Functionalized KIT-6/Polysulfone Mixed Matrix Membranes for Enhanced CO2/CH4 Gas Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials Used
2.2. Functionlization of KIT-6
2.3. Preparation of the Membranes
2.4. Characterizations of KIT-6, NH2KIT-6, and the Membranes
2.5. Gas Permeation and Separation Studies
3. Results and Discussion
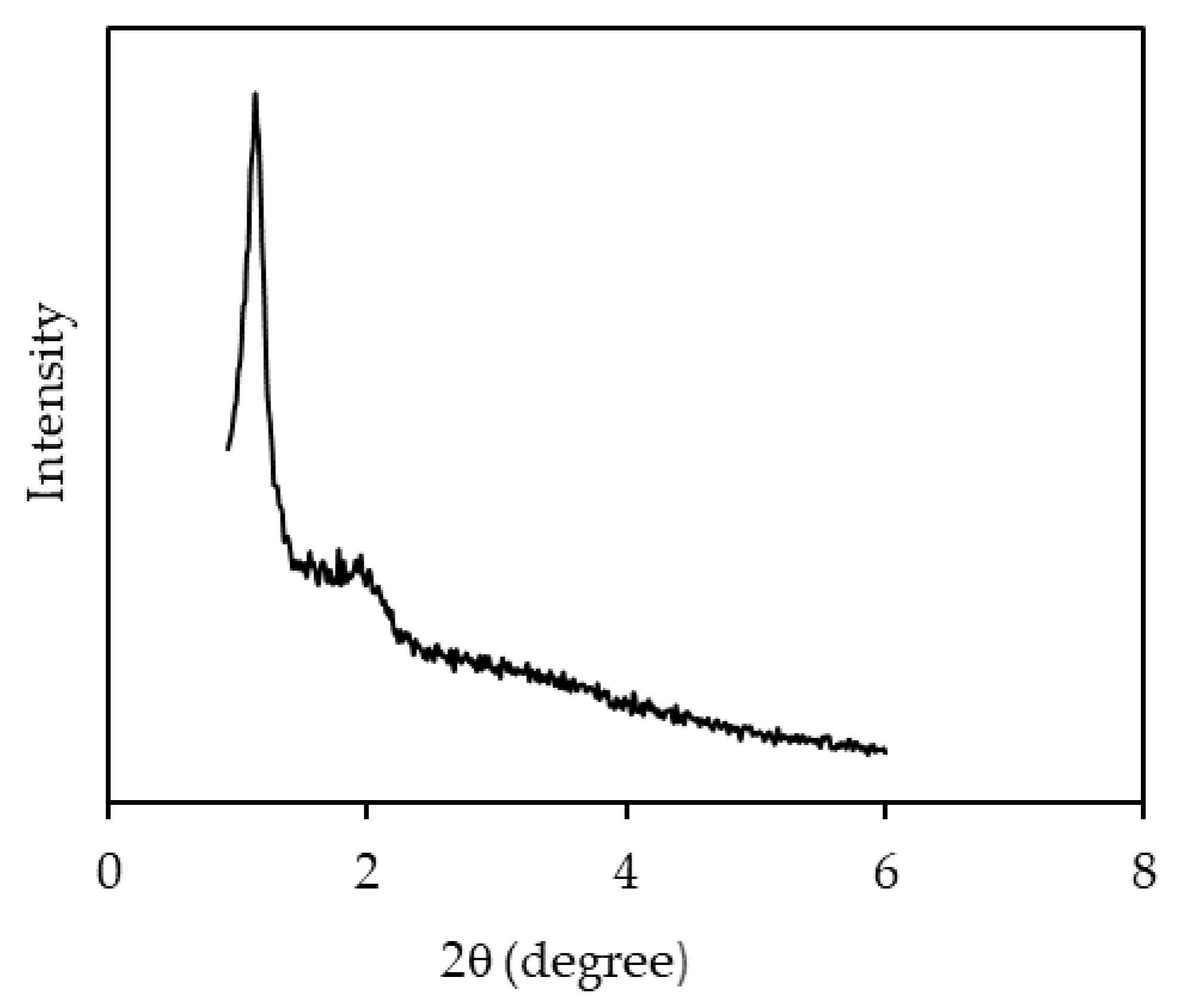
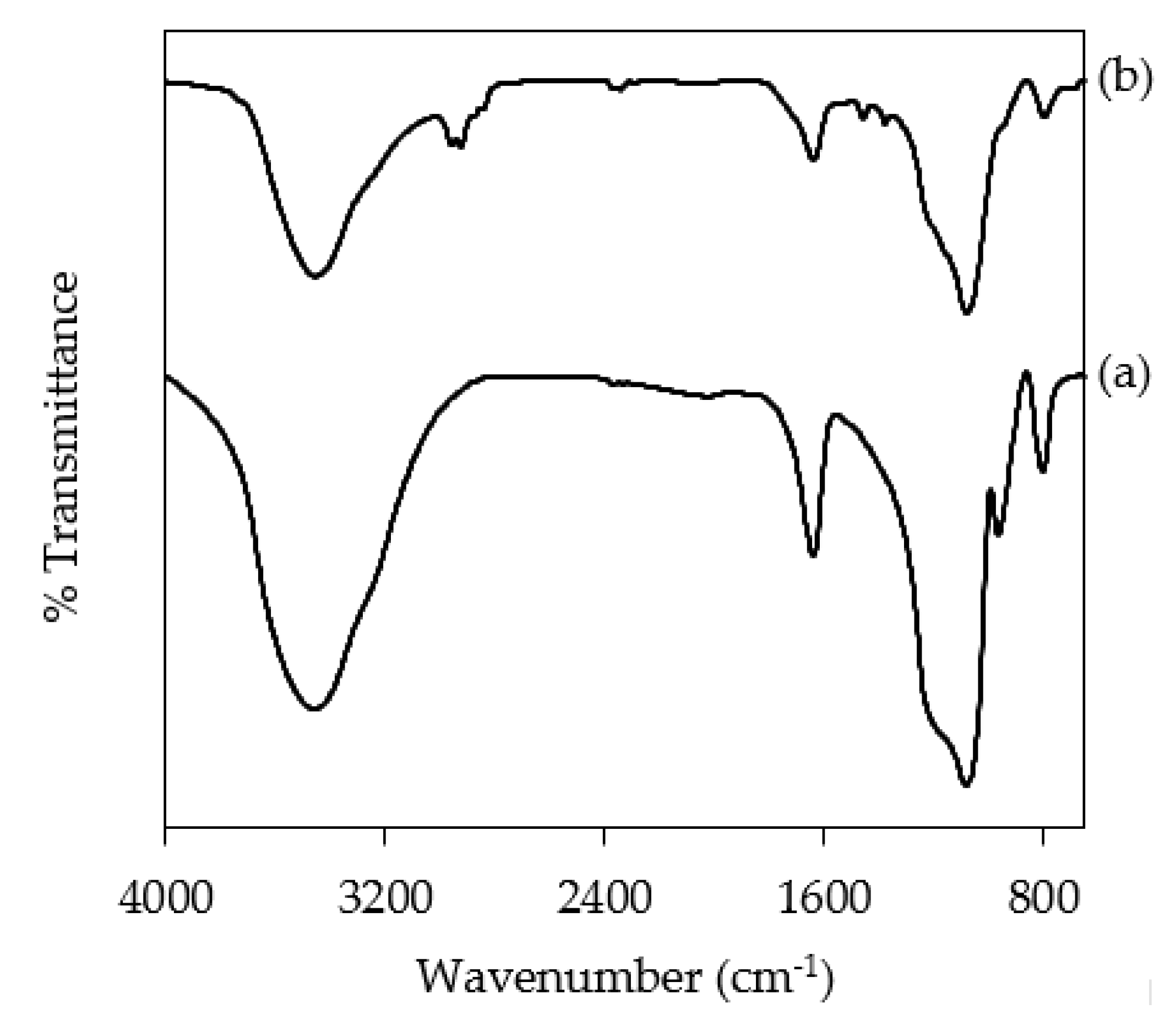
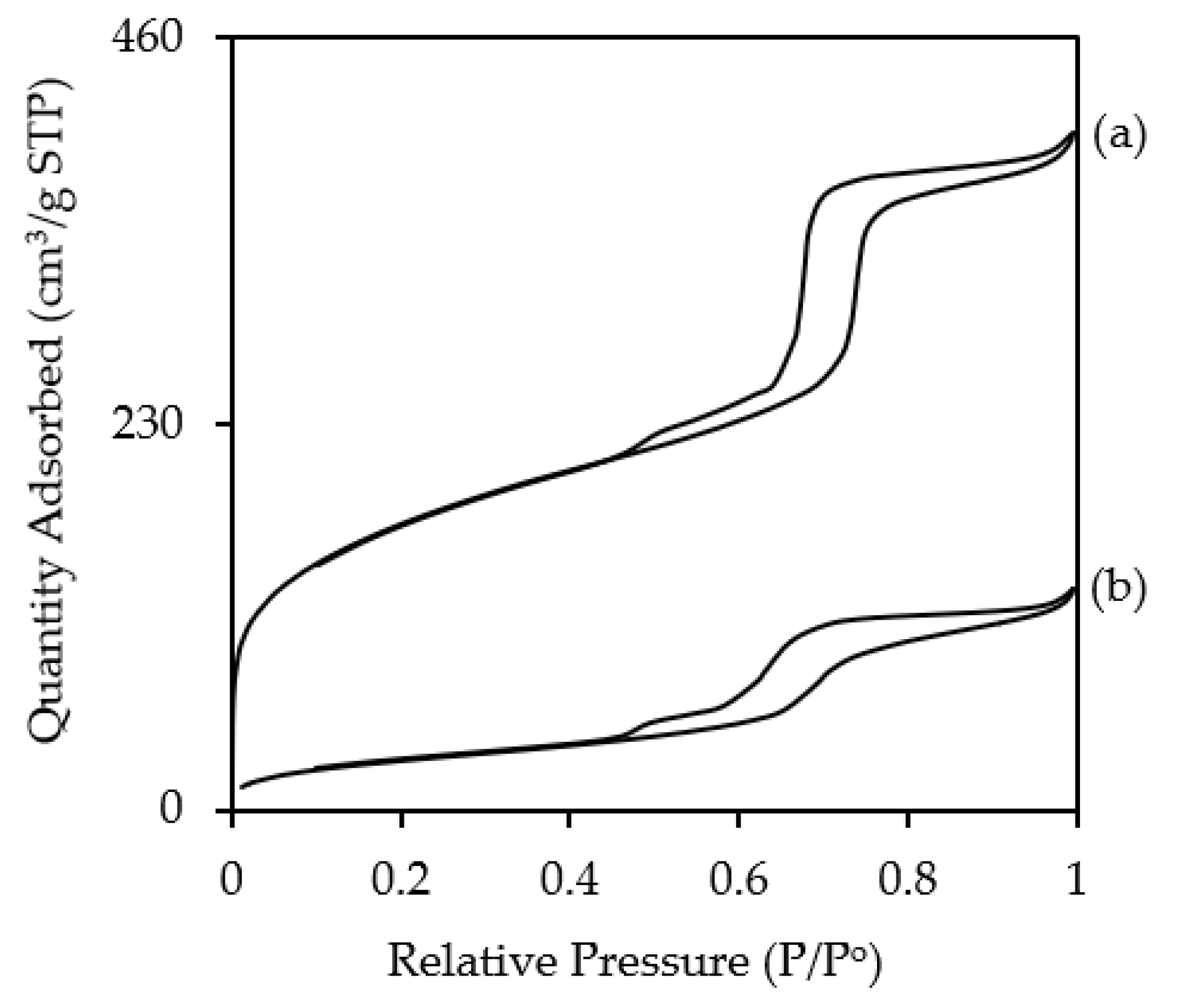
3.1. Characterizations of NH2KIT-6
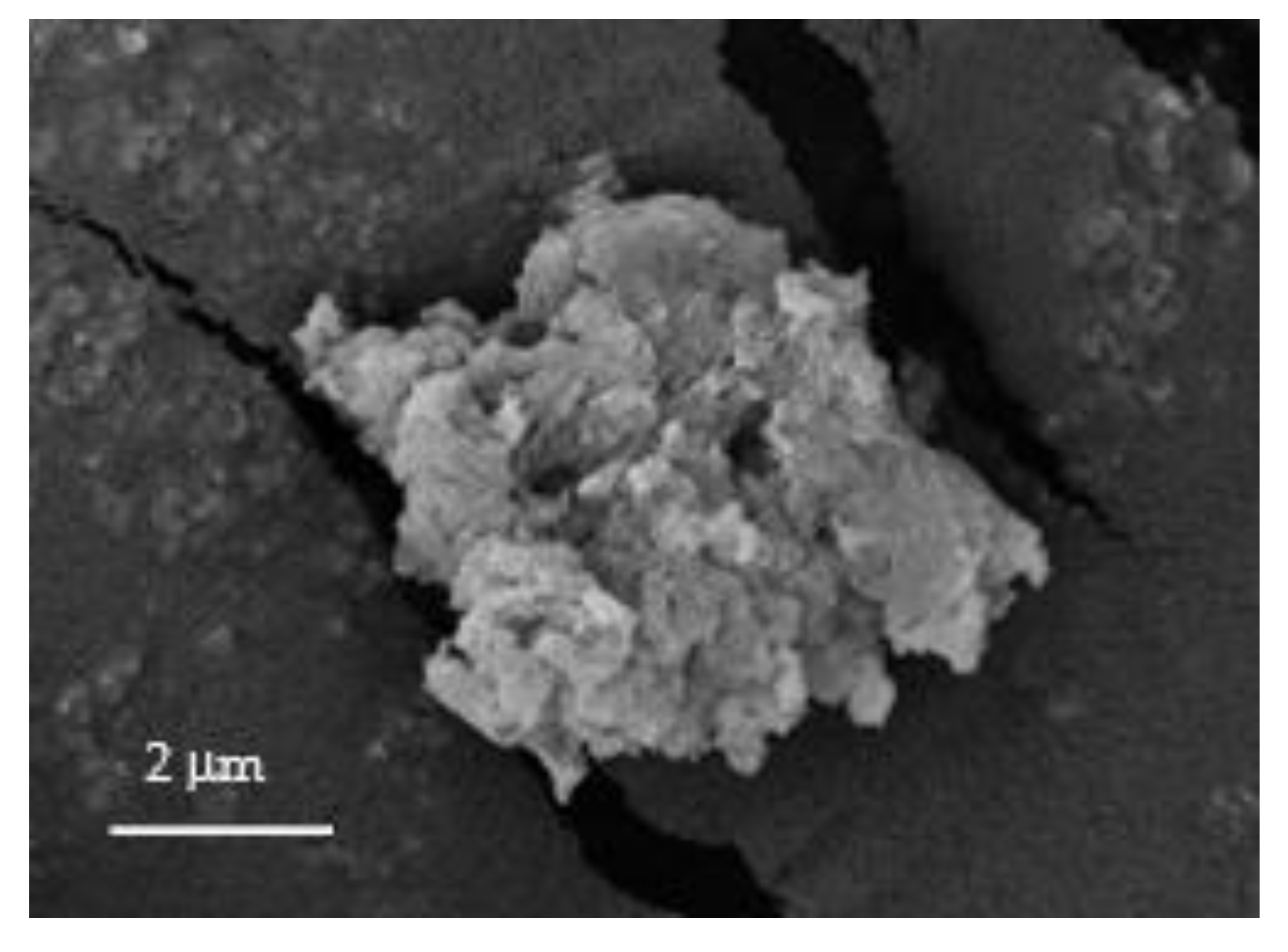
3.2. Characterizations of the Membranes
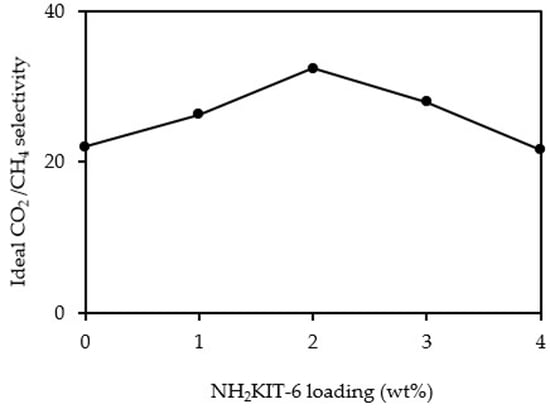
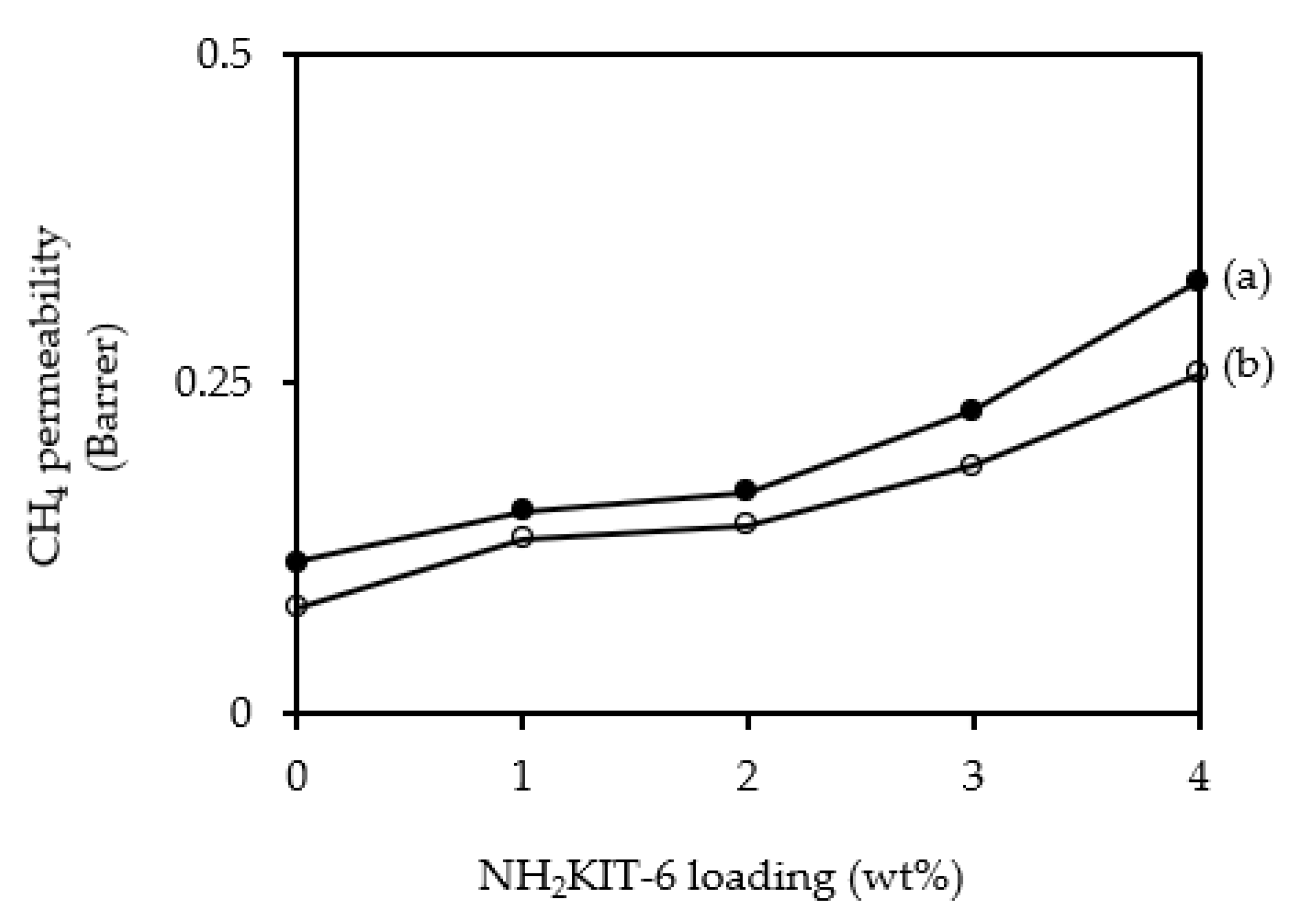
3.3. Gas Permeation and Separation Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waheed, N.; Mushtaq, A.; Tabassum, S.; Gilani, M.A.; Ilyas, A.; Ashraf, F.; Jamal, Y.; Bilad, M.R.; Khan, A.U.; Khan, L.A. Mixed matrix membranes based on polysulfone and rice husk extracted silica for CO2 separation. Sep. Purif. Technol. 2016, 170, 122–129. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Sadrzadeh, M.; Matsuura, T. Thermally stable polymers for advanced high-performance gas separation membranes. Prog. Energy Combust. Sci. 2018, 66, 1–41. [Google Scholar] [CrossRef]
