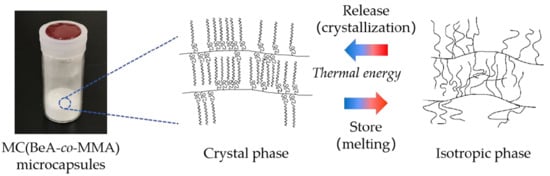
Crystal Transition Behavior and Thermal Properties of Thermal-Energy-Storage Copolymer Materials with an n-Behenyl Side-Chain
Abstract
1. Introduction
2. Materials and Experiments
2.1. Materials
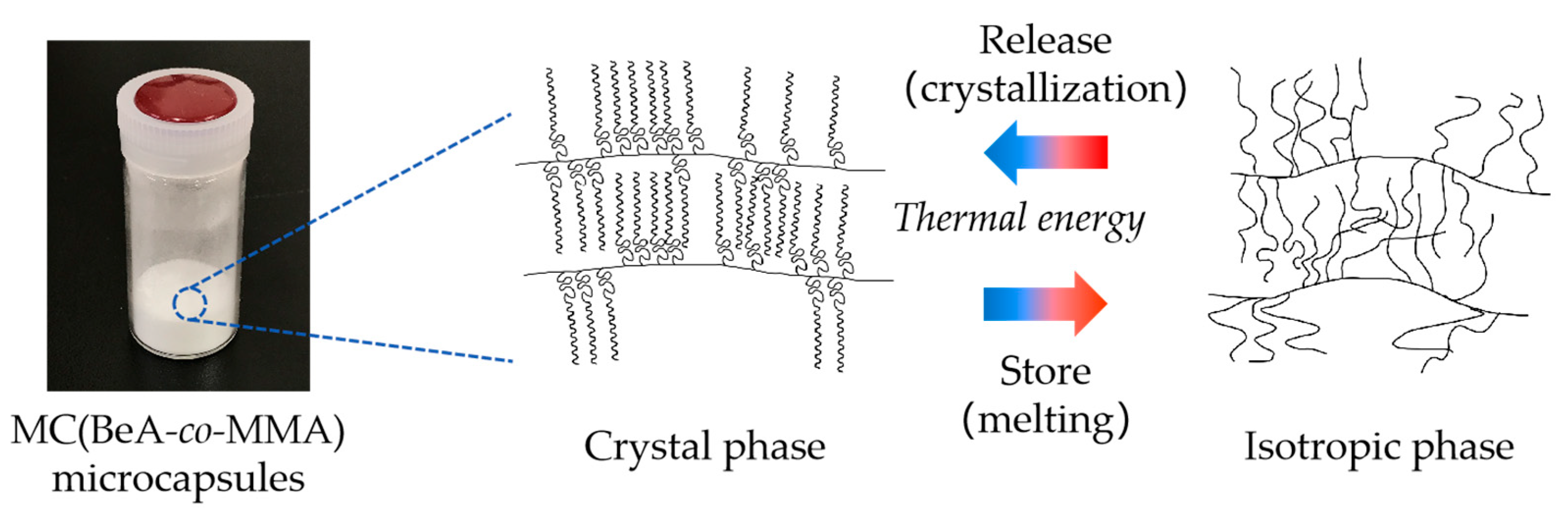
2.2. Preparation of Copolymer Microcapsules
2.3. Preparation of Copolymer Sheets
2.4. Characterization of Copolymer Microcapsules and Sheets
2.4.1. Differential Scanning Calorimetry (DSC)
2.4.2. Small-Angle X-ray Scattering (SAXS)
2.4.3. Wide-Angle X-ray Scattering (WAXS)
2.4.4. Fourier Transform Infrared Spectrometer (FTIR)
3. Results and Discussion
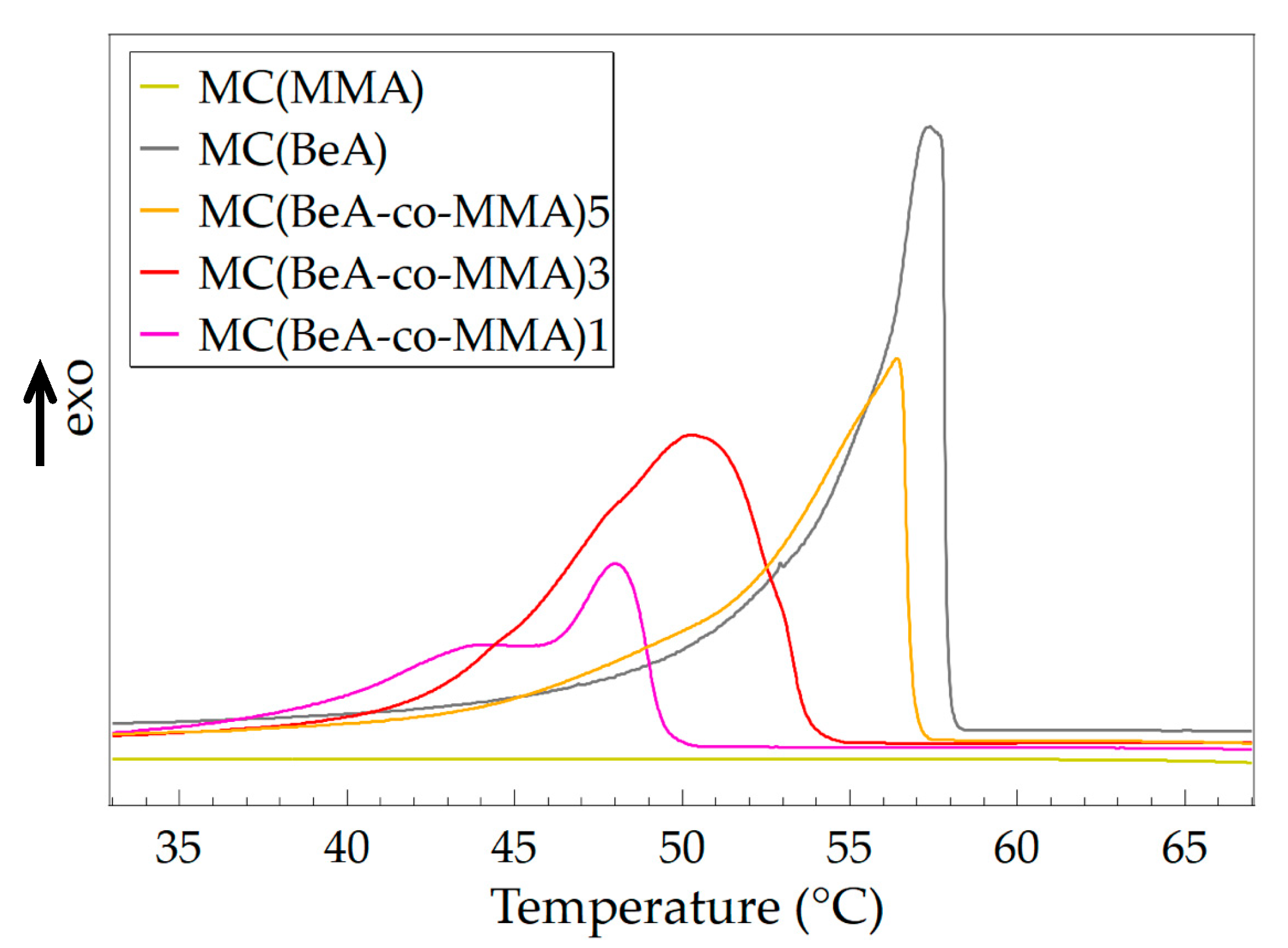
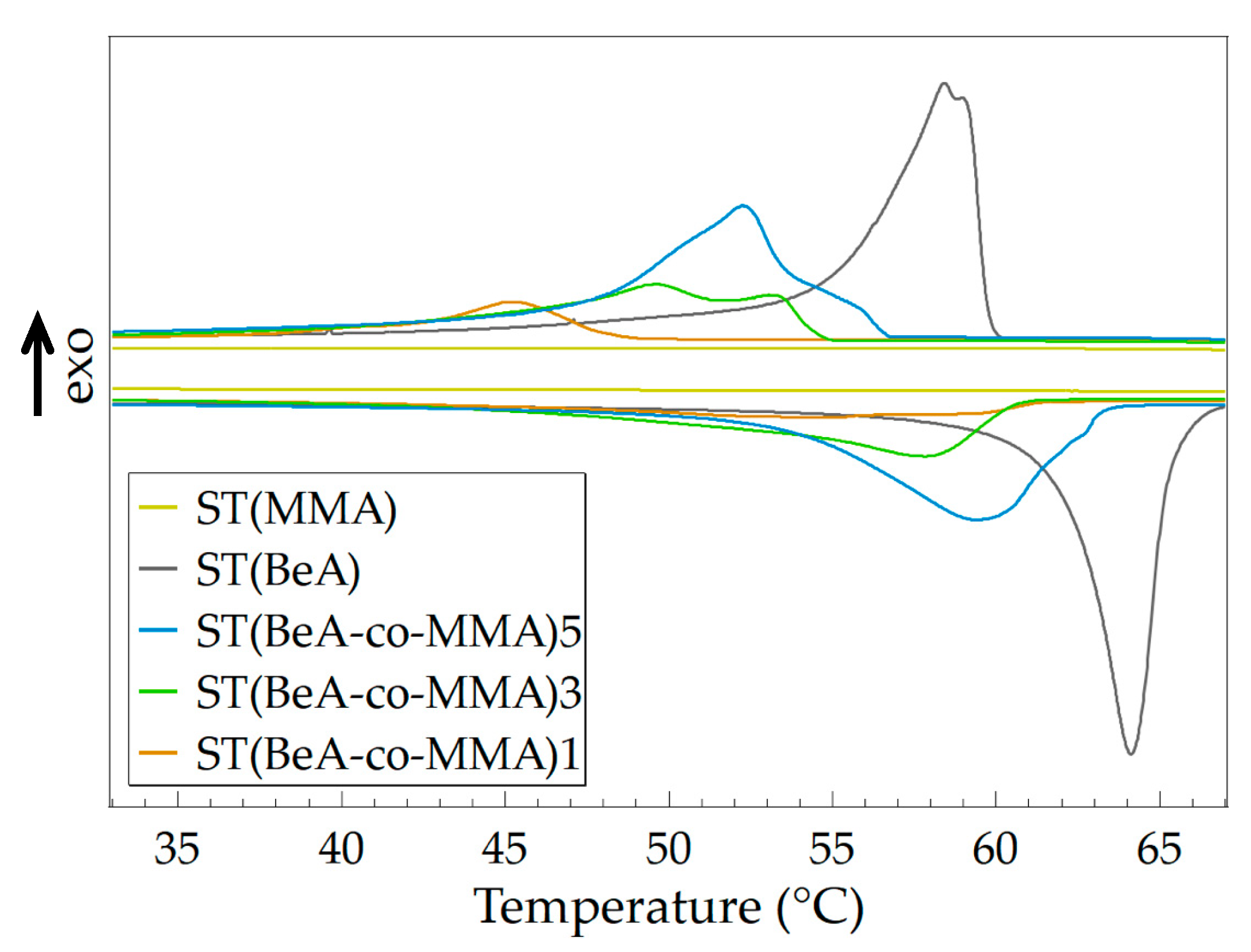
3.1. Effect of Monomer Ratio
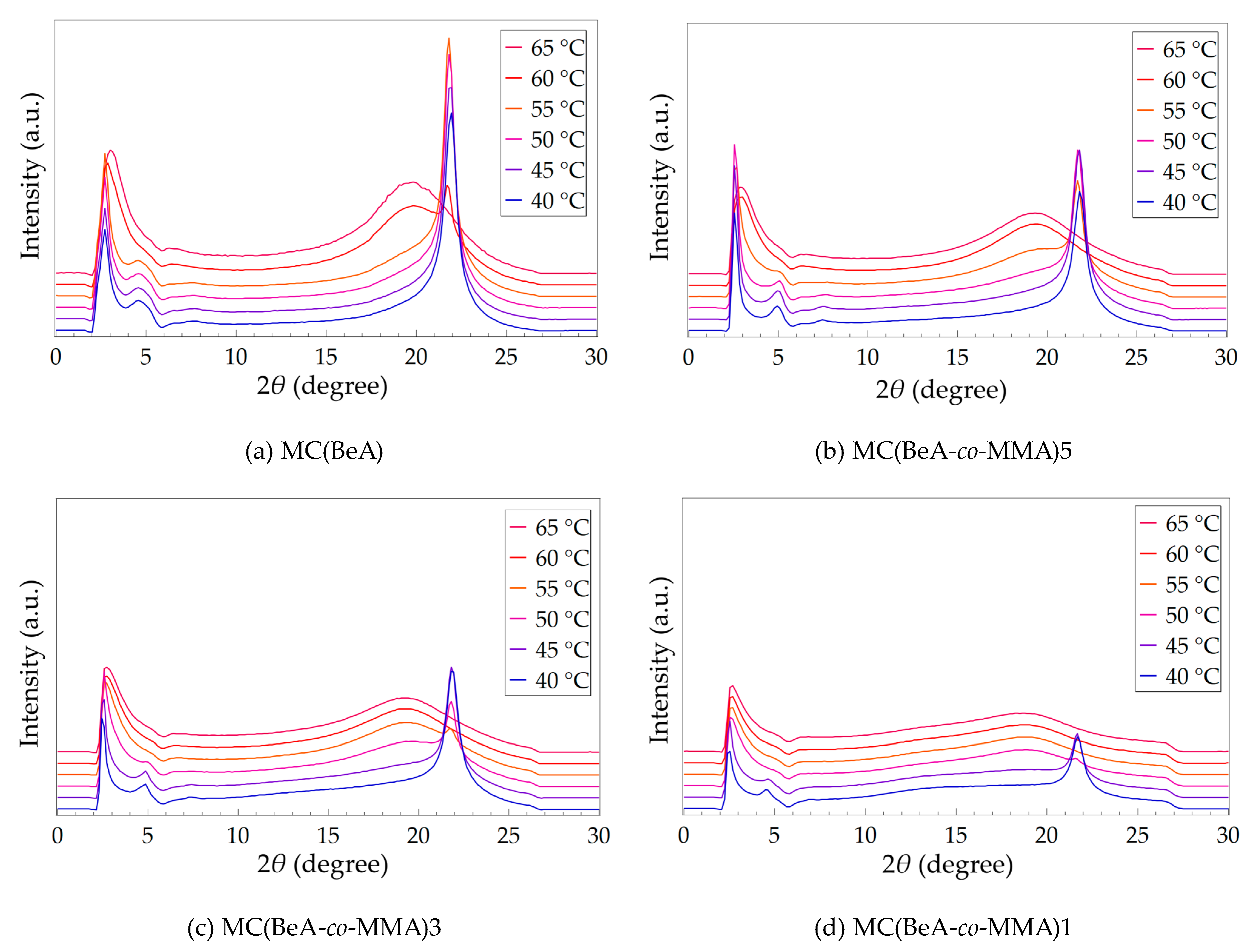
3.2. Effect of Temperature Changing
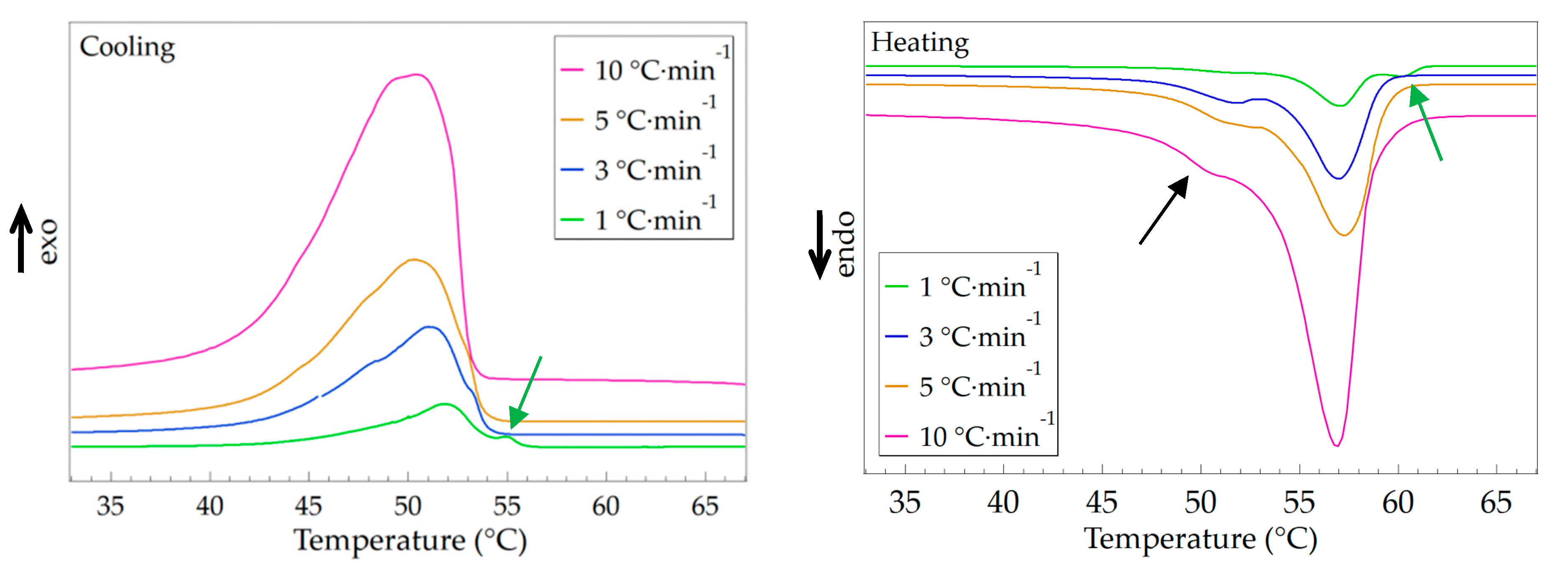
3.3. Effect of Temperature Changing Rate
3.4. Effect of Synthesis Method
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yataganbaba, A.; Ozkahraman, B.; Kurtbas, I. Worldwide trends on encapsulation of phase change materials: A bibliometric analysis (1990–2015). Appl. Energy 2017, 185, 720–731. [Google Scholar] [CrossRef]
- Castellon, C.; Martorell, I.; Cabeza, L.F.; Fernandez, A.I.; Manich, A.M. Compatibility of plastic with phase change materials (pcm). Int. J. Energ. Res. 2011, 35, 765–771. [Google Scholar] [CrossRef]
