Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Feather Keratin Solution
2.3. Preparation of Dialdehyde Carboxymethyl Cellulose
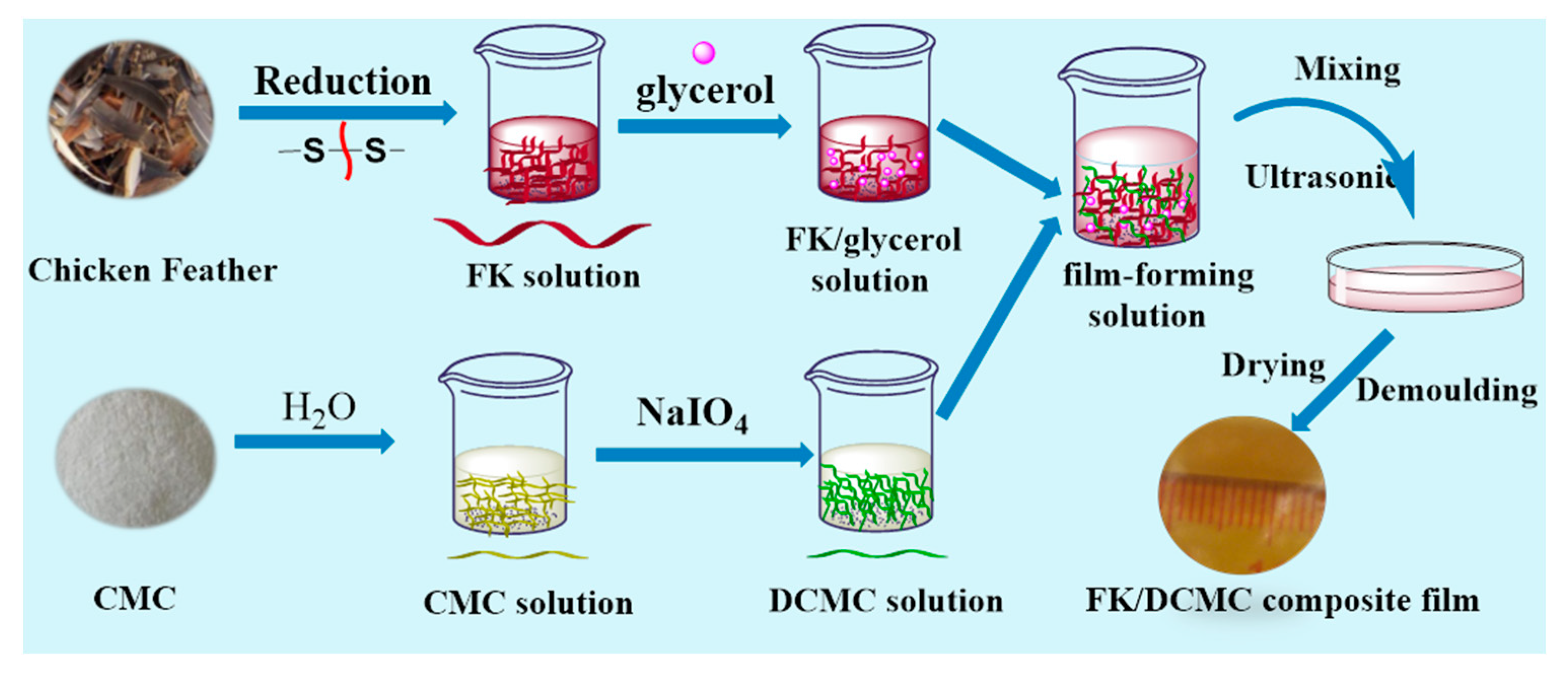
2.4. Preparation of FK/DCMC Composite Films
2.5. Characterization
3. Results and Discussion
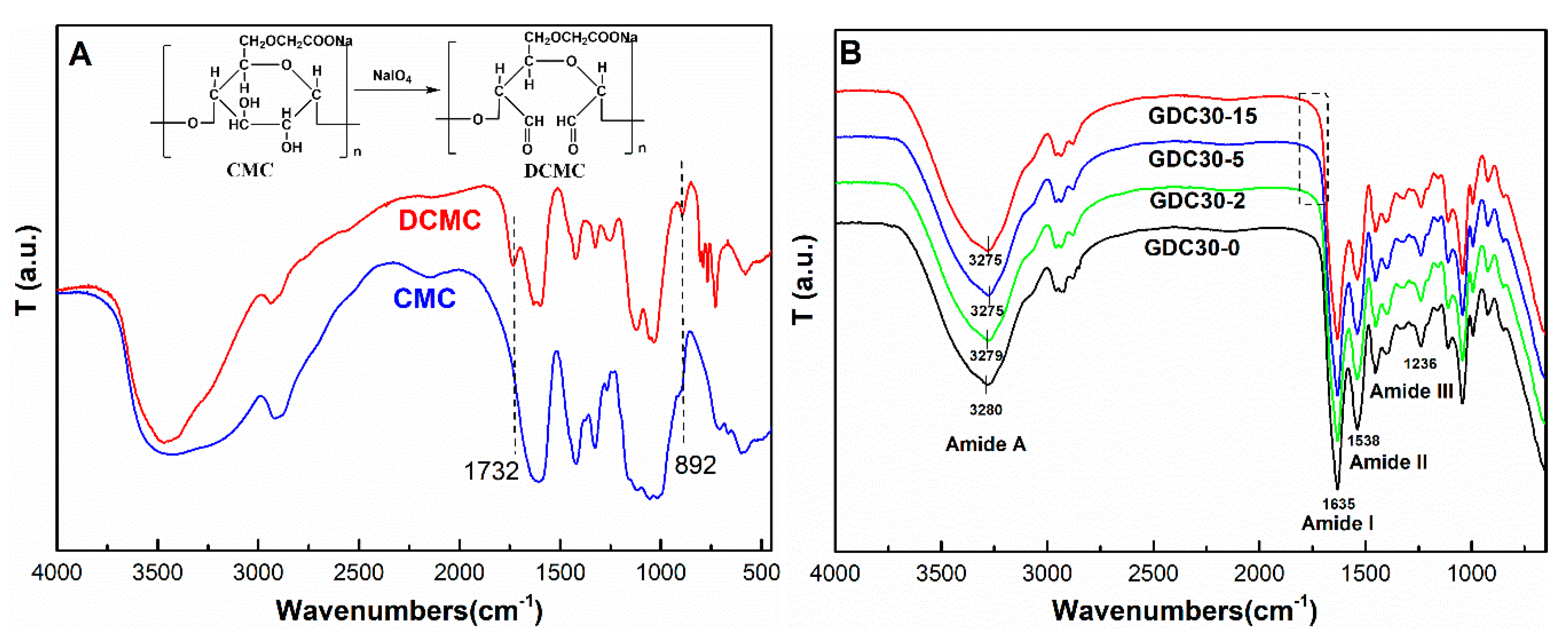
3.1. FTIR Spectra Analysis
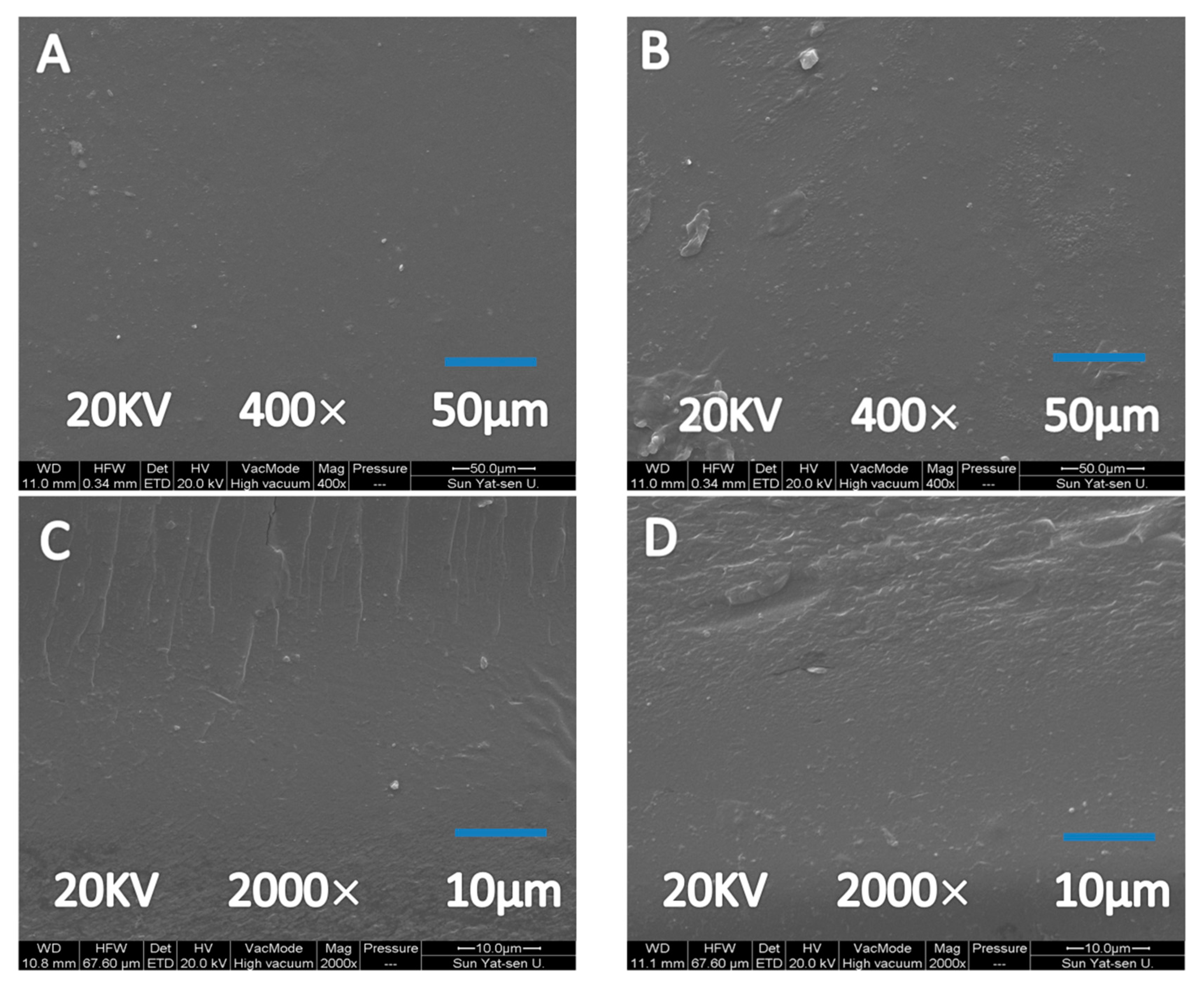
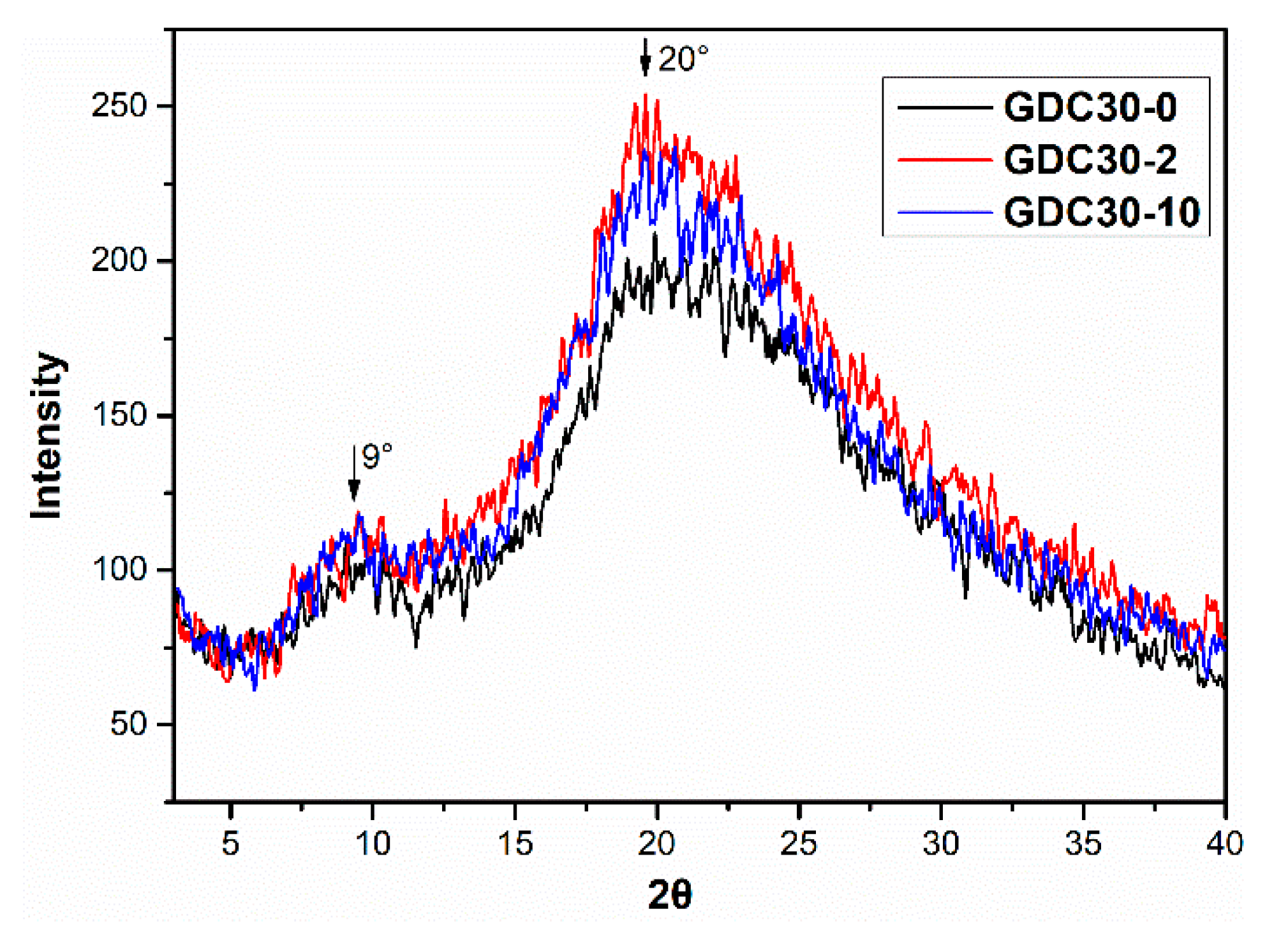
3.2. Film Morphology, Transparency and Aggregate Structure
3.3. Tensile Properties
3.4. Water Resistance
3.5. Water Vapor Permeability (WVP)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Zhang, C.; Deng, Y.; Wang, R.; Ma, E.; Wang, J.; Bai, J.; Wu, J.; Zhou, Y. Microplastics in the surface water of small-scale estuaries in Shanghai. Mar. Pollut. Bull. 2019, 149, 110569. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Wang, J. Current practices and future perspectives of microplastic pollution in freshwater ecosystems in China. Sci. Total Environ. 2019, 691, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, D.; Pinheiro, C.; Amorim, J.; Oliva-Teles, L.; Guilhermino, L.; Vieira, M.N. Microplastic pollution in commercial salt for human consumption: A review. Estuar. Coast. Shelf Sci. 2019, 219, 161–168. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Dick Vethaak, A.; Lavorante, B.; Lundebye, A.K.; Guilhermino, L. Marine microplastic debris: An emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018, 133, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2019, 702, 134455. [Google Scholar] [CrossRef]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Tanaka, M. Effects of plasticizers on the properties of edible films from skin gelatin of bigeye snapper and brownstripe red snapper. Eur. Food Res. Technol. 2006, 222, 229–235. [Google Scholar] [CrossRef]
