Insight into Hyper-Branched Aluminum Phosphonate in Combination with Multiple Phosphorus Synergies for Fire-Safe Epoxy Resin Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
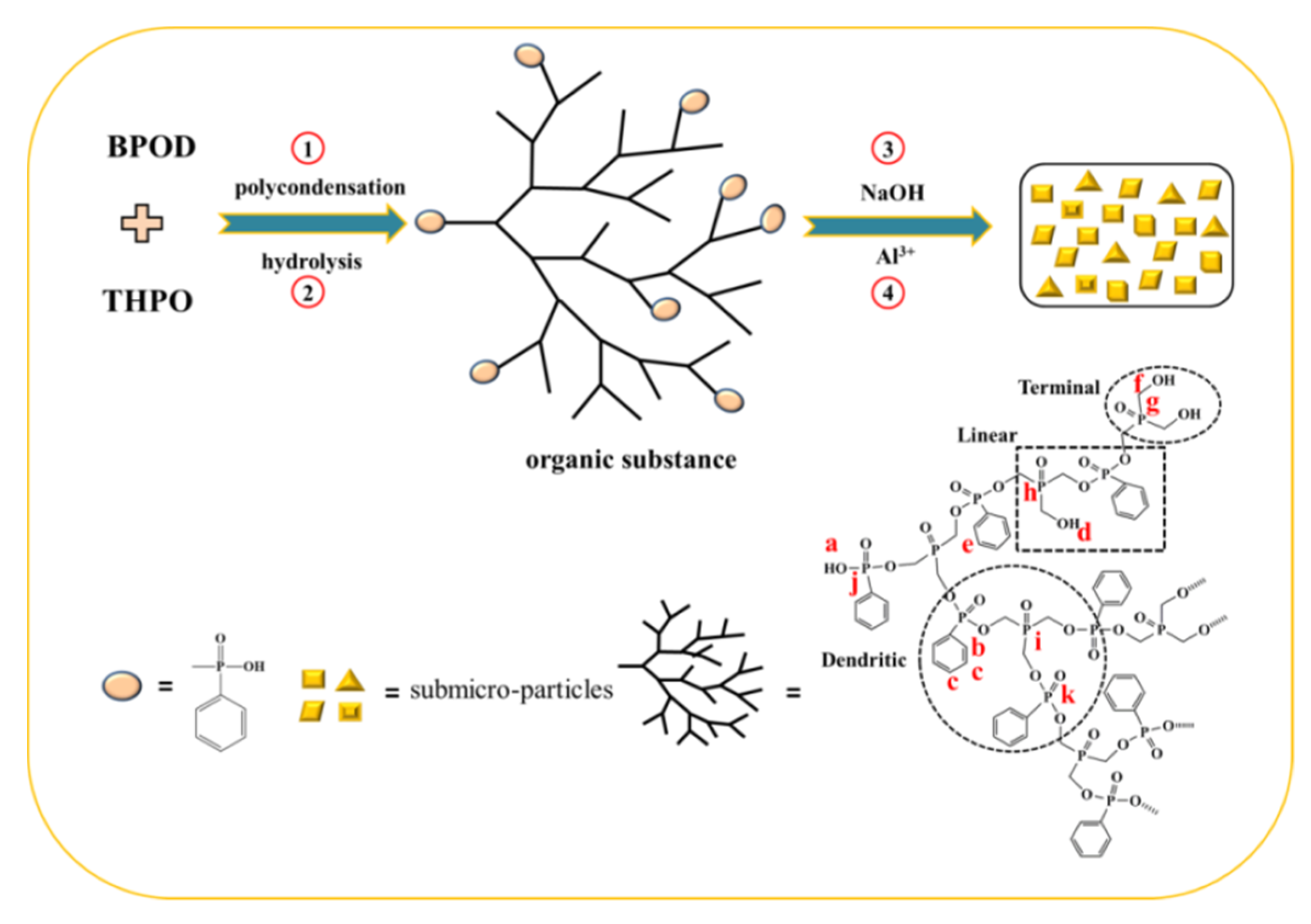
2.2. Synthesis of the Precursor
2.3. Synthesis of AHPP Submicro-Particles
2.4. Fabrication of EP/DOPO-AHPP Composites
3. Results and Discussion
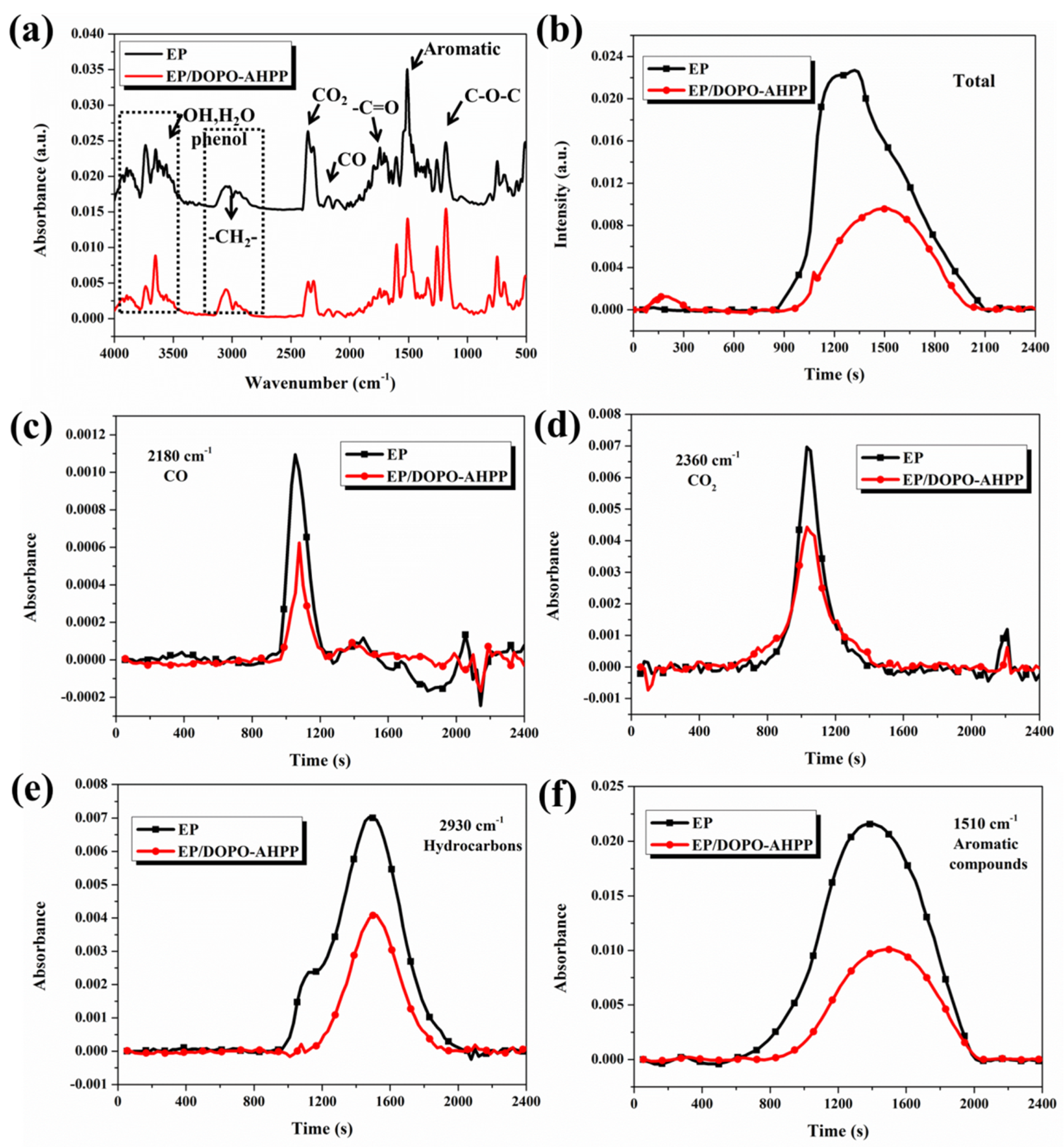
3.1. Structural Characterizations
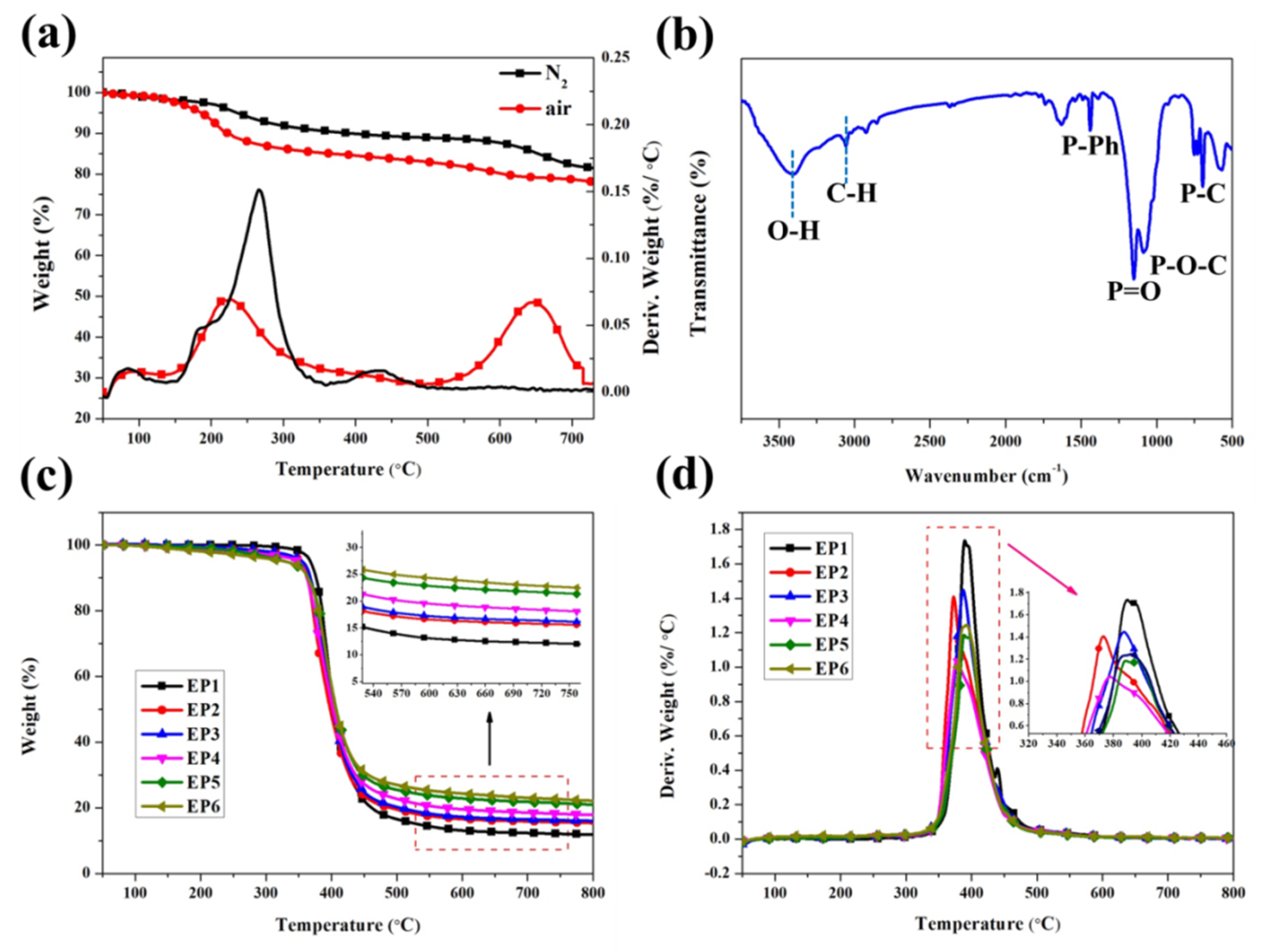
3.2. Characterization of the Degradation Behavior
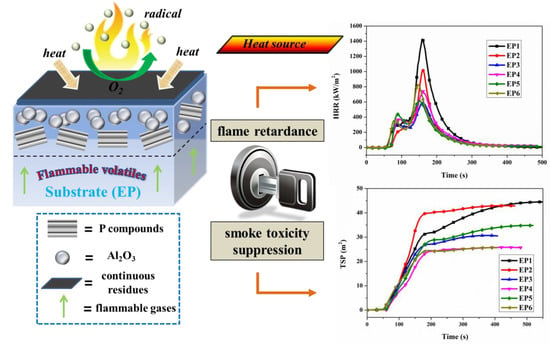
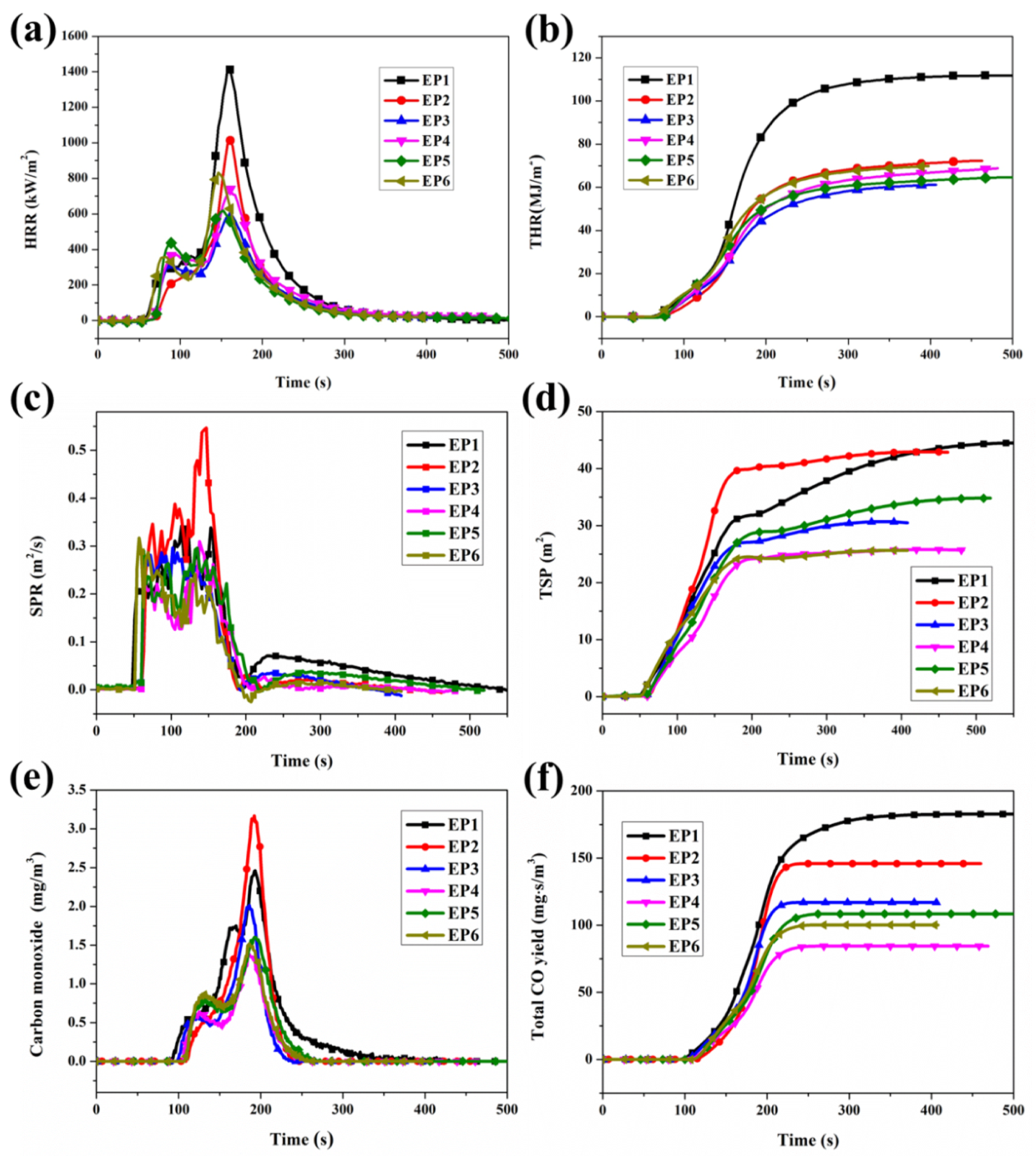
3.3. Flame Retardance
3.4. Smoke and Toxicity Hazards Analysis
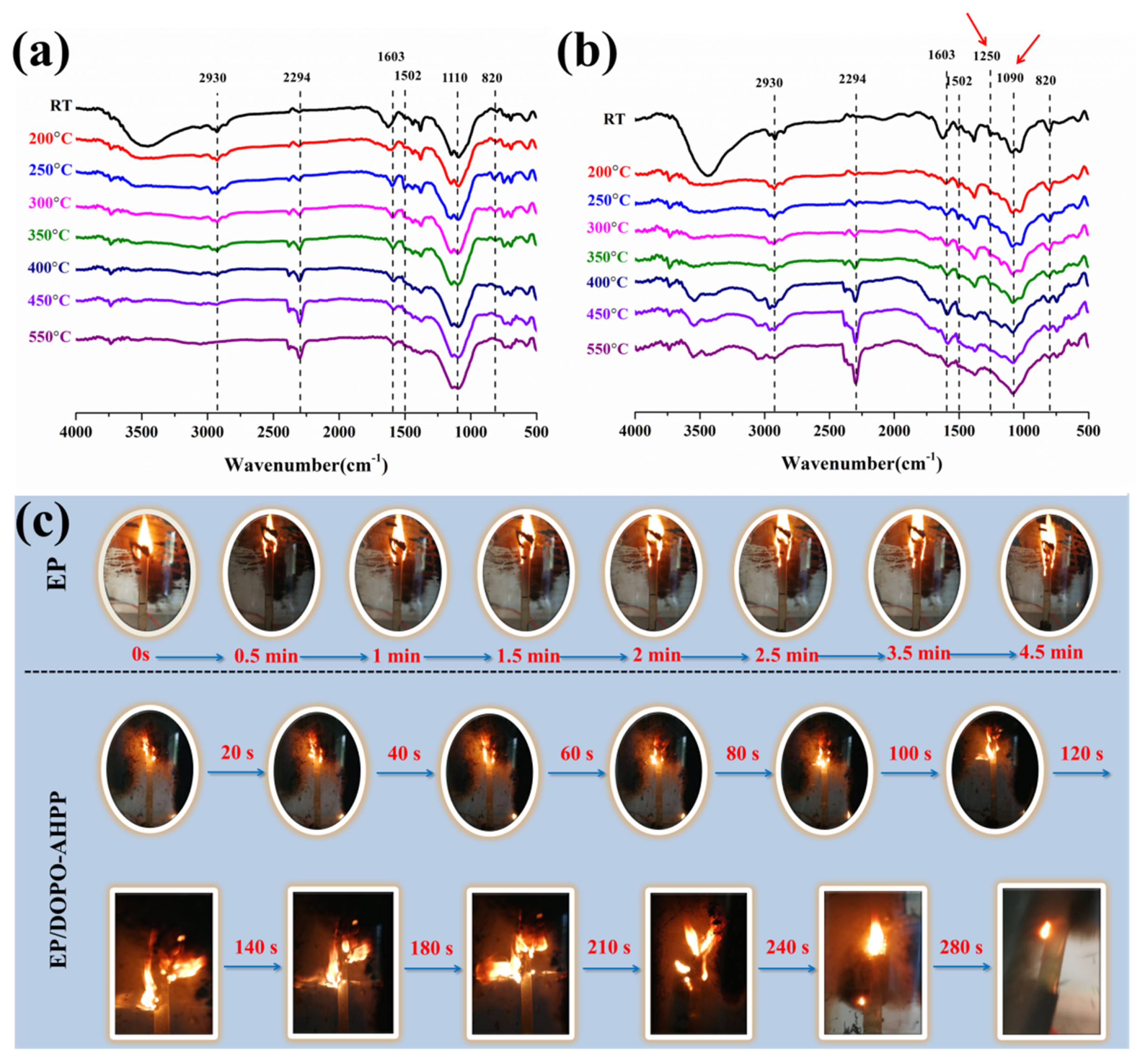
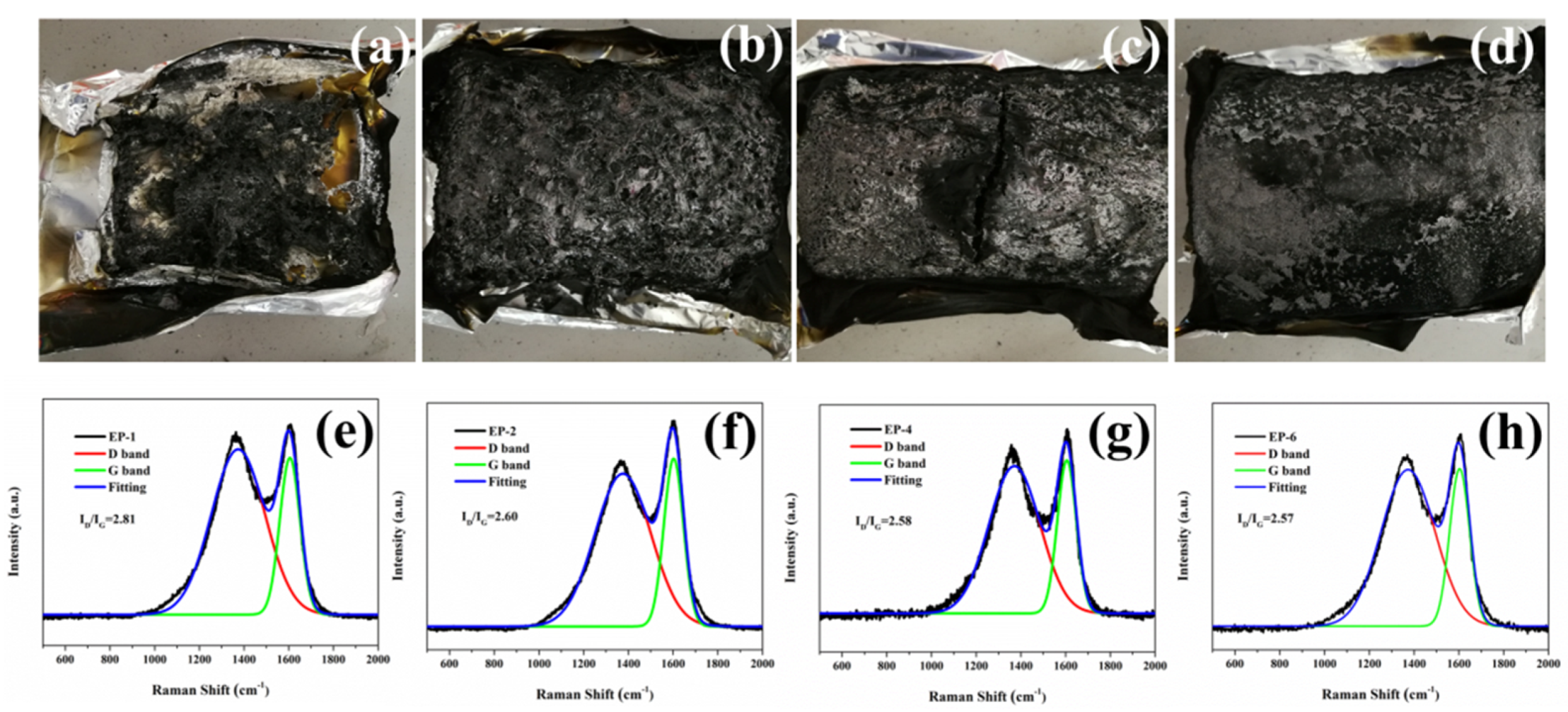
3.5. Flame Retardation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J. Vanillin-derived high-performance flame retardant epoxy resins: Facile synthesis and properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Auvergne, R.; Caillol, S.; David, G.; Boutevin, B.; Pascault, J.P. Biobased thermosetting epoxy: Present and future. Chem. Rev. 2013, 114, 1082–1115. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Huo, S.; Wang, M.; Cheng, L. Synthesis of a phosphorus/nitrogen-containing additive with multifunctional groups and its flame-retardant effect in epoxy resin. Ind. Eng. Chem. Res. 2015, 54, 7777–7786. [Google Scholar] [CrossRef]
- You, G.; Cheng, Z.; Tang, Y.; He, H. Functional group effect on char formation, flame retardancy and mechanical properties of phosphonate-triazine-based compound as flame retardant in epoxy resin. Ind. Eng. Chem. Res. 2015, 54, 7309–7319. [Google Scholar] [CrossRef]