- Lee, S.; Park, S.C.; Kim, T.Y.; Kang, S.W.; Kang, Y.S. Direct molecular interaction of CO2 with KTFSI dissolved in Pebax 2533 and their use in facilitated CO2 transport membranes. J. Membr. Sci. 2018, 548, 358–362. [Google Scholar] [CrossRef]
- Wang, J.H.; Li, Y.; Zhang, Z.S.; Hao, Z.P. Mesoporous KIT-6 silica–polydimethylsiloxane (PDMS) mixed matrix membranes for gas separation. J. Mater. Chem. Acad. 2015, 3, 8650–8658. [Google Scholar] [CrossRef]
- Jomekian, A.; Pakizeh, M.; Mansoori, S.A.A.; Poorafshari, M.; Hemmati, M.; Ataee, D.P. Gas transport behavior of novel modified MCM-48/ Polysulfone mixed matrix membrane coated by PDMS. J. Membr. Sci. Technol. 2011, 1, 1–6. [Google Scholar]
- Yavaria, M.; Okamotob, Y.; Lina, H. The role of halogens in polychlorotrifluoroethylene (PCTFE) in membrane gas separations. J. Membr. Sci. 2018, 548, 380–389. [Google Scholar] [CrossRef]
- Li, S.; Jiang, X.; Sun, H.; He, S.; Zhang, L.; Shao, L. Mesoporous dendritic fibrous nanosilica (DFNS) stimulating mix matrix membranes towards superior CO2 capture. J. Membr. Sci. 2019, 586, 185–191. [Google Scholar] [CrossRef]