- Su, J.-F.; Huang, Z.; Yuan, X.-Y.; Wang, X.-Y.; Li, M. Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr. Polym. 2010, 79, 145–153. [Google Scholar] [CrossRef]
- Fang, Y.; Tung, M.A.; Britt, I.J.; Yada, S.; Dalgleish, D.G. Tensile and barrier properties of edible films made from whey proteins. J. Food Sci. 2002, 67, 188–193. [Google Scholar] [CrossRef]
- Mojumdar, S.C.; Moresoli, C.; Simon, L.C.; Legge, R.L. Edible wheat gluten (WG) protein films. J. Therm. Anal. Calorim. 2011, 104, 929–936. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y. The structure, tensile properties and water resistance of hydrolyzed feather keratin-based bioplastics. Chin. J. Chem. Eng. 2016, 24, 415–420. [Google Scholar] [CrossRef]
- Reddy, N.; Chen, L.; Yang, Y. Biothermoplastics from hydrolyzed and citric acid Crosslinked chicken feathers. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Gong, J.; Fan, X.; Wang, P.; Wang, Q.; Qiu, Y. Transglutaminase-modified wool keratin film and its potential application in tissue engineering. Eng. Life Sci. 2013, 13, 149–155. [Google Scholar] [CrossRef]
- Tanabe, T.; Okitsu, N.; Yamauchi, K. Fabrication and characterization of chemically crosslinked keratin films. Mater. Sci. Eng. C 2004, 24, 441–446. [Google Scholar] [CrossRef]
- Tripathi, S.; Mehrotra, G.K.; Dutta, P.K. Physicochemical and bioactivity of cross-linked chitosan-PVA film for food packaging applications. Int. J. Biol. Macromol. 2009, 45, 372–376. [Google Scholar] [CrossRef]
- Hernandez-Munoz, P.; Villalobos, R.; Chiralt, A. Effect of cross-linking using aldehydes on properties of glutenin-rich films. Food Hydrocoll. 2004, 18, 403–411. [Google Scholar] [CrossRef]
- Pereda, M.; Aranguren, M.I.; Marcovich, N.E. Effect of Crosslinking on the Properties of Sodium Caseinate Films. J. Appl. Polym. Sci. 2010, 116, 18–26. [Google Scholar] [CrossRef]
