A Facile and Simple Method for Preparation of Novel High-Efficient Form-Stable Phase Change Materials Using Biomimetic–Synthetic Polydopamine Microspheres as a Matrix for Thermal Energy Storage
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
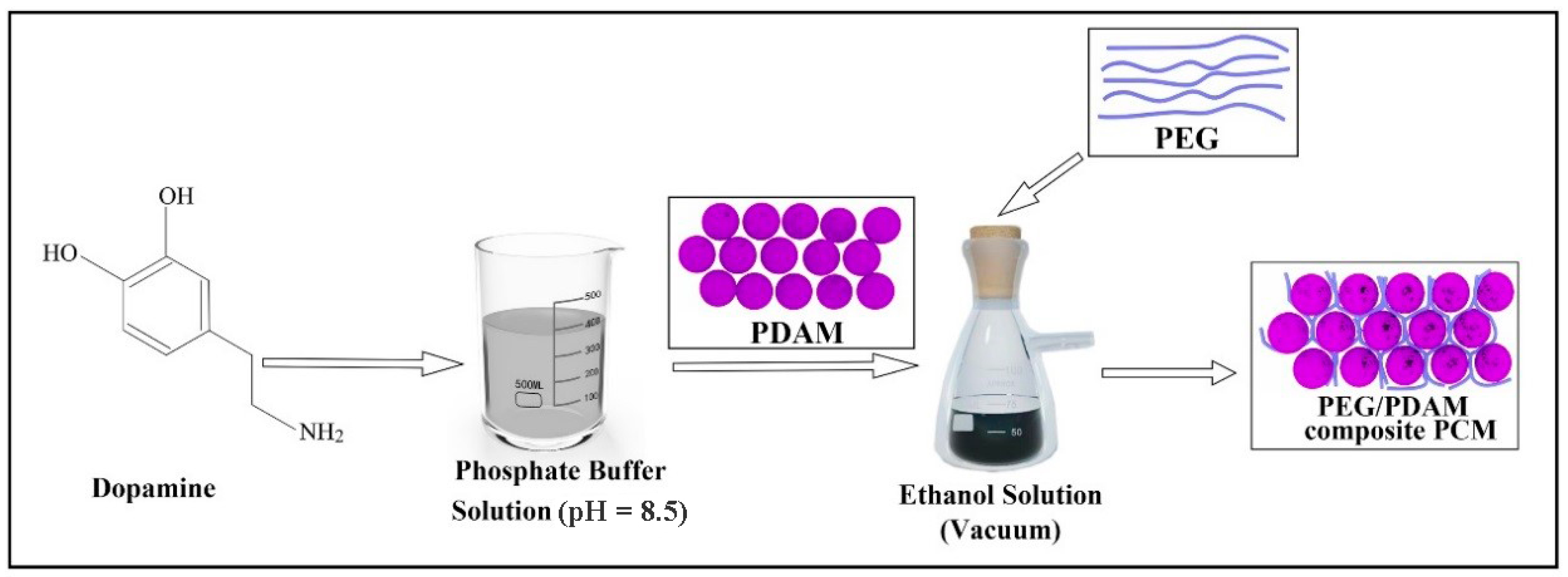
2.2. Preparation of PDAMs
2.3. Preparation of PEG/PDAM Composite Materials
2.4. Characterization
3. Results and Discussion
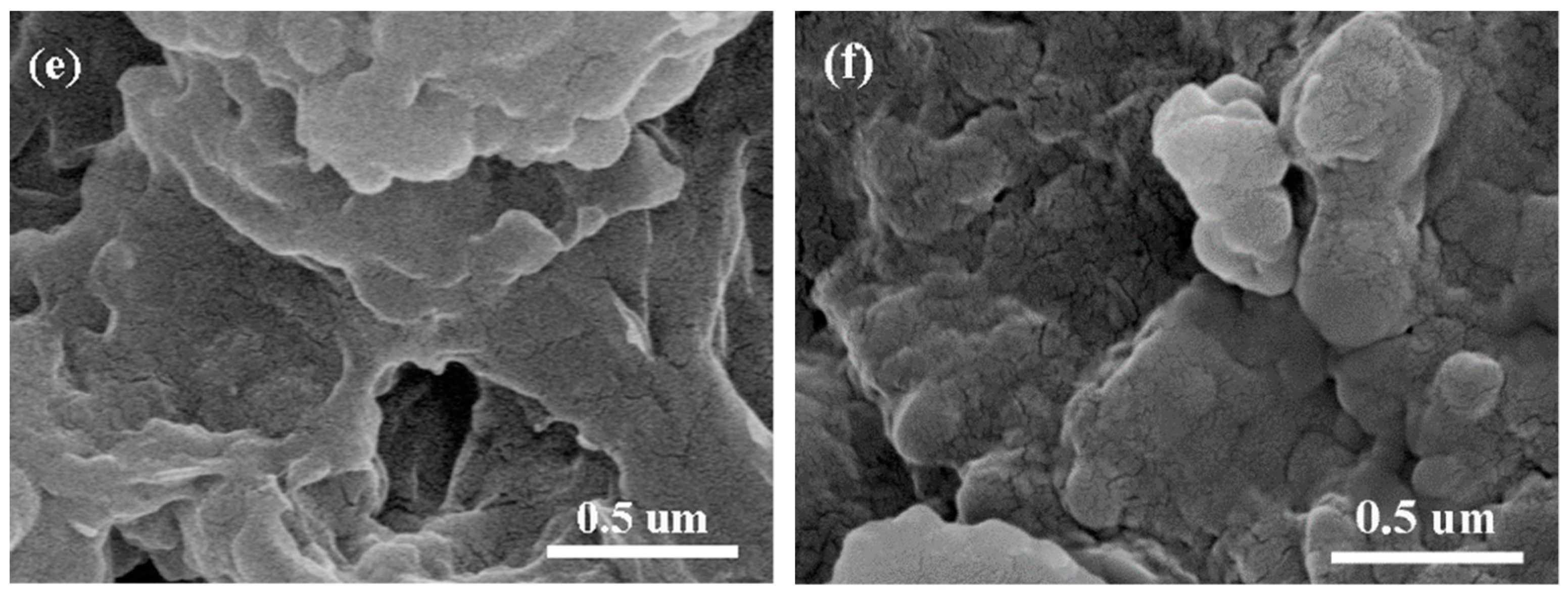
3.1. Characterization of PDAMs and PEG/PDAMs