- Mondal, S. Phase change materials for smart textiles–an overview. Appl. Therm. Eng. 2008, 28, 1536–1550. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M.M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Energy Convers. Manag. 2004, 45, 263–275. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Saktisahdan, T.J.; Jannifar, A.; Hasan, M.H.; Matseelar, H.S.C. A review of available methods and development on energy storage; technology update. Renew. Sustain. Energy Rev. 2014, 33, 532–545. [Google Scholar] [CrossRef]
- Tang, B.T.; Yang, Z.Y.; Zhang, S.F. Poly(polyethylene glycol methyl ether methacrylate) as novel solid-solid phase change material for thermal energy storage. J. Appl. Polym. Sci. 2012, 125, 1377–1381. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Biodegradable peo/cellulose-based solid-solid phase change materials. Polym. Adv. Technol. 2011, 22, 1633–1641. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. Synthesis and characterization of chain-extended and branched polyurethane copolymers as form stable phase change materials for solar thermal conversion storage. Sol. Energy Mater. Sol. Cells 2018, 186, 14–28. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Kenisarina, K.M. Form-stable phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2012, 16, 1999–2040. [Google Scholar] [CrossRef]
- Karaman, S.; Karaipekli, A.; Sari, A.; Bicer, A. Polyethylene glycol (peg)/diatomite composite as a novel form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2011, 95, 1647–1653. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Experiment study on the thermal properties of paraffin/kaolin thermal energy storage form-stable phase change materials. Appl. Energy 2016, 182, 475–487. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Tang, B.; Lu, R.; Zhang, S. Form-stable phase change materials with high phase change enthalpy from the composite of paraffin and cross-linking phase change structure. Appl. Energy 2016, 184, 241–246. [Google Scholar] [CrossRef]
- Inomata, K.; Sakamaki, Y.; Nose, T.; Sasaki, S. Solid-state structure of comb-like polymers having n-octadecyl side chains i. Cocrystallization of side chain with n-octadecanoic acid. Polym. J. 1996, 28, 986. [Google Scholar] [CrossRef][Green Version]
- Inomata, K.; Sakamaki, Y.; Nose, T.; Sasaki, S. Solid-state structure of comb-like polymers having n-octadecyl side chains ii. Crystalline-amorphous layered structure. Polym. J. 1996, 28, 992. [Google Scholar] [CrossRef]
- Wang, J.; Jian, Y.; Nie, J.; He, Y. Solid photopolymerization and polymer properties of octadecyl vinyl ether. J. Photochem. Photobiol. A 2013, 271, 105–110. [Google Scholar] [CrossRef]
- Fallahi, A.; Guldentops, G.; Tao, M.; Granados-Focil, S.; Van Dessel, S. Review on solid-solid phase change materials for thermal energy storage: Molecular structure and thermal properties. Appl. Therm. Eng. 2017, 127, 1427–1441. [Google Scholar] [CrossRef]