- Rhim, J.W.; Gennadios, A.; Weller, C.L.; Cezeirat, C.; Hanna, M.A. Soy protein isolate dialdehyde starch films. Ind. Crops Prod. 1998, 8, 195–203. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Z.; Peng, Y.; Han, B.; Li, Z.; Li, X.; Liu, W. Preparation, characterization and feasibility study of dialdehyde carboxymethyl cellulose as a novel crosslinking reagent. Carbohydr. Polym. 2016, 137, 632–641. [Google Scholar] [CrossRef]
- Guo, J.; Li, X.; Mu, C.; Zhang, H.; Qin, P.; Li, D. Freezing-thawing effects on the properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-MMT composite films. Food Hydrocoll. 2013, 33, 273–279. [Google Scholar] [CrossRef]
- Mu, C.; Guo, J.; Li, X.; Lin, W.; Li, D. Preparation and properties of dialdehyde carboxymethyl cellulose crosslinked gelatin edible films. Food Hydrocoll. 2012, 27, 22–29. [Google Scholar] [CrossRef]
- Wang, P.; He, H.; Cai, R.; Tao, G.; Yang, M.; Zuo, H.; Umar, A.; Wang, Y. Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application. Carbohydr. Polym. 2019, 212, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and moisture sensitivity of fully bio-based dialdehyde carboxymethyl cellulose cross-linked soy protein isolate films. Carbohydr. Polym. 2017, 157, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y.; Savina, I.N. Keratin/Polyvinyl Alcohol Blend Films Cross-Linked by Dialdehyde Starch and Their Potential Application for Drug Release. Polymers 2015, 7, 580–591. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, X.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and characterization of a dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015, 5, 27168–27174. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and Physicochemical Properties of Dialdehyde Starch Crosslinked Feather Keratin/PVA Composite Films. J. Macromol. Sci. A 2014, 51, 1009–1015. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Mechanical and Physical Properties of Pea Starch Edible Films in the Presence of Glycerol. J. Food Process. Preserv. 2016, 40, 1339–1351. [Google Scholar] [CrossRef]