- Wang, X.; Ding, X.; Zhao, H.; Fu, J.; Xin, Q.; Zhang, Y. Pebax-based mixed matrix membranes containing hollow polypyrrole nanospheres with mesoporous shells for enhanced gas permeation performance. J. Membr. Sci. 2020, 602, 117968. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Li, K.; Ma, C.; Zhang, J.; Li, H.; Chang, X.; Zhu, L.; Xue, Q. Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Sep. Purif. Technol. 2020, 248, 117080. [Google Scholar] [CrossRef]
- Guo, Z.; Zheng, W.; Yan, X.; Dai, Y.; Ruan, X.; Yang, X.; Li, X.; Zhang, N.; He, G. Ionic liquid tuning nanocage size of MOFs through a two-step adsorption/infiltration strategy for enhanced gas screening of mixed-matrix membranes. J. Membr. Sci. 2020, 605, 118101. [Google Scholar] [CrossRef]
- Kim, S.; Marand, E. High permeability nano-composite membranes based on mesoporous MCM-41 nanoparticles in a polysulfone matrix. Microporous Mesoporous Mater. 2008, 114, 129–136. [Google Scholar] [CrossRef]
- Kim, H.J.; Yang, H.C.; Chung, D.Y.; Yang, I.H.; Choi, Y.J.; Moon, J.K. Functionalized Mesoporous Silica Membranes for CO2 Separation Applications. J. Chem. 2015, 2015, 1–9. [Google Scholar]
- Khan, A.L.; Klaysom, C.; Gahlaut, A.; Vankelecom, I.F.J. Polysulfone acrylate membranes containing functionalized mesoporous MCM-41 for CO2 separation. J. Membr. Sci. 2013, 436, 145–153. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.Q.; Li, Y.F.; Wang, S.F.; Guo, R.L.; Jiang, Z.Y.; Wu, C.; Xin, Q.P.; Lu, X. Facilitated transport mixed matrix membranes incorporated with amine functionalized MCM-41 for enhanced gas separation properties. J. Membr. Sci. 2014, 465, 78–90. [Google Scholar] [CrossRef]
- Mohamad, M.B.; Fong, Y.Y.; Shariff, A. Gas separation of carbon dioxide from methane using polysulfone membrane incorporated with Zeolite-T. Procedia Eng. 2016, 148, 621–629. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Montazer-Rahmati, M.M.; Matsuura, T. Morphology and permeation properties of polysulfone membranes for gas separation: Effects of non-solvent additives and co-solvent. Sep. Purif. Technol. 2010, 72, 194–202. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Shahid, S.; Nijmeijer, K. Matrimid®/polysulfone blend mixed matrix membranes containing ZIF-8 nanoparticles for high pressure stability in natural gas separation. Sep. Purif. Technol. 2017, 189, 90–100. [Google Scholar] [CrossRef]
- Kishor, R.; Ghoshal, A.K. APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption. J. Chem. Eng. 2015, 262, 882–890. [Google Scholar] [CrossRef]
- Kim, W.G.; Lee, J.S.; Bucknall, D.G.; Koros, W.J.; Nair, S. Nanoporous layered silicate AMH-3/cellulose acetate nanocomposite membranes for gas separations. J. Membr. Sci. 2013, 441, 129–136. [Google Scholar] [CrossRef]
- Ayad, M.M.; Salahuddin, N.A.; El-Nasr, A.A.; Torad, N.L. Amine functionalized mesoporous silica KIT-6 as a controlled release drug delivery carrier. Microporous Mesoporous Mater. 2016, 229, 166–177. [Google Scholar] [CrossRef]
- Nigar, H. Amine-functionalized mesoporous silica: A Mater. capable of CO2 adsorption and fast regeneration by microwave heating. Am. Inst. Chem. Eng. 2016, 62, 547–555. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]
- Hafizi, H.; Chermahini, A.N.; Saraji, M.; Mohammadnezhad, G. The catalytic conversion of fructose into 5-hydroxymethylfurfural over acid-functionalized KIT-6, an ordered mesoporous silica. Chem. Eng. J. 2016, 294, 380–388. [Google Scholar] [CrossRef]
- Tzi, E.C.N.; Ching, O.P. Surface modification of AMH-3 for development of mixed matrix membranes. Procedia Eng. 2016, 148, 86–92. [Google Scholar] [CrossRef][Green Version]
- Richard, C.; Hing, K.; Schreiber, H.P. Interaction balances and properties of filled polymers. Polym. Compos. 1985, 6, 201–208. [Google Scholar] [CrossRef]
- Liu, C.S.; Pilania, G.; Wang, C.C.; Ramprasad, R. How critical are the van der Waals interactions in polymer crystals? J. Phys. Chem. 2012, 2012, 1–19. [Google Scholar] [CrossRef]
- Zornoza, B.; Irusta, S.; Tellez, C.; Coronas, J. Mesoporous silica sphere-polysulfone mixed matrix membranes for gas separation. Langmuir 2009, 25, 5903–5909. [Google Scholar] [CrossRef]
- Khdary, N.H.; Abdelsalam, M.E. Polymer-silica nanocomposite membranes for CO2 capturing. Arab. J. Chem. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Lasseuguette, E.; Malpass-Evans, R.; Carta, M.; McKeown, N.B.; Ferrari, M.