The Effect of Silica-Filler on Polyurethane Adhesives Based on Renewable Resource for Wood Bonding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adhesive Formulation
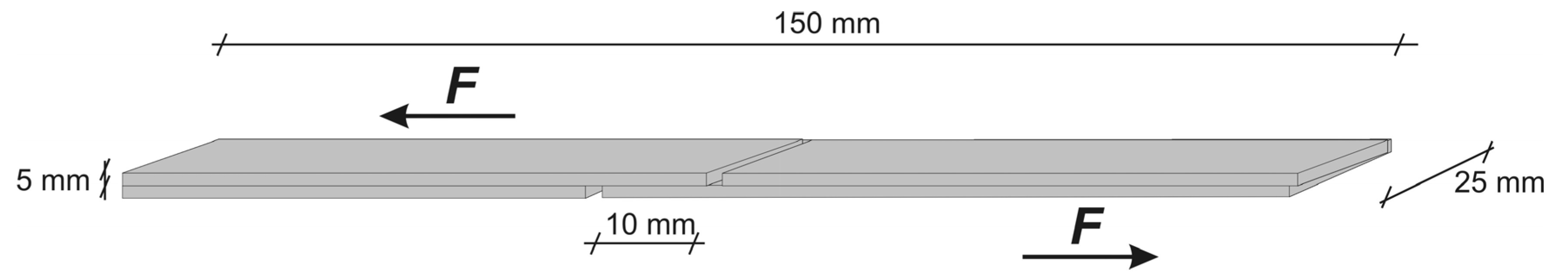
2.3. Lap Shear Strength
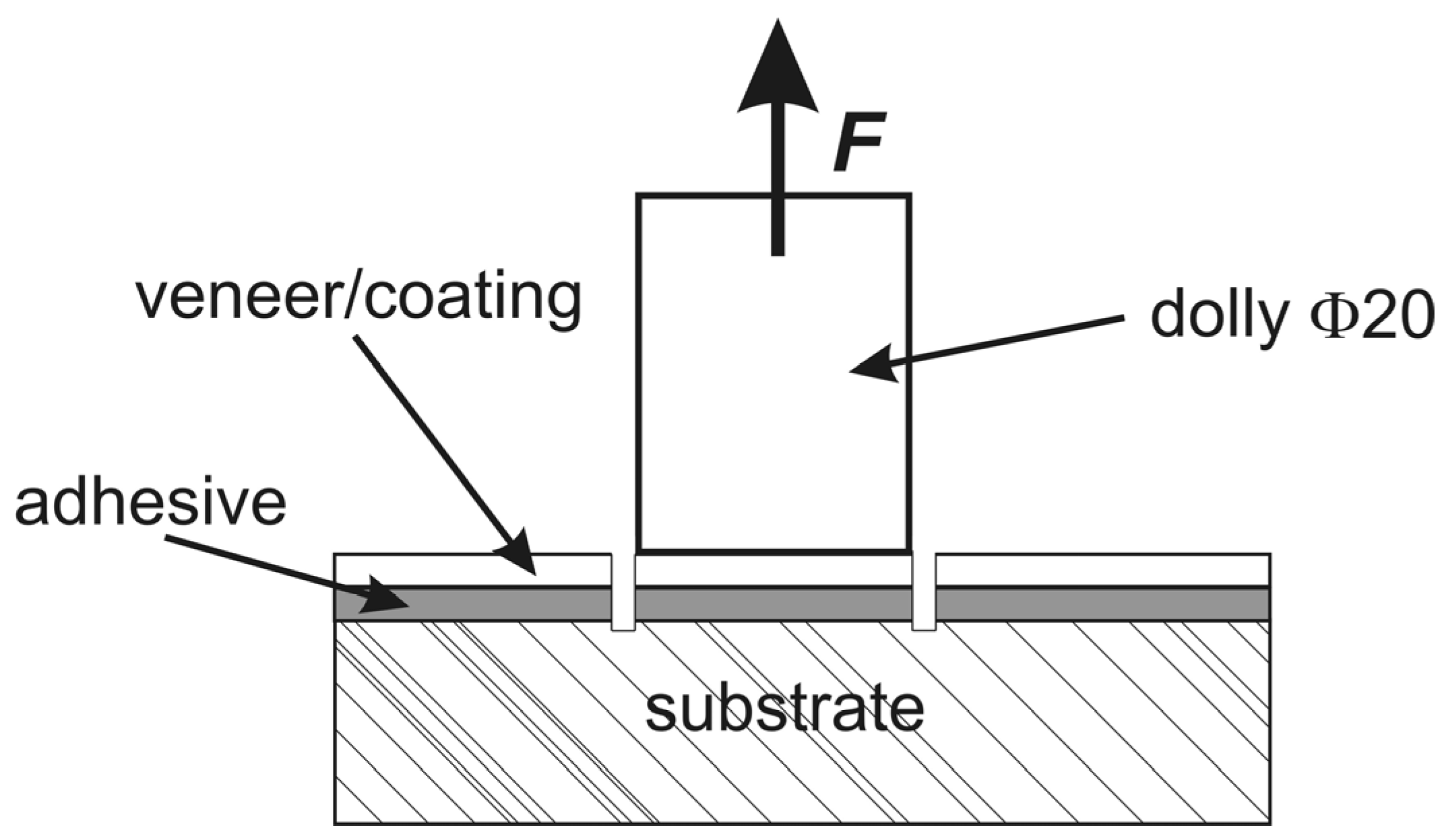
2.4. Pull-Off Adhesion Test
2.5. TG-MS Experiments
2.6. Contact Angle (θ), Surface Free Energy (γ), and Work of Adhesion (Wa)
3. Results and Discussion
3.1. Effect of Viscosity on Bonding
3.2. Reactivity
3.3. Bondline Shear Strength
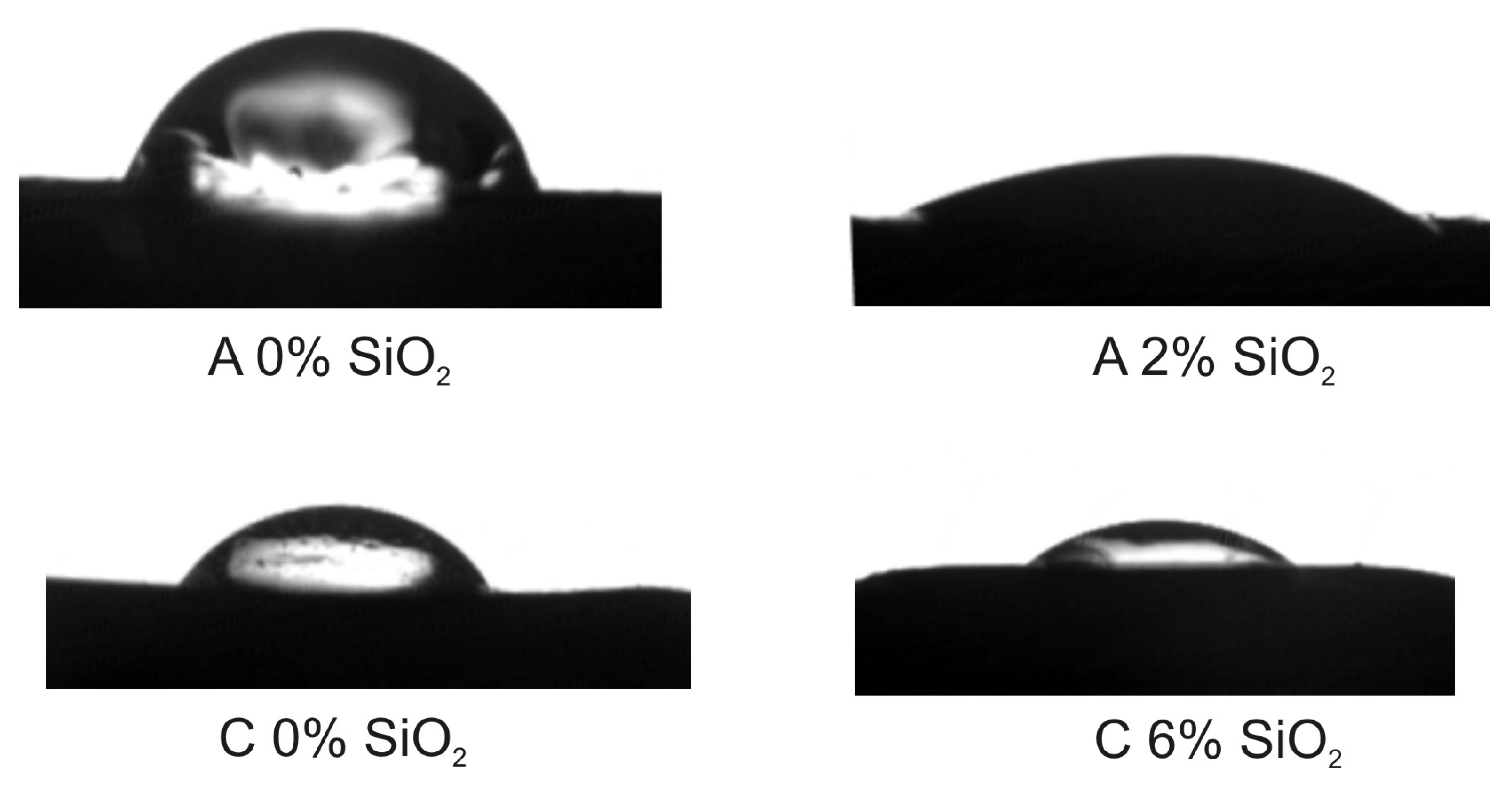
3.4. Wetting and Surface Properties
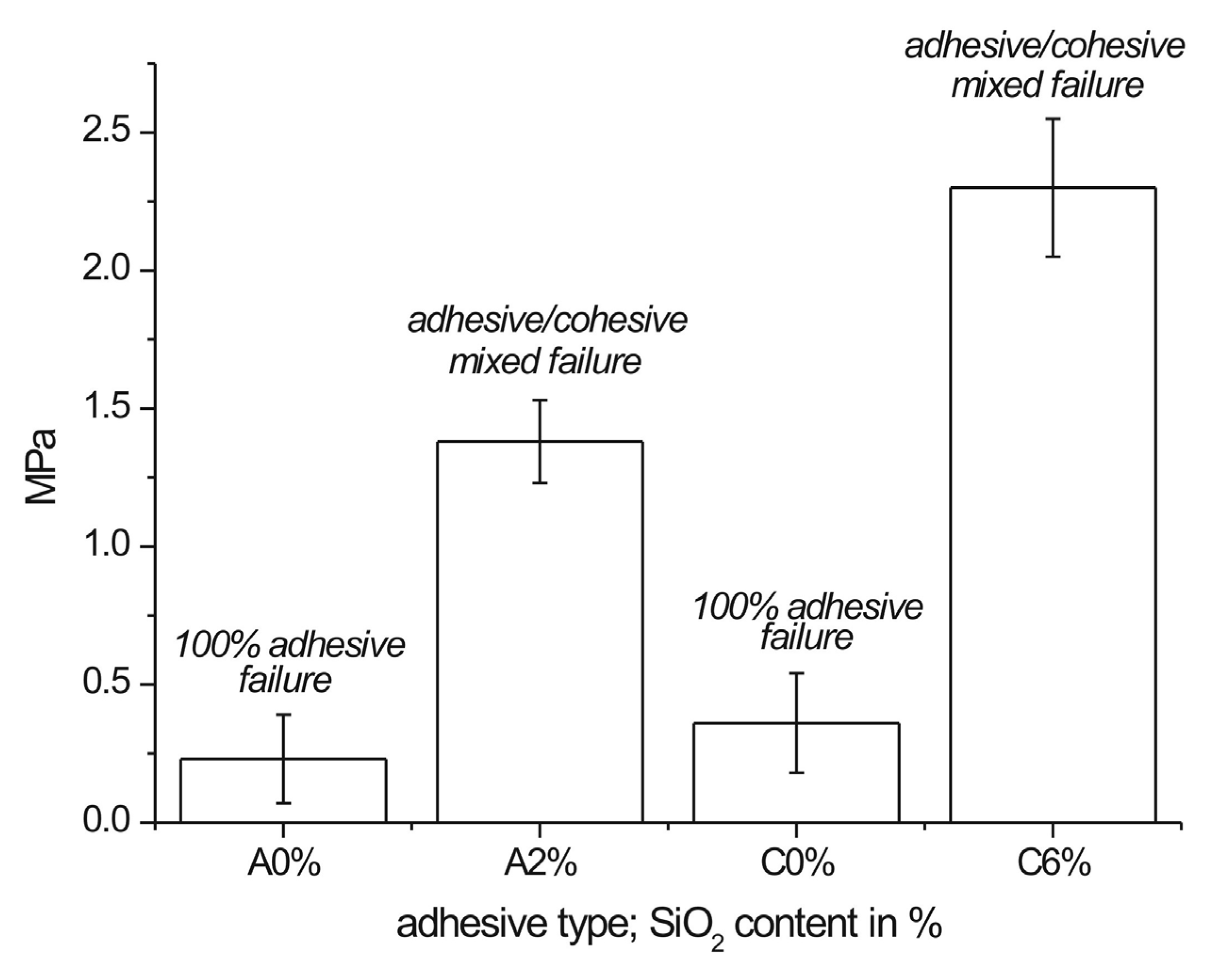
3.5. Pull-Off Adhesion Test
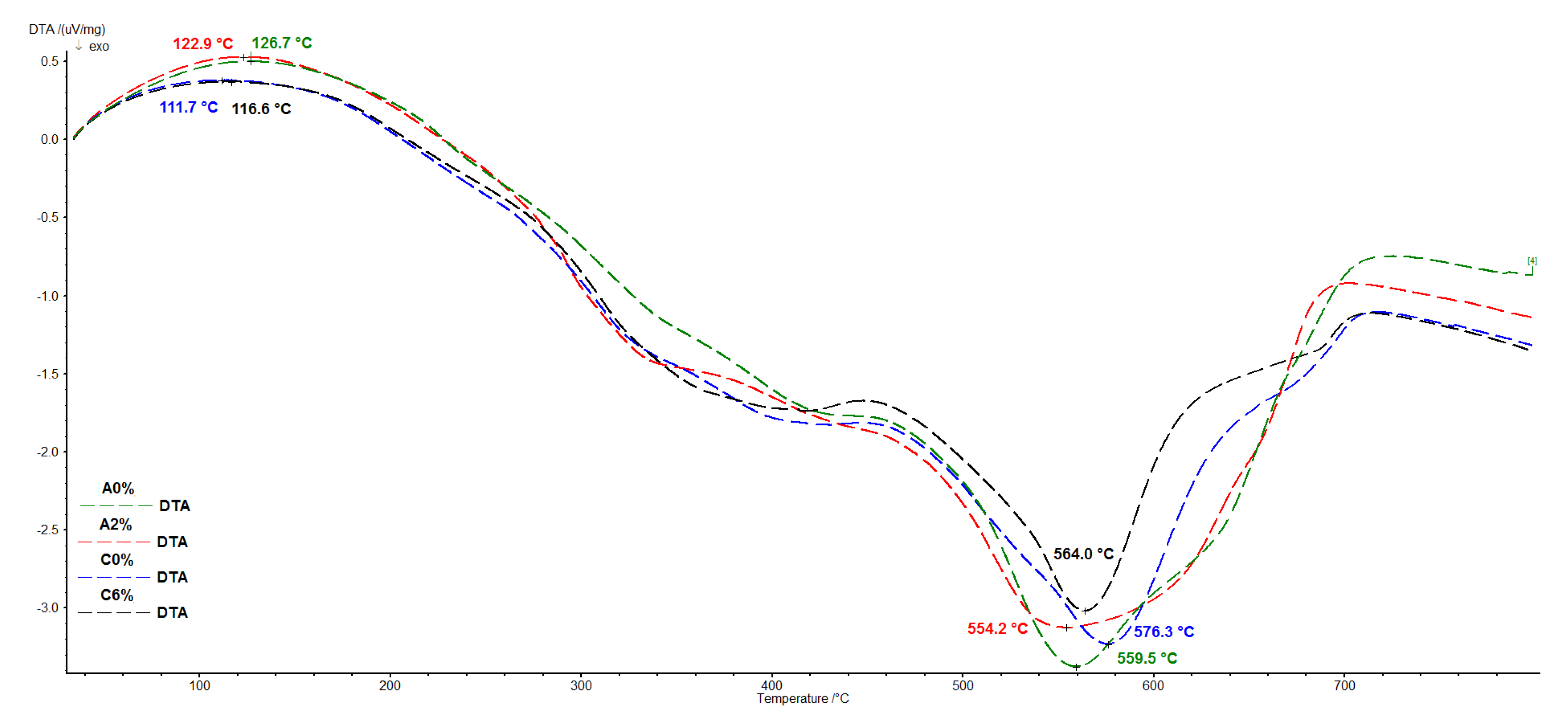