- Puscaselu, R.; Gutt, G.; Amariei, S. Biopolymer-Based Films Enriched with Stevia rebaudiana Used for the Development of Edible and Soluble Packaging. Coatings 2019, 9, 360. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Development of biocomposite films incorporated with different amounts of shellac, emulsifier, and surfactant. Food Hydrocoll. 2017, 72, 174–184. [Google Scholar] [CrossRef]
- Limpan, N.; Prodpran, T.; Benjakul, S.; Prasarpran, S. Influences of degree of hydrolysis and molecular weight of poly(vinyl alcohol) (PVA) on properties of fish myofibrillar protein/PVA blend films. Food Hydrocoll. 2012, 29, 226–233. [Google Scholar] [CrossRef]
- Vasconcelos, A.; Freddi, G.; Cavaco-Paulo, A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules 2008, 9, 1299–1305. [Google Scholar] [CrossRef]
- Ullah, A.; Wu, J. Feather Fiber-Based Thermoplastics: Effects of Different Plasticizers on Material Properties. Macromol. Mater. Eng. 2013, 298, 153–162. [Google Scholar] [CrossRef]
- Li, D.; Ye, Y.; Li, D.; Li, X.; Mu, C. Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin-PEG composite hydrogel fibers for wound dressings. Carbohydr. Polym. 2016, 137, 508–514. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, B.; Dou, Y.; Yin, G.; Cui, Y. Blend modification of feather keratin-based films using sodium alginate. J. Appl. Polym. Sci. 2017, 134, 44680. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Yi, M.; Ge, J.; Yin, G.; Li, X. Preparation and Physicochemical Properties of Blend Films of Feather Keratin and Poly(vinyl alcohol) Compatibilized by Tris(hydroxymethyl)aminomethane. Polymers 2018, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Nemet, N.; Soso, V.; Lazic, V. Effect of glycerol content and pH value of film-forming solution on the functional properties of protein-based edible films. Acta Period. Technol. 