- Wan, J.; Li, C.; Bu, Z.Y.; Xu, C.J.; Li, B.G.; Fan, H. A comparative study of epoxy resin cured with a linear diamine and a branched polyamine. Chem. Eng. J. 2012, 188, 160–172. [Google Scholar] [CrossRef]
- Jian, R.; Wang, P.; Duan, W.; Wang, J.; Zheng, X.; Weng, J. Synthesis of a novel P/N/S-containing flame retardant and its application in epoxy resin: Thermal property, flame retardance, and pyrolysis behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Li, Z.; Wang, X.; Wang, D.Y. A novel biobased epoxy resin with high mechanical stiffness and low flammability: Synthesis, characterization and properties. J. Mater. Chem. A 2015, 3, 21907–21921. [Google Scholar] [CrossRef]
- Starost, K.; Frijns, E.; Van Laer, J.; Faisal, N.; Egizabal, A.; Elizextea, C.; Blazquez, M.; Nelissen, I.; Njuguna, J. Assessment of nanoparticles release into the environment during drilling of carbon nanotubes/epoxy and carbon nanofibres/epoxy nanocomposites. J. Hazard. Mater. 2017, 340, 57–66. [Google Scholar] [CrossRef]
- Yu, B.; Shi, Y.; Yuan, B.; Qiu, S.; Xing, W.; Hu, W.; Song, L.; Lo, S.; Hu, Y. Enhanced thermal and flame retardant properties of flame-retardant-wrapped graphene/epoxy resin nanocomposites. J. Mater. Chem. A 2015, 3, 8034–8044. [Google Scholar] [CrossRef]
- Wang, W.; Kan, Y.; Liu, J.; Liew, K.M.; Liu, L.; Hu, Y. Self-assembly of zinc hydroxystannate on amorphous hydrous TiO2 solid sphere for enhancing fire safety of epoxy resin. J. Hazard. Mater. 2017, 340, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, X.; Wu, D. Novel spirocyclic phosphazene-based epoxy resin for halogen-free fire resistance: Synthesis, curing behaviors, and flammability characteristics. ACS Appl. Mater. Interfaces 2012, 4, 4047–4061. [Google Scholar] [CrossRef] [PubMed]
