Fabrication of Antibacterial Nanofibrous Membrane Infused with Essential Oil Extracted from Tea Tree for Packaging Applications
Abstract
1. Introduction
2. Materials and Method
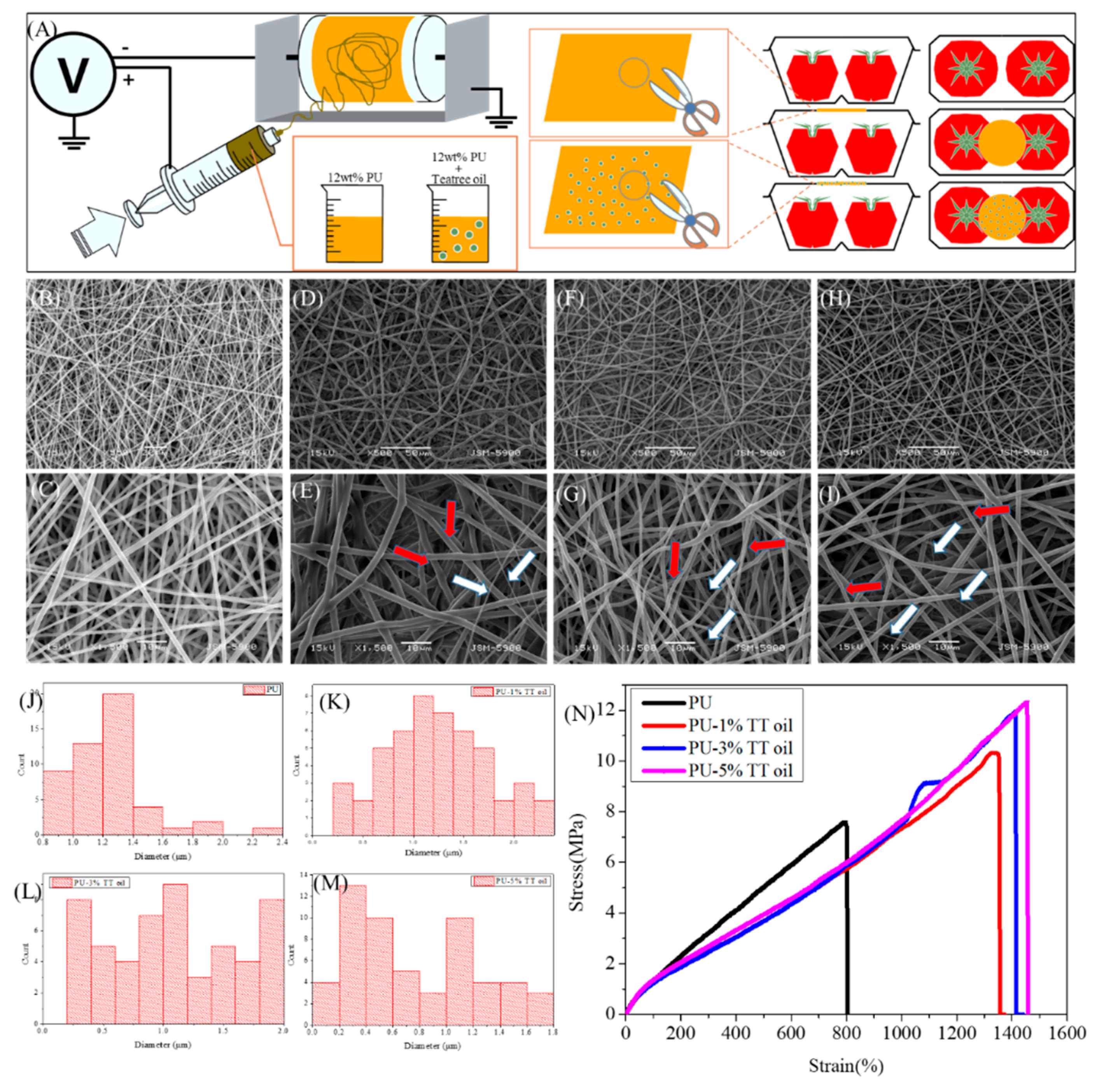
2.1. Preparation of Packaging Samples
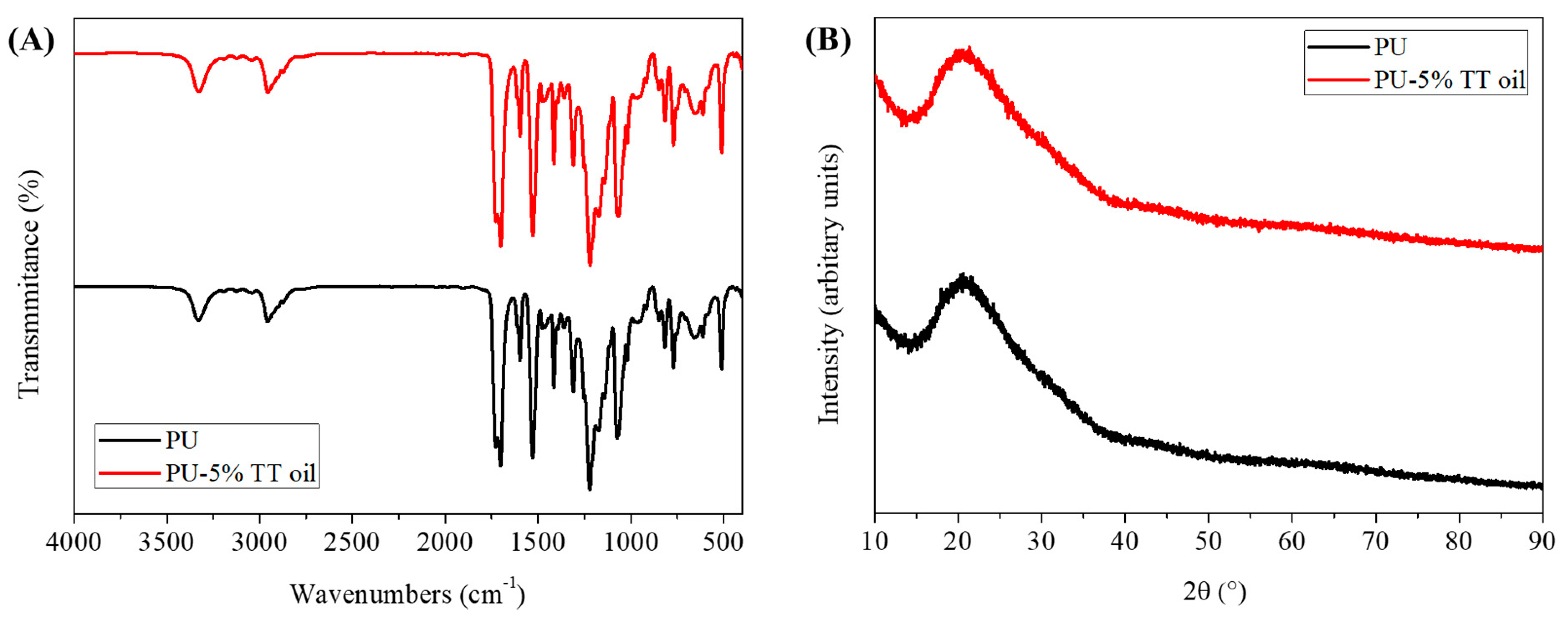
2.2. Characterization
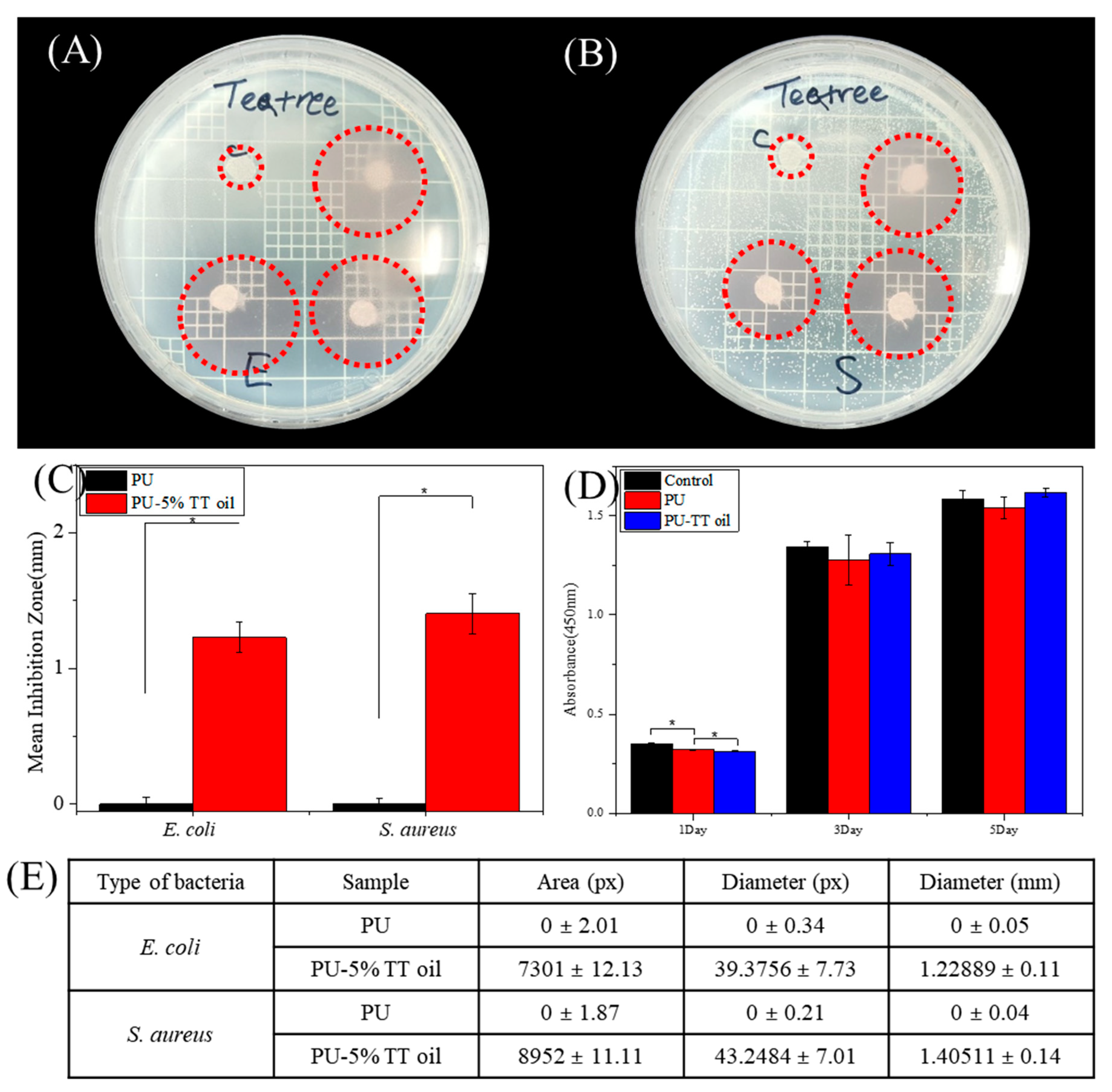
2.3. Antibacterial Test
2.4. In Vitro Biocompatibility Test
2.5. Evaluation of Efficacy Using Tomatoes
2.6. Image and Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Ero-Phillips, O.; Jenkins, M.; Stamboulis, A. Tailoring Crystallinity of Electrospun Plla Fibres by Control of Electrospinning Parameters. Polymers 2012, 4, 1331–1348. [Google Scholar] [CrossRef]
- Vitkova, L.; Musilova, L.; Achbergerova, E.; Minarik, A.; Smolka, P.; Wrzecionko, E.; Mracek, A. Electrospinning of Hyaluronan Using Polymer Coelectrospinning and Intermediate Solvent. Polymers 2019, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Lung, Y.C.; Kuo, C.C.; Liang, F.C.; Tsai, T.L.; Jiang, D.H.; Satoh, T.; Jeng, R.J. Novel Multifunctional Luminescent Electrospun Fluorescent Nanofiber Chemosensor-Filters and Their Versatile Sensing of pH, Temperature, and Metal Ions. Polymers 2018, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Cheung, K.C. Biodegradable Cell-Seeded Nanofiber Scaffolds for Neural Repair. Polymers 2011, 3, 1684–1733. [Google Scholar] [CrossRef]
- Kakinoki, S.; Uchida, S.; Ehashi, T.; Murakami, A.; Yamaoka, T. Surface Modification of Poly(L-lactic acid) Nanofiber with Oligo(D-lactic acid) Bioactive-Peptide Conjugates for Peripheral Nerve Regeneration. Polymers 2011, 3, 820–832. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.J. TiO2 NPs Assembled into a Carbon Nanofiber Composite Electrode by a One-Step Electrospinning Process for Supercapacitor Applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jang, S.; Aguilar, L.E.; Park, C.H.; Kim, C.S. Structural Packaging Technique Using Biocompatible Nanofiber with Essential Oil to Prolong the Shelf-Life of Fruit. J. Nanosci. Nanotechnol. 2019, 19, 2228–2231. [Google Scholar] [CrossRef]
- Yadav, E.; Kumar, S.; Mahant, S.; Khatkar, S.; Rao, R. Tea tree oil: A promising essential oil. J. Essent. Oil Res. 2017, 29, 201–213. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Effects of Melaleuca alternifolia (tea tree) essential oil and the major monoterpene component terpinen-4-ol on the development of single- and multistep antibiotic resistance and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2012, 56, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.K.; Yang, H.J.; Jung, H.; Yoon, D.J.; Sang, M.K.; Jeun, Y.C. Application of Volatile Antifungal Plant Essential Oils for Controlling Pepper Fruit Anthracnose by Colletotrichum gloeosporioides. Plant Pathol. J. 2015, 31, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Safety, efficacy and provenance of tea tree (Melaleuca alternifolia) oil. Contact Dermat. 2001, 45, 65–67. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Southwell, I.A.; Maddox, C.D.A.; Zalucki, M.P. Metabolism of 1,8-Cineole in Tea Tree (Melaleuca-Alternifolia and Melaleuca-Linariifolia) by Pyrgo Beetle (Paropsisterna-Tigrina). J. Chem. Ecol. 1995, 21, 439–453. [Google Scholar] [CrossRef]
- Russell, M.F.; Southwell, I.A. Monoterpenoid accumulation in 1,8-cineole, terpinolene and terpinen-4-ol chemotypes of Melaleuca alternifolia seedlings. Phytochemistry 2003, 62, 683–689. [Google Scholar] [CrossRef]