2010, 41, 57–67. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Baraniak, B. Effects of plasticizers, pH and heating of film-forming solution on the properties of pea protein isolate films. J. Food Eng. 2011, 105, 295–305. [Google Scholar] [CrossRef]
- Mauri, A.N.; Anon, M.C. Effect of solution pH on solubility and some structural properties of soybean protein isolate films. J. Sci. Food Agric. 2006, 86, 1064–1072. [Google Scholar] [CrossRef]
- Moore, G.R.P.; Martelli, S.M.; Gandolfo, C.; do Amaral Sobral, P.J.; Laurindo, J.B. Influence of the glycerol concentration on some physical properties of feather keratin films. Food Hydrocoll. 2006, 20, 975–982. [Google Scholar] [CrossRef]
- Wang, H.; Yin, G.; Feng, G.; Dou, Y.; He, M.; Deng, X. Preparation and Properties of Feather Keratin and Sodium Carboxy Methyl Cellulose Blend Films. Polym. Mater. Sci. Eng. 2014, 30, 139–143. [Google Scholar]
- Deng, X.; Yin, G.; Zhang, B.; Dou, Y.; He, M.; Wang, H. Preparation and property of compression-molding feather keratin films. New Chem. Mater. 2015, 43, 60–68. [Google Scholar]
- Chen, Y.; Ye, R.; Li, X.; Wang, J. Preparation and characterization of extruded thermoplastic zein-poly(propylene carbonate) film. Ind. Crops Prod. 2013, 49, 81–87. [Google Scholar] [CrossRef]
- Martucci, J.F.; Ruseckaite, R.A. Tensile Properties, Barrier Properties, and Biodegradation in Soil of Compression-Molded Gelatin-Dialdehyde Starch Films. J. Appl. Polym. Sci. 2009, 112, 2166–2178. [Google Scholar] [CrossRef]
- Jane, J.; Spence, K.E. Biodegradable Thermoplastic Composition of Aldehyde Starch and Protein. U.S. Patent US 5,397,834, 14 March 1995. [Google Scholar]