- Mao, Y.; Gong, J.; Zhu, M.; Ito, H. Excellent thermal stability p(bea-co-mma) microcapsules with high thermal energy storage capacity. Polymer 2018, 150, 267–274. [Google Scholar] [CrossRef]
- Gong, J.; Hosaka, E.; Sakai, K.; Ito, H.; Shibata, Y.; Sato, K.; Nakanishi, D.; Ishihara, S.; Hamada, K. Processing and thermal response of temperature-sensitive-gel(tsg)/polymer composites. Polymers 2018, 10, 486. [Google Scholar] [CrossRef]
- Mao, Y.; Miyazaki, T.; Sakai, K.; Gong, J.; Zhu, M.; Ito, H. A 3d printable thermal energy storage crystalline gel using mask-projection stereolithography. Polymers 2018, 10, 1117. [Google Scholar] [CrossRef]
- Mao, Y.; Miyazaki, T.; Gong, J.; Zhu, M. Energy storage crystalline gel materials for 3d printing application. Proc. SPIE 2017. [Google Scholar] [CrossRef]
- Gong, J.; Azusa, S.; Makino, M.; Kawakami, M.; Furukawa, H. 3d printing of medical and edible gels. J. Jpn. Soc. Abrasive Technol. 2016, 60, 134–137. [Google Scholar]
- Gong, J.; Furukawa, H. Smart optical device of varifocal lens developed with high transparent shape memory gels. Expected Mater. Future 2013, 13, 5–8. [Google Scholar]
- Gong, J.; Igarashi, S.; Sawamura, K.; Makino, M.; Hasnat Kabir, M.; Furukawa, H. Gel engineering materials meso-decorated with polymorphic crystals. Adv. Mater. Res. 2013, 746, 325–329. [Google Scholar] [CrossRef]