- Jarimopas, B.; Singh, S.P.; Sayasoonthorn, S.; Singh, J. Comparison of package cushioning materials to protect post-harvest impact damage to apples. Packag. Technol. Sci. 2007, 20, 315–324. [Google Scholar] [CrossRef]
- Ravindra, M.R.; Goswami, T.K. Post-harvest handling and storage of mangoes—An overview. J. Food Sci. Technol. Mys. 2007, 44, 449–458. [Google Scholar]
- Hsu, Y.H.; Lin, C.T.; Yu, Y.H.; Chou, Y.C.; Liu, S.J.; Chan, E.C. Dual delivery of active antibactericidal agents and bone morphogenetic protein at sustainable high concentrations using biodegradable sheath-core-structured drug-eluting nanofibers. Int. J. Nanomed. 2016, 11, 3927–3937. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Park, K.E.; Kim, M.H.; You, H.K.; Lee, J.; Park, W.H. Effect of nanofiber content on bone regeneration of silk fibroin/poly(epsilon-caprolactone) nano/microfibrous composite scaffolds. Int. J. Nanomed. 2015, 10, 485–502. [Google Scholar] [CrossRef]
- Rogalski, J.J.; Zhang, H.; Yao, J.; Bastiaansen, C.W.M.; Peijs, T. High-modulus rotary jet spun co-polyimide nanofibers and their composites. Nanocomposites 2019. [Google Scholar] [CrossRef]
- Seo, D.K.; Jeun, J.P.; Bin Kim, H.; Kang, P.H. Preparation and Characterization of the Carbon Nanofiber Mat Produced from Electrospun Pan/Lignin Precursors by Electron Beam Irradiation. Rev. Adv. Mater. Sci. 2011, 28, 31–34. [Google Scholar]
- Strotmann, U.J.; Eglsaer, H.; Pagga, U. Development and Evaluation of a Growth-Inhibition Test with Sewage Bacteria for Assessing Bacterial Toxicity of Chemical-Compounds. Chemosphere 1994, 28, 755–766. [Google Scholar] [CrossRef]