3.6. Thermal Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tohmura, S.; Li, G.; Qin, E. Preparation and characterization of wood polyalcohol-based isocyanate adhesives. J. Appl. Polym. Sci. 2005, 98, 791–795. [Google Scholar] [CrossRef]
- Juhaida, M.F.; Paridah, M.T.; Mohd Hilmi, M.; Sarni, Z.; Jalaluddin, H.; Mohamad Zaki, A.R. Liquefaction of kenaf (Hibiscus cannabinus L.) core for wood laminating adhesive. Bioresour. Technol. 2010, 101, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zheng, Z.; Hse, C.Y. Microwave-assisted liquefaction of wood with polyhydric alcohols and its application in preparation of polyurethane (PU) foams. Eur. J. Wood Prod. 2012, 70, 461–470. [Google Scholar] [CrossRef]
- Lee, W.J.; Chao, C.Y. Effect of containing polyhydric alcohol liquefied wood on the properties of thermoplastic polyurethane resins. Eur. J. Wood Prod. 2018, 76, 1745–1752. [Google Scholar] [CrossRef]
- Borowicz, M.; Paciorek-Sadowska, J.; Lubczak, J.; Czupryński, B. Biodegradable, flame-retardant, and bio-based rigid polyurethane/polyisocyanurate foams for thermal insulation application. Polymers 2019, 11, 1816. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, C.; Chen, Q.; Ingram, I.D.V.; Zeng, X.; Ren, T.; Xie, H. Sustainable aromatic aliphatic polyesters and polyurethanes prepared from vanillin-derived diols via green catalysis. Polymers 2020, 12, 586. [Google Scholar] [CrossRef]
- Ivdre, A.; Abolins, A.; Sevastyanova, I.; Kirpluks, M.; Cabulis, U.; Merijs-Meri, R. Rigid polyurethane foams with various isocyanate indices based on polyols from rapeseed oil and waste PET. Polymers 2020, 12, 738. [Google Scholar] [CrossRef]