- Parris, N.; Coffin, D.R. Composition factors affecting the water vapor permeability and tensile properties of hydrophilic zein films. J. Agric. Food Chem. 1997, 45, 1596–1599. [Google Scholar] [CrossRef]
- Rhim, J.W.; Gennadios, A.; Handa, A.; Weller, C.L.; Hanna, M.A. Solubility, tensile, and color properties of modified soy protein isolate films. J. Agric. Food Chem. 2000, 48, 4937–4941. [Google Scholar] [CrossRef] [PubMed]
- Martelli, S.M.; Moore, G.R.P.; Laurindo, J.B. Mechanical Properties, Water Vapor Permeability and Water Affinity of Feather Keratin Films Plasticized with Sorbitol. J. Polym. Environ. 2006, 14, 215–222. [Google Scholar] [CrossRef]
- Saberi, B.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical and mechanical properties of a new edible film made of pea starch and guar gum as affected by glycols, sugars and polyols. Int. J. Biol. Macromol. 2017, 104, 345–359. [Google Scholar] [CrossRef]
- Irissin-Mangata, J.; Bauduin, G.; Boutevin, B.; Gontard, N. New plasticizers for wheat gluten films. Eur. Polym. J. 2001, 37, 1533–1541. [Google Scholar] [CrossRef]
| Films | Transmission (%) | Transparency | ||||||
|---|---|---|---|---|---|---|---|---|
| 200 nm | 280 nm | 400 nm | 500 nm | 600 nm | 700 nm | 800 nm | ||
| GDC30-0 | 0.001 ± 0.001 | 0.022 ± 0.011 | 19.14 ± 3.55 | 38.64 ± 2.37 | 61.25 ± 2.15 | 72.21 ± 1.90 | 77.50 ± 1.54 | 1.63 ± 0.42 |
| GDC30-2 | 0.001 ± 0.001 | 0.013 ± 0.009 | 15.71 ± 2.41 | 25.51 ± 3.85 | 53.25 ± 1.76 | 56.32 ± 2.31 | 69.46 ± 2.38 | 1.82 ± 0.22 |
| GDC30-5 | 0.003 ± 0.001 | 0.041 ± 0.015 | 13.64 ± 2.73 | 28.35 ± 5.10 | 55.86 ± 2.11 | 69.31 ± 2.42 | 74.69 ± 1.65 | 1.81 ± 0.37 |
| GDC30-10 | 0.002 ± 0.002 | 0.024 ± 0.010 | 15.36 ± 3.19 | 17.20 ± 3.14 | 44.18 ± 3.20 | 58.78 ± 2.90 | 65.64 ± 1.74 | 1.97 ± 0.27 |
| GDC40-0 | 0.001 ± 0.001 | 0.053 ± 0.021 | 20.11 ± 3.13 | 35.37 ± 2.69 | 60.28 ± 5.45 | 67.70 ± 1.75 | 75.38 ± 1.67 | 1.78 ± 0.59 |