- Allauddin, S.; Narayan, R.; Raju, K. Synthesis and properties of alkoxysilane castor oil and their polyurethane/urea–silica hybrid coating films. ACS Sustain. Chem. Eng. 2013, 1, 910–918. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, Y.; Liu, Y.; Wang, Q.; He, Y.; Wang, B. Intergrowth charring for flame-retardant glass fabric-reinforced epoxy resin composites. J. Mater. Chem. A 2015, 3, 4284–4290. [Google Scholar] [CrossRef]
- Chen, X.; Ye, J.; Yuan, L.; Liang, G.; Gu, A. Multi-functional ladderlike polysiloxane: Synthesis, characterization and its high performance flame retarding bismaleimide resins with simultaneously improved thermal resistance, dimensional stability and dielectric properties. J. Mater. Chem. A 2014, 2, 7491–7501. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Duan, L.; Gui, Z.; Wang, B.; Hu, Y.; Yuen, R.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stöß, K.; Ciesielski, M.; Diederichs, J.; Döring, M.; Krämer, J.; Altstädt, V. A New Halogen-Free Flame Retardant Based on 9, 10-Dihydro-9-oxa-10-vphosphaphenanthrene-10-oxide for Epoxy Resins and their Carbon Fiber Composites for the Automotive and Aviation Industries. Macromol. Mater. Eng. 2011, 296, 14–30. [Google Scholar] [CrossRef]
- Müller, P.; Bykov, Y.; Döring, M. New star-shaped phosphorus-containing flame retardants based on acrylates for epoxy resins. Polym. Adv. Technol. 2013, 24, 834–840. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, Z.; Qian, L.; Hao, J. Pyrolysis and flame retardant behavior of a novel compound with multiple phosphaphenanthrene groups in epoxy thermosets. J. Anal. Appl. Pyrolysis 2017, 127, 23–30. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.; Altstädt, V. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Mariappan, T.; Zhou, Y.; Hao, J.; Wilkie, C.A. Influence of oxidation state of phosphorus on the thermal and flammability of polyurea and epoxy resin. Eur. Polym. J. 2013, 49, 3171–3180. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Yang, J.; Zeng, Z.; Chen, Y. Self-initiated photopolymerization of hyperbranched acrylates. Polymer 2009, 50, 4325–4333. [Google Scholar] [CrossRef]
- Liu, J.; Huang, W.; Zhou, Y.; Yan, D. Synthesis of hyperbranched polyphosphates by self-condensing ring-opening polymerization of HEEP without catalyst. Macromolecules 2009, 42, 4394–4399. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Z.; Hong, C.; Liu, Y.; Pan, C. Michael addition polymerizations of difunctional amines (AA′) and triacrylamides (B3). J. Polym. Sci. Part A Polym. Chem. 2006, 44, 6226–6242. [Google Scholar] [CrossRef]
- Yang, W.; Yuen, R.K.; Hu, Y.; Lu, H.; Song, L. Development and characterization of fire retarded glass-fiber reinforced poly (1, 4-butylene terephthalate) composites based on a novel flame retardant system. Ind. Eng. Chem. Res. 2011, 50, 11975–11981. [Google Scholar] [CrossRef]
- Pinto, U.A.; Visconte, L.L.Y.; Nunes, R.C.R. Mechanical properties of thermoplastic polyurethane elastomers with mica and aluminum trihydrate. Eur. Polym. J. 2001, 37, 1935–1937. [Google Scholar] [CrossRef]
- Yuan, Y.; Shi, Y.; Yu, B.; Zhan, J.; Zhang, Y.; Song, L.; Ma, C.; Hu, Y. Facile synthesis of aluminum branched oligo (phenylphosphonate) submicro-particles with enhanced flame retardance and smoke toxicity suppression for epoxy resin composites. J. Hazard. Mater. 2020, 381, 121233. [Google Scholar] [CrossRef]
- Xie, J.; Hu, L.; Shi, W.; Deng, X.; Cao, Z.; Shen, Q. Synthesis and characterization of hyperbranched polytriazole via an ‘A2+ B3’approach based on click chemistry. Polym. Int. 2008, 57, 965–974. [Google Scholar] [CrossRef]