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers 2019, 11, 1202. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; p. 12. [Google Scholar]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilization of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Nagy, L.; Nagy, M.; Vadkerti, B.; Daróczi, L.; Deák, G.; Zsuga, M.; Kéki, S. Designed polyurethanes for potential biomedical and pharmaceutical applications: Novel synthetic strategy for preparing sucrose containing biocompatible and biodegradable polyurethane networks. Polymers 2019, 11, 825. [Google Scholar] [CrossRef]
- Gama, N.V.; Amaral, C.; Silva, T.; Vicente, R.; Pereira Coutinho, J.A.; Barros-Timmons, A.; Ferreira, A. Thermal energy storage and mechanical performance of crude glycerol polyurethane composite foams containing phase change materials and expandable graphite. Materials 2018, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, Y. Two-step sequential liquefaction of lignocellulosic biomass by crude glycerol for the production of polyols and polyurethane foams. Bioresour. Technol. 2014, 161, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Savelyev, Y.; Markovskaya, L.; Savelyeva, O.; Akhranovich, E.; Parkhomenko, N.; Travinskaya, T. Degradable polyurethane foams based on disaccharides. J. Appl. Polym. Sci. 2015, 132, 42131. [Google Scholar] [CrossRef]
- Globe Newswire. Industrial Sugar Market to Reach USD 45.6 Billion by 2027. Reports and Data. 29 July 2020. Available online: https://www.globenewswire.com/news-release/2020/07/29/2069858/0/en/Industrial-Sugar-Market-To-Reach-USD-45-6-Billion-By-2027-Reports-and-Data.html (accessed on 25 August 2020).