| GDC40-2 | 0.002 ± 0.001 | 0.031 ± 0.017 | 19.19 ± 4.27 | 24.50 ± 2.50 | 56.35 ± 1.18 | 72.27 ± 2.12 | 78.43 ± 2.89 | 1.23 ± 0.24 |
| GDC40-5 | 0.002 ± 0.002 | 0.011 ± 0.007 | 22.56 ± 5.36 | 39.28 ± 3.37 | 65.10 ± 2.44 | 74.59 ± 2.85 | 77.89 ± 1.30 | 1.55 ± 0.16 |
| GDC40-10 | 0.001 ± 0.001 | 0.042 ± 0.023 | 16.56 ± 4.20 | 38.74 ± 4.78 | 62.64 ± 2.59 | 71.14 ± 2.15 | 74.06 ± 2.53 | 1.69 ± 0.35 |
| Samples | εb (%) | σb (MPa) | MC (%) | Solubility (%) | WVP (10−10 gm/m2 s Pa) |
|---|---|---|---|---|---|
| GDC30-0 | 17.6 ± 3.0 | 4.0 ± 0.9 | 20.6 ± 2.1 | 46.2 ± 4.5 | 3.7 ± 0.3 |
| GDC30-2 | 26.8 ± 5.7 | 2.1 ± 0.8 | 17.9 ± 0.7 | 43.8 ± 2.4 | 3.3 ± 0.2 |
| GDC30-5 | 28.3 ± 9.1 | 1.6 ± 0.4 | 18.7 ± 0.5 | 42.2 ± 2.1 | 3.6 ± 0.3 |
| GDC30-10 | 27.5 ± 3.7 | 1.2 ± 0.6 | 19.3 ± 0.8 | 45.5 ± 3.3 | 3.8 ± 0.1 |
| GDC40-0 | 31.0 ± 6.4 | 3.1 ± 1.1 | 22.4 ± 1.6 | 59.2 ± 2.7 | 5.0 ± 0.6 |
| GDC40-2 | 29.1 ± 5.1 | 0.9 ± 0.7 | 19.5 ± 1.7 | 48.9 ± 4.3 | 3.6 ± 0.5 |
| GDC40-5 | 29.6 ± 2.1 | 1.0 ± 0.3 | 18.1 ± 1.2 | 51.0 ± 3.8 | 3.8 ± 0.4 |
| GDC40-10 | 30.8 ± 4.6 | 0.7 ± 0.4 | 21.6 ± 0.9 | 53.3 ± 3.6 | 4.2 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers 2020, 12, 158. https://doi.org/10.3390/polym12010158
Dou Y, Zhang L, Zhang B, He M, Shi W, Yang S, Cui Y, Yin G. Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers. 2020; 12(1):158. https://doi.org/10.3390/polym12010158
Chicago/Turabian StyleDou, Yao, Liguang Zhang, Buning Zhang, Ming He, Weimei Shi, Shiqing Yang, Yingde Cui, and Guoqiang Yin. 2020. "Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging" Polymers 12, no. 1: 158. https://doi.org/10.3390/polym12010158
APA StyleDou, Y., Zhang, L., Zhang, B., He, M., Shi, W., Yang, S., Cui, Y., & Yin, G. (2020). Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers, 12(1), 158. https://doi.org/10.3390/polym12010158