- Daasch, L.; Smith, D. Infrared spectra of phosphorus compounds. Anal. Chem. 1951, 23, 853–868. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, H.; Yu, B.; Shi, Y.; Wang, W.; Song, L.; Hu, Y.; Zhang, Y. Phosphorus and nitrogen-containing polyols: Synergistic effect on the thermal property and flame retardancy of rigid polyurethane foam composites. Ind. Eng. Chem. Res. 2016, 55, 10813–10822. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, W. Photopolymerization and thermal behaviors of acrylated benzenephosphonates/epoxy acrylate as flame retardant resins. Eur. Polym. J. 2006, 42, 2261–2269. [Google Scholar] [CrossRef]
- Xu, W.; Wirasaputra, A.; Liu, S.; Yuan, Y.; Zhao, J. Highly effective flame retarded epoxy resin cured by DOPO-based co-curing agent. Polym. Degrad. Stab. 2015, 122, 44–51. [Google Scholar] [CrossRef]
- Perret, B.; Schartel, B.; Stöß, K.; Ciesielski, M.; Diederichs, J.; Döring, M.; Krämer, J.; Altstädt, V. Novel DOPO-based flame retardants in high-performance carbon fibre epoxy composites for aviation. Eur. Polym. J. 2011, 47, 1081–1089. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, Y.; Yang, L.; Cai, Y.; Song, L.; Chen, Z.; Fan, W. Fire retardant synergism between melamine and triphenyl phosphate in poly (butylene terephthalate). Polym. Degrad. Stab. 2006, 91, 2093–2100. [Google Scholar] [CrossRef]
- Wang, G.A.; Cheng, W.M.; Tu, Y.L.; Wang, C.C.; Chen, C.Y. Characterizations of a new flame-retardant polymer. Polym. Degrad. Stab. 2006, 91, 3344–3353. [Google Scholar] [CrossRef]
- Liu, C.; Chen, T.; Yuan, C.; Song, C.; Chang, Y.; Chen, G.; Xu, Y.; Dai, L. Modification of epoxy resin through the self-assembly of a surfactant-like multi-element flame retardant. J. Mater. Chem. A 2016, 4, 3462–3470. [Google Scholar] [CrossRef]