- Deng, S.; Pizzi, A.; Du, G.; Lagel, M.C.; Delmotte, L.; Abdalla, S. Synthesis, structure characterization and application of melamine-glyoxal adhesive resins. Eur. J. Wood Prod. 2018, 76, 283–296. [Google Scholar] [CrossRef]
- Santiago-Medina, F.; Foyer, G.; Pizzi, A.; Caillol, S.; Delmotte, L. Lignin-derived non-toxic aldehydes for ecofriendly tannin adhesives for wood panels. Int. J. Adhes. Adhes. 2016, 70, 239–248. [Google Scholar] [CrossRef]
- Zhao, Z.; Sakai, S.; Wu, D.; Chen, Z.; Zhu, N.; Huang, C.; Sun, S.; Zhang, M.; Umemura, K.; Yong, Q. Further exploration of sucrose–citric acid adhesive: Investigation of optimal hot-pressing conditions for plywood and curing behavior. Polymers 2019, 11, 1996. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, Z.; Umemura, K. Further Exploration of sucrose-citric acid adhesive: Synthesis and application on plywood. Polymers 2019, 11, 1875. [Google Scholar] [CrossRef]
- Jasiūnas, L.; Peck, G.; Bridžiuvienė, D.; Miknius, L. Mechanical, thermal properties and stability of high renewable content liquefied residual biomass derived bio-polyurethane wood adhesives. Int. J. Adhes. Adhes. 2020, 101, 102618. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Carmen Sánchez, M.; Franco, J.M. Preparation, characterization and mechanical properties of bio-based polyurethane adhesives from isocyanate-functionalized cellulose acetate and castor oil for bonding wood. Polymers 2017, 9, 132. [Google Scholar] [CrossRef]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef]
- EN 312 Particleboards—Specifications; European Committee for Standardization: Brussels, Belgium, 2011.
- EN 622 Fiberboards—Specifications; European Committee for Standardization: Brussels, Belgium, 2006.
- Moubarik, A.; Pizzi, A.; Allal, A.; Charrier, F.; Khoukh, A.; Charrier, B. Cornstarch—Mimosa tannin—Urea formaldehyde resins as adhesives in the particleboard production. Starch-Stӓrke 2010, 62, 131–138. [Google Scholar] [CrossRef]
- Bahattab, M.A.; Donate-Robles, J.; García-Pacios, V.; Martín-Martínez, J.M. Characterization of polyurethane adhesives containing nanosilicas of different particle size. Int. J. Adhes. Adhes. 2011, 31, 97–103. [Google Scholar] [CrossRef]
- Randall, D.; Lee, S. (Eds.) The Polyurethanes Book; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Evance, A.G. The strength of brittle materials containing second phase dispersions. Phil. Mag. 1972, 26, 1327–1344. [Google Scholar] [CrossRef]
- Green, D.G.; Nicholson, P.S.; Embury, J.D. Fracture of a brittle particulate composite, Part 2: Theoretical aspects. J. Mater. Sci. 1979, 14, 1657–1661. [Google Scholar] [CrossRef]
- Imanaka, M.; Takeuchi, Y.; Nakamura, Y.; Nishimura, A.; Iida, T. Fracture toughness of spherical silica-filled epoxy adhesives. Int. J. Adhes. Adhes. 2001, 21, 389–396. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Javni, I.; Waddon, A.; Banhegyi, G. Structure and properties of polyurethane—Silica nanocomposites, J. Appl. Polym. Sci. 2000, 76, 133–151. [Google Scholar] [CrossRef]
- He, H.; Pang, Y.; Duan, Z.; Luo, N.; Wang, Z. The Strengthening and toughening of biodegradable poly (lactic acid) using the SiO2-PBA core—Shell nanoparticle. Materials 2019, 12, 2510. [Google Scholar] [CrossRef]
- Torró-Palau, A.M.; Fernández-García, J.C.; Orgilés-Barceló, A.C.; Martín-Martínez, J.M. Characterization of polyurethanes containing different silicas. Int. J. Adhes. Adhes. 2001, 21, 1–9. [Google Scholar] [CrossRef]
- Kuriyagawa, M.; Kawamura, T.; Hayashi, S.; Nitta, K.-H. Reinforcement of polyurethane-based shape memory polymer by hindered phenol compounds and silica particles. J. Appl. Polym. Sci. 2010, 117, 1695–1702. [Google Scholar] [CrossRef]
- Rokicki, G.; Rakoczy, P.; Parzuchowski, P.; Sobiecki, M. Hyperbranched aliphatic polyethers obtained from environmentally benign monomer: Glycerol carbonate. Green Chem. 2005, 7, 529–539. [Google Scholar] [CrossRef]
- Mamiński, M.; Parzuchowski, P.; Trojanowska, A.; Dziewulski, S. Fast-curing polyurethane adhesives derived from environmentally friendly hyperbranched polyglycerols—The effect of macromonomer structure. Biomass Bioenerg. 2011, 35, 4461–4468. [Google Scholar] [CrossRef]
- EN 205 Adhesives. Wood Adhesives for Non-Structural Applications. Determination of Tensile Shear Strength of Lap Joints; European Committee for Standardization: Brussels, Belgium, 2003. [Google Scholar]
- EN 311 Wood-Based Panels—Surface Soundness—Test Method; European Committee for Standardization: Brussels, Belgium, 2002.