- Hu, W. Polymer Physics-a Molecular Approach; Springer: New York, NY, USA, 2013. [Google Scholar]
- Ruland, W. X-ray determination of crystallinity and diffuse disorder scattering. Acta Crystallogr. 1961, 14, 1180–1185. [Google Scholar] [CrossRef]
- Ryan, A.J.; Bras, W.; Mant, G.R.; Derbyshire, G.E. A direct method to determine the degree of crystallinity and lamellar thickness of polymers: Application to polyethylene. Polymer 1994, 35, 4537–4544. [Google Scholar] [CrossRef]
- Yoshio, S.; Kiyoshige, F. Solid-state polymerization of long-chain vinyl compounds. Iv. Effects of chain length on the polymorphic behavior and postpolymerization of n-alkyl methacrylates. J. Polym. Sci. 1980, 18, 2437–2494. [Google Scholar]
- Shibasaki, Y.; Fukuda, K. Solid-state polymerization of long-chain vinyl compounds. Ii. Effect of molecular arrangement on polymerizability of octadecyl acrylate. J. Polym. Sci. 1979, 17, 2947–2959. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wu, D. Design and fabrication of dual-functional microcapsules containing phase change material core and zirconium oxide shell with fluorescent characteristics. Sol. Energy Mater. Sol. Cells 2015, 133, 56–68. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Phase transitions among the rotator phases of the normal alkanes: A review. Phys. Rep. 2015, 588, 1–54. [Google Scholar] [CrossRef]
