174. [Google Scholar]
- Shamsudin, R.; Azam, F.A.; Hamid, M.A.A.; Ismail, H. Bioactivity and Cell Compatibility of β-Wollastonite Derived from Rice Husk Ash and Limestone. Materials 2017, 10, 1188. [Google Scholar] [CrossRef]
- Tytgat, L.; Kollert, M.R.; Van Damme, L.; Thienpont, H.; Ottevaere, H.; Duda, G.N.; Geissler, S.; Dubruel, P.; Van Vlierberghe, S.; Qazi, T.H. Evaluation of 3D Printed Gelatin-Based Scaffolds with Varying Pore Size for MSC-Based Adipose Tissue Engineering. Macromol. Biosci. 2020, 20, 1900364. [Google Scholar] [CrossRef]
- Negrini, N.C.; Celikkin, N.; Tarsini, P.; Farè, S.; Święszkowski, W. Three-dimensional printing of chemically crosslinked gelatin hydrogels for adipose tissue engineering. Biofabrication 2020, 12, 025001. [Google Scholar] [CrossRef]
- Lin, J.; Pan, D.; Sun, Y.; Ou, C.; Wang, Y.; Cao, J. The modification of gelatin films: Based on various cross-linking mechanism of glutaraldehyde at acidic and alkaline conditions. Food Sci. Nutr. 2019, 7, 4140–4146. [Google Scholar] [CrossRef]
- Stoch, A.; Jastrzębski, W.; Brożek, A.; Trybalska, B.; Cichocińska, M.; Szarawara, E. FTIR monitoring of the growth of the carbonate containing apatite layers from simulated and natural body fluids. J. Mol. Struct. 1999, 511–512, 287–294. [Google Scholar] [CrossRef]




| Code | CS:FG (wt:wt) |
|---|---|
| CS–FG1 | 68:32 |
| CS–FG2 | 74:26 |
| CS–FG3 | 77:23 |
| CS–FG4 | 81:19 |
| Code | Needle Diameter (mm) = 0.25 | ||
|---|---|---|---|
| Pressure (kPa) | Feed Rate (mm s−1) | Evaluation | |
| CS–FG1 | 250–300 | 1–10 | - |
| CS–FG2 | 300–350 | 1–10 | - |
| CS–FG3 | 450–500 | 4–6 | + |
| CS–FG4 | 550 | 0.5–3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curti, F.; Stancu, I.-C.; Voicu, G.; Iovu, H.; Dobrita, C.-I.; Ciocan, L.T.; Marinescu, R.; Iordache, F. Development of 3D Bioactive Scaffolds through 3D Printing Using Wollastonite–Gelatin Inks. Polymers 2020, 12, 2420. https://doi.org/10.3390/polym12102420
Curti F, Stancu I-C, Voicu G, Iovu H, Dobrita C-I, Ciocan LT, Marinescu R, Iordache F. Development of 3D Bioactive Scaffolds through 3D Printing Using Wollastonite–Gelatin Inks. Polymers. 2020; 12(10):2420. https://doi.org/10.3390/polym12102420
Chicago/Turabian StyleCurti, Filis, Izabela-Cristina Stancu, Georgeta Voicu, Horia Iovu, Cristina-Ioana Dobrita, Lucian Toma Ciocan, Rodica Marinescu, and Florin Iordache. 2020. "Development of 3D Bioactive Scaffolds through 3D Printing Using Wollastonite–Gelatin Inks" Polymers 12, no. 10: 2420. https://doi.org/10.3390/polym12102420
APA StyleCurti, F., Stancu, I.-C., Voicu, G., Iovu, H., Dobrita, C.-I., Ciocan, L. T., Marinescu, R., & Iordache, F. (2020). Development of 3D Bioactive Scaffolds through 3D Printing Using Wollastonite–Gelatin Inks. Polymers, 12(10), 2420. https://doi.org/10.3390/polym12102420