- Wolkenhauer, A.; Avramidis, G.; Hauswald, E.; Militz, H.; Viöl, W. Sanding vs. plasma preatment of aged wood: A comparison with respect to surface energy. Int. J. Adhes. Adhes. 2009, 29, 18–22. [Google Scholar] [CrossRef]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloid. Surface. A 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Chiacchiarelli, L.M.; Puri, I.; Puglia, D.; Kenny, J.M.; Torre, L. Cure kinetics of a highly reactive silica—Polyurethane nanocomposite. Thermochim. Acta 2012, 549, 172–178. [Google Scholar] [CrossRef]
- Mamiński, M. Effect of hydroxyl functionality of branched polyol component on PUR curing thermal effect. Ann. Warsaw Univ. Life Sci. SGGW For. Wood Technol. 2012, 78, 238–242. [Google Scholar]
- Lengyel, K.; Barbu, M.C.; Campean, M.; Badin, N.; Bedelean, B. Improving properties of particleboards with reduced density. Bioresources 2018, 13, 1289–1302. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, J.; Chen, S.; Huang, Y.; Ding, F.; Guo, W.; Lei, D.; Yang, L.; Qing, F.-L.; Sou, Z. CO2-based poly (propylene carbonate) with various carbonate linkage content for reactive hot-melt polyurethane adhesives. Int. J. Adhes. Adhes. 2020, 96, 102456. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Huang, J.-Q.; Sun, L.-J.; Lei, D.; Cao, J.; Chen, S.; Shih, W.-C.; Qing, F.-L.; Sou, Z.-W. PPC-based reactive hot melt polyurethane adhesive (RHMPA)—Efficient glues for multiple types of substrates. Chin. J. Polym. Sci. 2018, 36, 58–64. [Google Scholar] [CrossRef]
- Malaki, M.; Hashemzadeh, Y.; Karevan, M. Effect of nano-silica on the mechanical properties of acrylic-polyurethane coatings. Prog. Org. Coat. 2016, 101, 477–485. [Google Scholar] [CrossRef]
- Sernek, M.; Boonstra, M.; Pizzi, A.; Despres, A.; Gerardin, P. Bonding performance of heat treated wood with structural adhesives. Holz Roh. Werkst. 2008, 66, 173–180. [Google Scholar] [CrossRef]
- Nuñes, R.; Fonseca, J.; Pereira, M. Polymer-filler interactions and mechanicalproperties of a polyurethane elastomer. Polym. Test. 2000, 19, 93–103. [Google Scholar] [CrossRef]
- Serkis, M.; Špirková, M.; Kredatusova, J.; Hodan, J.; Bureš, R. Organic-inorganic nanocomposite films made from polyurethane dispersions and colloidal silica particles. Compos. Interface 2016, 23, 157–173. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Jia, Z.; Luo, Y.; Jia, D. Use of precipitated silica with silanol groups as an inorganic chain extender in polyurethane. Mater. Design. 2015, 87, 324–330. [Google Scholar] [CrossRef]
- Beck, G.; Hill, C.; Cocher, P.M.; Alfredsen, G. Accessibility of hydroxyl groups in furfurylated wood at different weight percent gains and during Rhodonia placenta decay. Eur. J. Wood Prod. 2019, 77, 953–955. [Google Scholar] [CrossRef]
- Qin, R.-Y.; Schreiber, H.P. Adhesion at partially restructured polymer surfaces. Colloid. Surface 1999, 156, 85–93. [Google Scholar] [CrossRef]