- Shibasaki, Y.; Nakahara, H.; Fukuda, K. Solid-state polymerization of long-chain vinyl compounds. I. Effect of molecular arrangement on polymerizability of octadecyl methacrylate. J. Polym. Sci. 1979, 17, 2387–2400. [Google Scholar] [CrossRef]
- Sari, A.; Alkan, C.; Bicer, A. Synthesis and thermal properties of polystyrene-graft-peg copolymers as new kinds of solid-solid phase change materials for thermal energy storage. Mater. Chem. Phys. 2012, 133, 87–94. [Google Scholar] [CrossRef]
- Şentürk, S.B.; Kahraman, D.; Alkan, C.; Gökçe, İ. Biodegradable peg/cellulose, peg/agarose and peg/chitosan blends as shape stabilized phase change materials for latent heat energy storage. Carbohydr. Polym. 2011, 84, 141–144. [Google Scholar] [CrossRef]
- Chen, C.; Liu, W.; Yang, H.; Zhao, Y.; Liu, S. Synthesis of solid–solid phase change material for thermal energy storage by crosslinking of polyethylene glycol with poly(glycidyl methacrylate). Sol. Energ. 2011, 85, 2679–2685. [Google Scholar] [CrossRef]
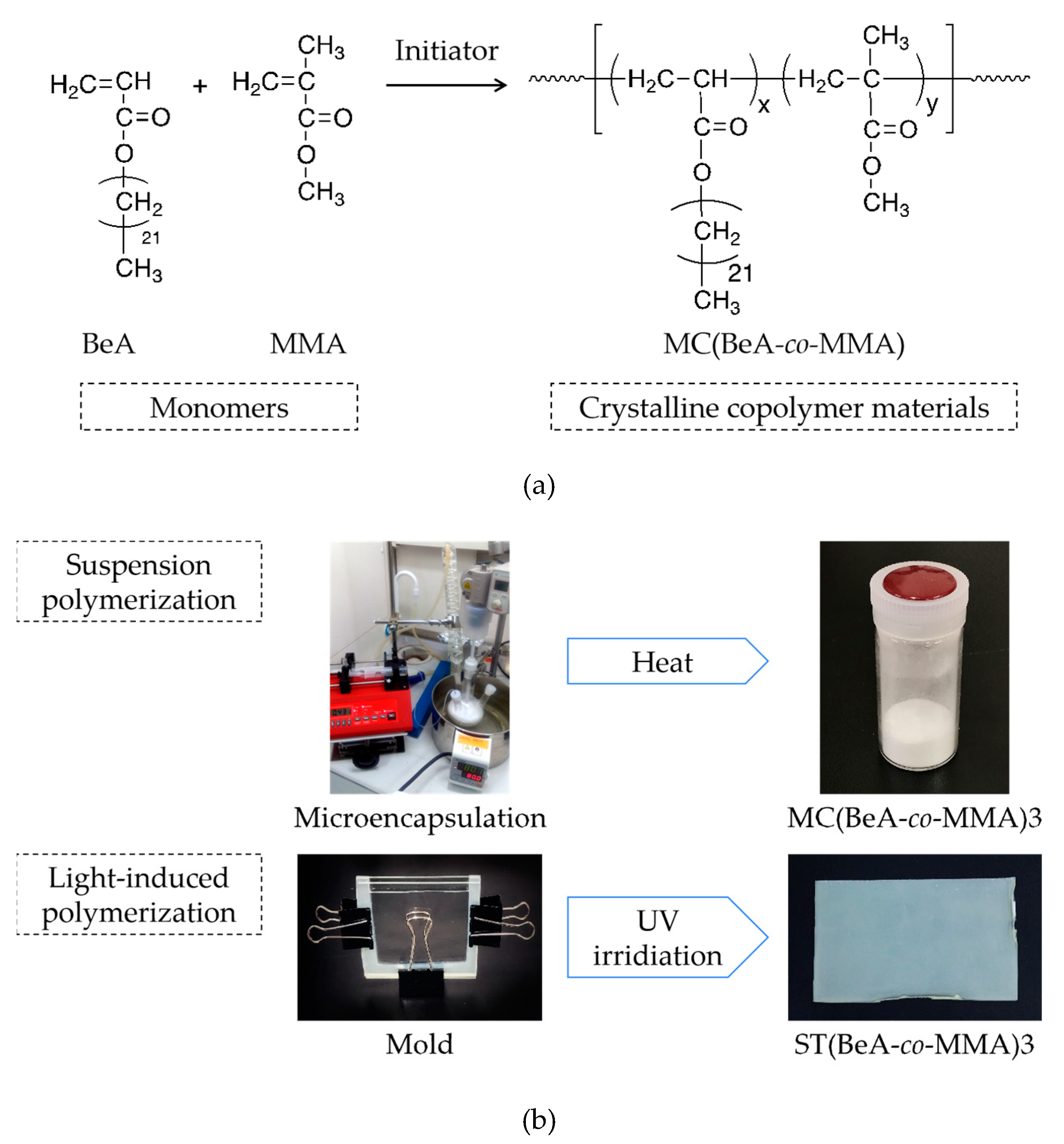

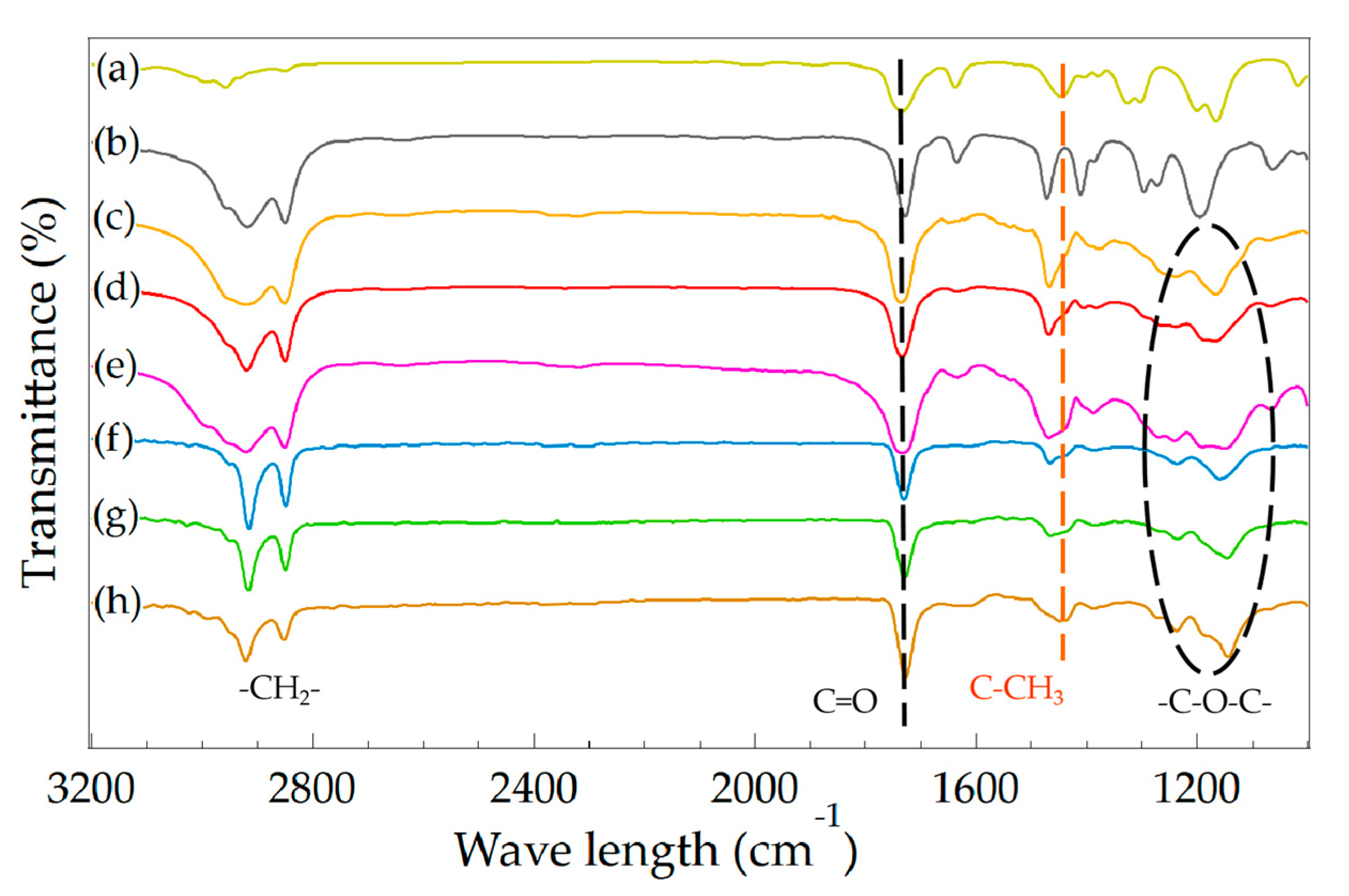
| Sample | Synthesis Method | Monomers | Monomer Ratio | |
|---|---|---|---|---|
| Microcapsule | MC(MMA) | Heat-initiated suspension polymerization | MMA | – |
| MC(BeA) | BeA | – | ||
| MC(BeA-co-MMA)1 | BeA:MMA | 1:1 | ||
| MC(BeA-co-MMA)3 | BeA:MMA | 3:1 | ||
| MC(BeA-co-MMA)5 | BeA:MMA | 5:1 | ||
| Sheet | ST(MMA) | Light-induced polymerization | MMA | – |
| ST(BeA) | BeA | – | ||