- Xi, W.; Qian, L.; Chen, Y.; Wang, J.; Liu, X. Addition flame-retardant behaviors of expandable graphite and [bis (2-hydroxyethyl) amino]-methyl-phosphonic acid dimethyl ester in rigid polyurethane foams. Polym. Degrad. Stab. 2015, 122, 36–43. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J.; Wu, P. Preparation of organically dispersible graphene nanosheet powders through a lyophilization method and their poly (lactic acid) composites. Carbon 2010, 48, 3834–3839. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Z. Facile synthesis of a novel magnesium amino-tris-(methylenephosphonate)-reduced graphene oxide hybrid and its high performance in mechanical strength, thermal stability, smoke suppression and flame retardancy in phenolic foam. J. Hazard. Mater. 2018, 357, 89–99. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire. Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Feng, X.; Xing, W.; Song, L.; Hu, Y. In situ synthesis of a MoS2/CoOOH hybrid by a facile wet chemical method and the catalytic oxidation of CO in epoxy resin during decomposition. J. Mater. Chem. A 2014, 2, 13299–13308. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, J.; Xue, Y.; He, C.; Zhou, X.; Xie, X.; Ye, Y.; Mai, Y.W. Simultaneous improvement in the flame resistance and thermal conductivity of epoxy/Al2O3 composites by incorporating polymeric flame retardant-functionalized graphene. J. Mater. Chem. A 2017, 5, 13544–13556. [Google Scholar] [CrossRef]
- Wawrzyn, E.; Schartel, B.; Ciesielski, M.; Kretzschmar, B.; Braun, U.; Döring, M. Are novel aryl phosphates competitors for bisphenol A bis (diphenyl phosphate) in halogen-free flame-retarded polycarbonate/acrylonitrile-butadiene-styrene blends? Eur. Polym. J. 2012, 48, 1561–1574. [Google Scholar] [CrossRef]