-C. Temperature and pressure dependence of gas permeation in a microporous Troger’s base polymer. Membranes 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chung, T.S.; Paul, D.R. Gas sorption and permeation in PIM-1. J. Membr. Sci. 2013, 432, 50–57. [Google Scholar] [CrossRef]
- Biondo, L.D.; Duarte, J.; Zeni, M.; Godinho, M.A. Dual-mode interpretation of CO2/CH4 permeability in polysulfone membranes at low pressures. Anais Acad. Bras. Cienc. 2018, 90, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.H.; Ng, T.Y.S.; Chew, T.L.; Oh, P.C.; Ahmad, A.L.; Ho, C.-D. Evaluation of the properties, gas permeability and selectivity of mixed matrix membrane based on polysulfone polymer matrix incorporated with KIT-6 silica. Polymers 2019, 11, 1732. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]




| Polymer/Filler | Amine Group Used for the Filler Functionalization | Filler Loading (wt%) | CO2 Permeability (Barrer) | Ideal CO2/CH4 Selectivity | Reference |
|---|---|---|---|---|---|
| Pebax/MCM-41 | Polyethylenimine | 5 | ~87 | ~23 | [14] |
| PSF/RHS | 4-aminophenazone | 10 | ~5.6 | ~28.1 | [1] |
| PSF/MCM-41 | Aminopropyltrimethoxysilane | 10 | ~6.2 | ~28.0 | [13] |