| ST(BeA-co-MMA)1 | BeA:MMA | 1:1 | ||
| ST(BeA-co-MMA)3 | BeA:MMA | 3:1 | ||
| ST(BeA-co-MMA)5 | BeA:MMA | 5:1 | ||
| Sample | Crystallization | Melting | Degree of Supercooling (°C) | ||
|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J·g–1) | Tm (°C) | ΔHm (J·g–1) | ||
| MC(MMA) | - | - | - | - | - |
| MC(BeA) | 57.4 | 115.6 | 61.8 | 114.3 | 4.4 |
| MC(BeA-co-MMA)5 | 56.4 | 98.2 | 61.8 | 97.0 | 5.4 |
| MC(BeA-co-MMA)3 | 50.4 | 105.2 | 57.3 | 104.9 | 6.9 |
| MC(BeA-co-MMA)1 | 48.0 | 56.1 | 54.1 | 56.5 | 6.1 |
| Sample | Peak I (2θ) | d1 (Å) | Peak II (2θ) | d2 (Å) | Peak III (2θ) | d3 (Å) |
|---|---|---|---|---|---|---|
| MC(BeA) | 2.72 | 32.45 | 4.58 | 19.28 | 21.95 | 4.05 |
| MC(BeA-co-MMA)5 | 2.55 | 34.62 | 4.93 | 17.91 | 21.82 | 4.07 |
| MC(BeA-co-MMA)3 | 2.44 | 36.18 | 4.86 | 18.17 | 21.82 | 4.07 |
| MC(BeA-co-MMA)1 | 2.40 | 36.78 | 4.49 | 19.67 | 21.69 | 4.09 |
| Temperature Changing Rate | Crystallization | Melting | ||
|---|---|---|---|---|
| Tc (°C) | ΔHc (J·g–1) | Tm (°C) | ΔHm (J·g–1) | |
| 1 °C·min–1 | 51.8 | 112.5 | 57.1 | 114.7 |
| 3 °C·min–1 | 51.1 | 105.8 | 56.9 | 106.3 |
| 5 °C·min–1 | 50.4 | 105.2 | 57.3 | 104.9 |
| 10 °C·min–1 | 50.3 | 102.1 | 56.9 | 103.1 |
| Sample | Crystallization | Melting | Degree of Supercooling (°C) | ||
|---|---|---|---|---|---|
| Tc (°C) | ΔHc (J·g–1) | Tm (°C) | ΔHm (J·g–1) | ||
| ST(MMA) | - | - | - | - | - |
| ST(BeA) | 58.4 | 115.2 | 64.1 | 115.9 | 5.7 |