- Rytwo, G.; Zakai, R.; Wicklein, B. The Use of ATR-FTIR Spectroscopy for Quantification of Adsorbed Compounds. J. Spectrosc. 2015. [Google Scholar] [CrossRef]
- Strotmann, U.J.; Pagga, U. A growth inhibition test with sewage bacteria—Results of an international ring test 1995. Chemosphere 1996, 32, 921–933. [Google Scholar] [CrossRef]
- Tarus, B.K.; Mwasiagi, J.I.; Fadel, N.; Al-Oufy, A.; Elmessiry, M. Electrospun cellulose acetate and poly(vinyl chloride) nanofiber mats containing silver nanoparticles for antifungi packaging. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Lee, J.; Ko, S.W.; Son, B.C.; Lee, J.H.; Kim, C.S.; Park, C.H. Fabrication of Antibacterial Nanofibrous Membrane Infused with Essential Oil Extracted from Tea Tree for Packaging Applications. Polymers 2020, 12, 125. https://doi.org/10.3390/polym12010125
Lee JY, Lee J, Ko SW, Son BC, Lee JH, Kim CS, Park CH. Fabrication of Antibacterial Nanofibrous Membrane Infused with Essential Oil Extracted from Tea Tree for Packaging Applications. Polymers. 2020; 12(1):125. https://doi.org/10.3390/polym12010125
Chicago/Turabian StyleLee, Ji Yeon, Joshua Lee, Sung Won Ko, Byeong Cheol Son, Jun Hee Lee, Cheol Sang Kim, and Chan Hee Park. 2020. "Fabrication of Antibacterial Nanofibrous Membrane Infused with Essential Oil Extracted from Tea Tree for Packaging Applications" Polymers 12, no. 1: 125. https://doi.org/10.3390/polym12010125
APA StyleLee, J. Y., Lee, J., Ko, S. W., Son, B. C., Lee, J. H., Kim, C. S., & Park, C. H. (2020). Fabrication of Antibacterial Nanofibrous Membrane Infused with Essential Oil Extracted from Tea Tree for Packaging Applications. Polymers, 12(1), 125. https://doi.org/10.3390/polym12010125