| PSF/MCM-41 | 3-aminopropyltriethoxysilane | 20 | ~7.3 | ~28.1 | [11] |
| PSF/NH2KIT-6 | (3-Aminopropyl) triethoxysilane | 2 | ~5.4 | ~32.4 | Current study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chew, T.L.; Ding, S.H.; Oh, P.C.; Ahmad, A.L.; Ho, C.-D. Functionalized KIT-6/Polysulfone Mixed Matrix Membranes for Enhanced CO2/CH4 Gas Separation. Polymers 2020, 12, 2312. https://doi.org/10.3390/polym12102312
Chew TL, Ding SH, Oh PC, Ahmad AL, Ho C-D. Functionalized KIT-6/Polysulfone Mixed Matrix Membranes for Enhanced CO2/CH4 Gas Separation. Polymers. 2020; 12(10):2312. https://doi.org/10.3390/polym12102312
Chicago/Turabian StyleChew, Thiam Leng, Sie Hao Ding, Pei Ching Oh, Abdul Latif Ahmad, and Chii-Dong Ho. 2020. "Functionalized KIT-6/Polysulfone Mixed Matrix Membranes for Enhanced CO2/CH4 Gas Separation" Polymers 12, no. 10: 2312. https://doi.org/10.3390/polym12102312
APA StyleChew, T. L., Ding, S. H., Oh, P. C., Ahmad, A. L., & Ho, C.-D. (2020). Functionalized KIT-6/Polysulfone Mixed Matrix Membranes for Enhanced CO2/CH4 Gas Separation. Polymers, 12(10), 2312. https://doi.org/10.3390/polym12102312