- Meera, K.M.S.; Sankar, M.R.; Paul, J.; Jaisankar, S.N.; Manda, A.B. The influence of applied silica nanoparticles on a bio-renewable castor oil based polyurethane nanocomposite and its physicochemical properties. Phys. Chem. Chem. Phys. 2014, 16, 9276–9288. [Google Scholar] [CrossRef]
- Vega-Baudrit, J.; Sibaja-Ballestero, M.; Vázquez, P.; Nuñez, S.; Martín-Martínez, J.M.; Benavides-Rodríguez, L. Kinetics of isothermal degradation studies in adhesives by thermogravimetric data: Effect of hydrophilic nanosilica fillers on the thermal properties of thermoplastic polyurethane-silica nanocomposites. Recent Pat. Nanotech. 2008, 2, 220–226. [Google Scholar] [CrossRef]


| Polyol | Hydroxyl Number [mg KOH/g] | Hydroxyl Functionality | Viscosity at 25 °C [Pa·s] | |
|---|---|---|---|---|
| - | - | - | Non-filled | SiO2-filled |
| A | 694 | 9 a | 10 | 400 |
| C | 425 | 5 | 0.70 | 275 |
| Liquid | γ | γD | γP |
|---|---|---|---|
| Diiodomethane | 50.80 | 50.80 | 0.00 |
| Water | 72.80 | 21.90 | 51.00 |
| Polyol | SiO2-Filler Addition [wt%] | Gelling Time [s] |
|---|---|---|
| A | 0 | 510 |
| A | 2.0 | 600 |
| C | 0 | 600 |
| C | 6.0 | 2100 |
| Characteristics | - | Polyol Used | |||
|---|---|---|---|---|---|
| - | A0% SiO2 | A2% SiO2 | C0% SiO2 | C6% SiO2 | |
| contact angle θ [°] | water | 56.9 ± 2.3 | 23.1 ± 4.0 | 53.5 ± 1.6 | 27.7 ± 0.7 |
| diiodomethane | 45.3 ± 2.4 | 17.8 ± 0.3 | 38.1 ± 0.9 | 38.0 ± 2.2 | |
| surface free energy [mJ/m2] | 51.7 ± 2.0 | 75.0 ± 1.3 | 55.0 ± 1.7 | 70.7 ± 1.8 | |
| work of adhesion [mJ/m2] | 112.0 ± 1.5 | 139.7 ± 1.8 | 116.5 ± 1.6 | 137.4 ± 2.0 | |
| Sample | Temperature of Weight Loss [°C] | Residual Weight at 800 °C [%] | ||
|---|---|---|---|---|
| 5% | 50% | 80% | ||
| A0% SiO2 | 207.8 | 421.2 | 592.4 | 1.21 |
| A2% SiO2 | 189.0 | 406.1 | 580.4 | 3.87 |
| C0% SiO2 | 163.3 | 387.3 | 567.3 | 1.20 |
| C6% SiO2 | 156.2 | 367.7 | 559.5 | 6.25 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamiński, M.Ł.; Więcław-Midor, A.M.; Parzuchowski, P.G. The Effect of Silica-Filler on Polyurethane Adhesives Based on Renewable Resource for Wood Bonding. Polymers 2020, 12, 2177. https://doi.org/10.3390/polym12102177
Mamiński MŁ, Więcław-Midor AM, Parzuchowski PG. The Effect of Silica-Filler on Polyurethane Adhesives Based on Renewable Resource for Wood Bonding. Polymers. 2020; 12(10):2177. https://doi.org/10.3390/polym12102177
Chicago/Turabian StyleMamiński, Mariusz Ł., Anna M. Więcław-Midor, and Paweł G. Parzuchowski. 2020. "The Effect of Silica-Filler on Polyurethane Adhesives Based on Renewable Resource for Wood Bonding" Polymers 12, no. 10: 2177. https://doi.org/10.3390/polym12102177
APA StyleMamiński, M. Ł., Więcław-Midor, A. M., & Parzuchowski, P. G. (2020). The Effect of Silica-Filler on Polyurethane Adhesives Based on Renewable Resource for Wood Bonding. Polymers, 12(10), 2177. https://doi.org/10.3390/polym12102177