| ST(BeA-co-MMA)5 | 52.3 | 91.0 | 59.4 | 91.0 | 7.1 |
| ST(BeA-co-MMA)3 | 49.7 | 61.6 | 57.8 | 61.4 | 8.1 |
| ST(BeA-co-MMA)1 | 45.2 | 23.7 | 55.0 | 25.2 | 9.8 |
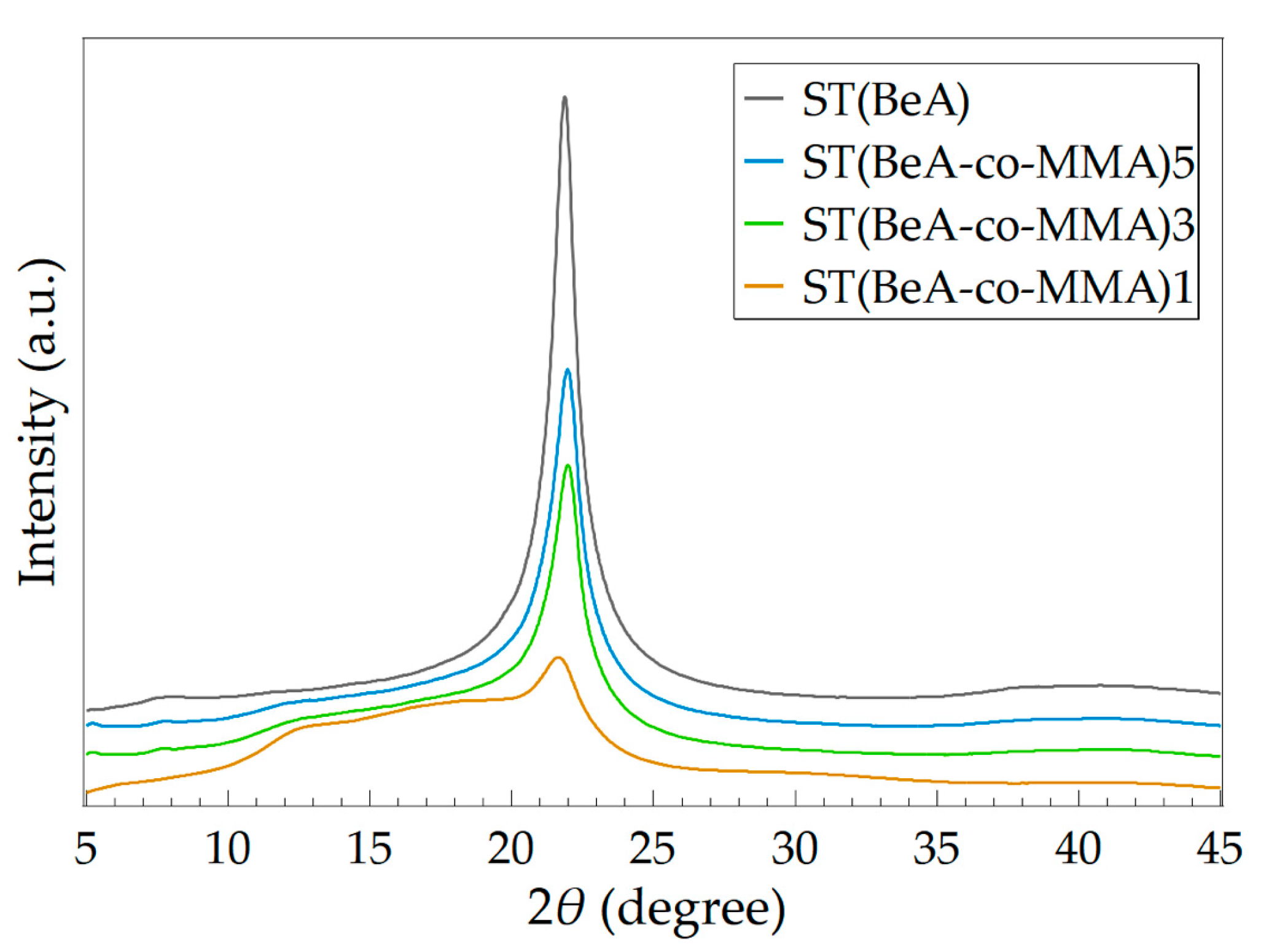
| Sample | Peak (2θ) | d (Å) | Wc (%) |
|---|---|---|---|
| ST(BeA) | 21.86 | 4.06 | 56.67 |
| ST(BeA-co-MMA)5 | 21.98 | 4.04 | 38.23 |
| ST(BeA-co-MMA)3 | 21.98 | 4.04 | 29.78 |
| ST(BeA-co-MMA)1 | 21.69 | 4.10 | 13.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Gong, J.; Zhu, M.; Ito, H. Crystal Transition Behavior and Thermal Properties of Thermal-Energy-Storage Copolymer Materials with an n-Behenyl Side-Chain. Polymers 2019, 11, 1512. https://doi.org/10.3390/polym11091512
Mao Y, Gong J, Zhu M, Ito H. Crystal Transition Behavior and Thermal Properties of Thermal-Energy-Storage Copolymer Materials with an n-Behenyl Side-Chain. Polymers. 2019; 11(9):1512. https://doi.org/10.3390/polym11091512
Chicago/Turabian StyleMao, Yuchen, Jin Gong, Meifang Zhu, and Hiroshi Ito. 2019. "Crystal Transition Behavior and Thermal Properties of Thermal-Energy-Storage Copolymer Materials with an n-Behenyl Side-Chain" Polymers 11, no. 9: 1512. https://doi.org/10.3390/polym11091512
APA StyleMao, Y., Gong, J., Zhu, M., & Ito, H. (2019). Crystal Transition Behavior and Thermal Properties of Thermal-Energy-Storage Copolymer Materials with an n-Behenyl Side-Chain. Polymers, 11(9), 1512. https://doi.org/10.3390/polym11091512