- Yu, B.; Tawiah, B.; Wang, L.Q.; Yuen, A.C.Y.; Zhang, Z.C.; Shen, L.L.; Lin, B.; Fei, B.; Yang, W.; Li, A. Interface decoration of exfoliated MXene ultra-thin nanosheets for fire and smoke suppressions of thermoplastic polyurethane elastomer. J. Hazard. Mater. 2019, 374, 110–119. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, W.; Shi, Y.; Song, L.; Ma, C.; Hu, Y. The influence of highly dispersed Cu2O-anchored MoS2 hybrids on reducing smoke toxicity and fire hazards for rigid polyurethane foam. J. Hazard. Mater. 2019, 382, 121028. [Google Scholar] [CrossRef]
- Schartel, B.; Braun, U.; Balabanovich, A.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.; Altstädt, V. Pyrolysis and fire behaviour of epoxy systems containing a novel 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-(DOPO)-based diamino hardener. Eur. Polym. J. 2008, 44, 704–715. [Google Scholar] [CrossRef]
- Yu, L.; Chen, L.; Dong, L.P.; Li, L.J.; Wang, Y.Z. Organic–inorganic hybrid flame retardant: Preparation, characterization and application in EVA. RSC Adv. 2014, 4, 17812–17821. [Google Scholar] [CrossRef]


| Samples | EP (g) | DDM (g) | DOPO (g) | AHPP (g) | Proportion (DOPO/AHPP) | Additive Loading (wt. %) |
|---|---|---|---|---|---|---|
| EP-1 | 100 | 21.8 | - | - | - | - |
| EP-2 | 100 | 21.8 | 6.41 | - | - | 5 |
| EP-3 | 100 | 21.8 | 4.27 | 2.13 | 2/1 | 5 |
| EP-4 | 100 | 21.8 | 2.13 | 4.27 | 1/2 | 5 |
| EP-5 | 100 | 21.8 | 3.21 | 3.21 | 1/1 | 5 |
| EP-6 | 100 | 21.8 | - | 6.41 | - | 5 |
| Samples | Nitrogen | ||
|---|---|---|---|
| Td (°C) | Tmax (°C) | Char (%) | |
| EP-1 | 367 | 391 | 11.8 |
| EP-2 | 279 | 372 | 12.4 |
| EP-3 | 356 | 388 | 15.6 |
| EP-4 | 321 | 378 | 21.0 |
| EP-5 | 350 | 390 | 17.5 |
| EP-6 | 329 | 393 | 21.1 |
| Samples | LOI (%) | UL-94 | t1/t2 (s/s) | PHRR (kW/m2) | THR (MJ/m2) | SPR (m2/s) | CO (mg/m3) | Residues (wt. %) |
|---|---|---|---|---|---|---|---|---|
| EP-1 | 23.5 | NR | - | 1422 | 111.9 | 0.343 | 2.43 | 4.3 |
| EP-2 | 28.5 | NR | - | 1015 | 72.6 | 0.546 | 3.18 | 14.5 |
| EP-3 | 32 | V-0 | 2.4/1.7 | 578 | 60.9 | 0.295 | 1.59 | 18.4 |
| EP-4 | 31 | V-1 | 6.5/8.2 | 732 | 69.1 | 0.307 | 1.36 | 22.4 |
| EP-5 | 31.5 | V-0 | 1.5/4.2 | 607 | 65.0 | 0.316 | 1.52 | 21.0 |
| EP-6 | 28 | NR | - | 832 | 69.4 | 0.294 | 2.00 | 18.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Y.; Yu, B.; Shi, Y.; Mao, L.; Xie, J.; Pan, H.; Liu, Y.; Wang, W. Insight into Hyper-Branched Aluminum Phosphonate in Combination with Multiple Phosphorus Synergies for Fire-Safe Epoxy Resin Composites. Polymers 2020, 12, 64. https://doi.org/10.3390/polym12010064
Yuan Y, Yu B, Shi Y, Mao L, Xie J, Pan H, Liu Y, Wang W. Insight into Hyper-Branched Aluminum Phosphonate in Combination with Multiple Phosphorus Synergies for Fire-Safe Epoxy Resin Composites. Polymers. 2020; 12(1):64. https://doi.org/10.3390/polym12010064
Chicago/Turabian StyleYuan, Yao, Bin Yu, Yongqian Shi, Long Mao, Jianda Xie, Haifeng Pan, Yuejun Liu, and Wei Wang. 2020. "Insight into Hyper-Branched Aluminum Phosphonate in Combination with Multiple Phosphorus Synergies for Fire-Safe Epoxy Resin Composites" Polymers 12, no. 1: 64. https://doi.org/10.3390/polym12010064
APA StyleYuan, Y., Yu, B., Shi, Y., Mao, L., Xie, J., Pan, H., Liu, Y., & Wang, W. (2020). Insight into Hyper-Branched Aluminum Phosphonate in Combination with Multiple Phosphorus Synergies for Fire-Safe Epoxy Resin Composites. Polymers, 12(1), 64. https://doi.org/10.3390/polym12010064