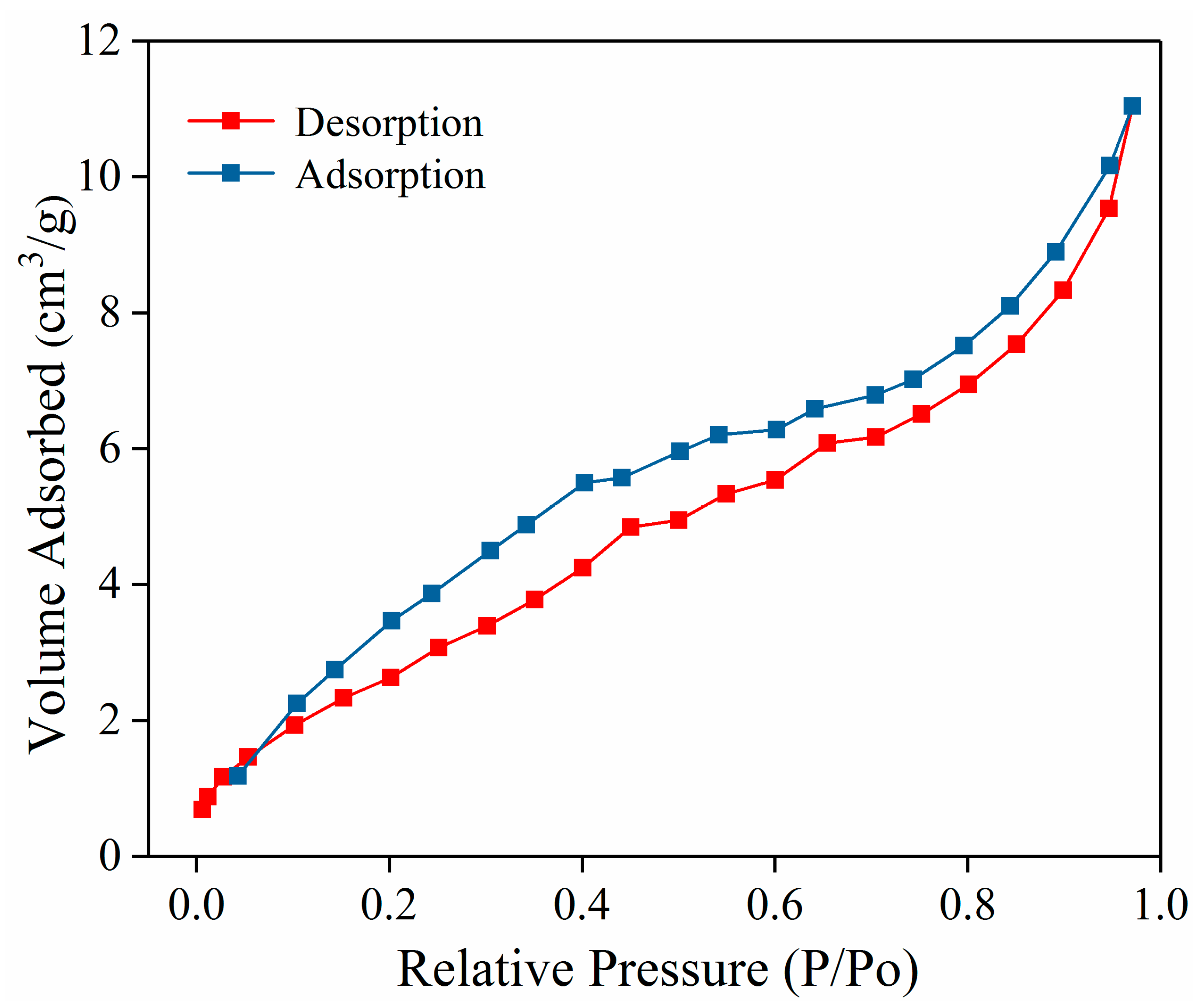
3.2. The Nitrogen Adsorption–Desorption Isotherm of the PDAMs
3.3. Leakage Tests of PEG and PEG/PDAM Composites
3.4. XRD Patterns of PEG, PDAM, and PEG/PDAM Composites
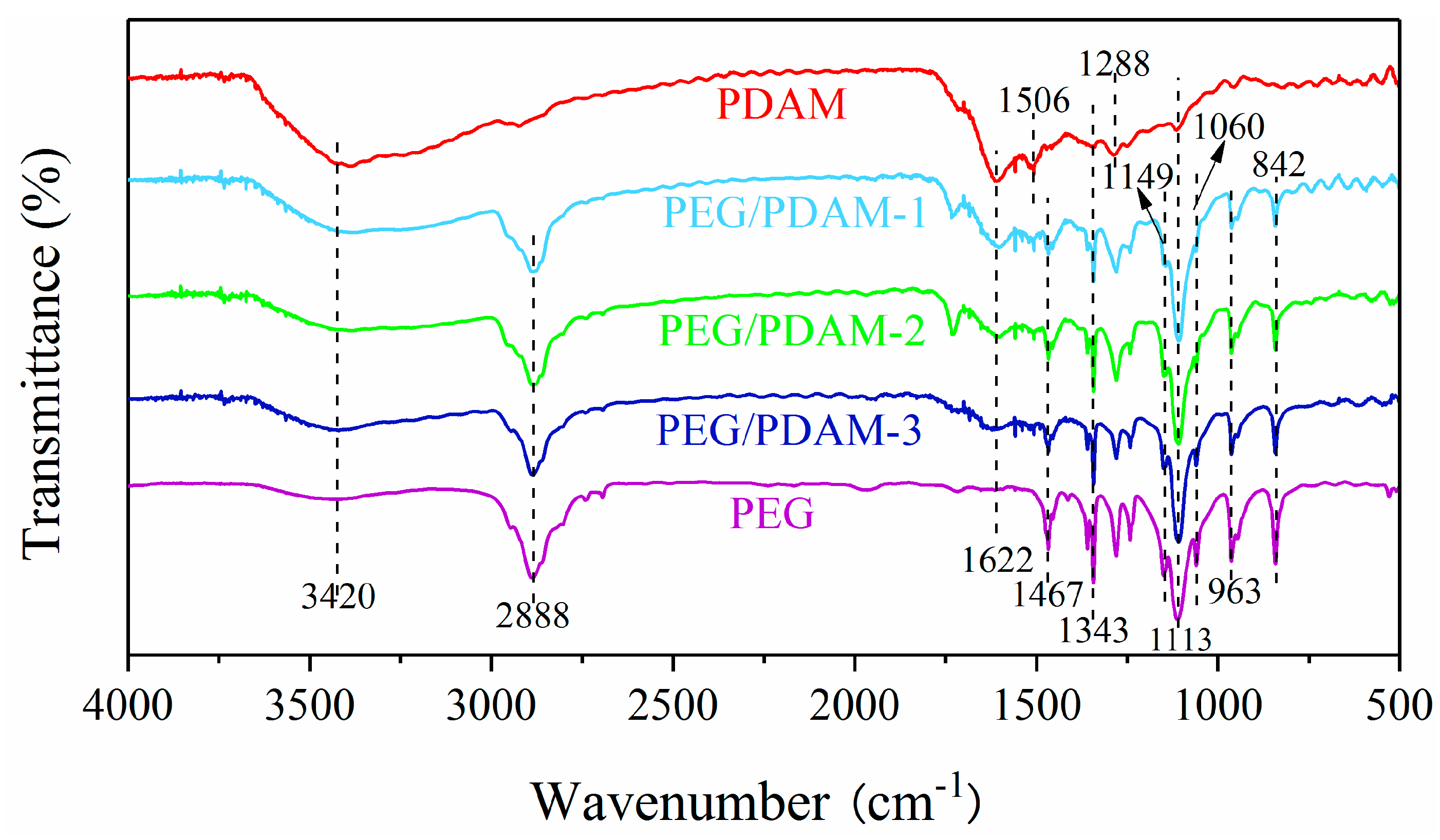
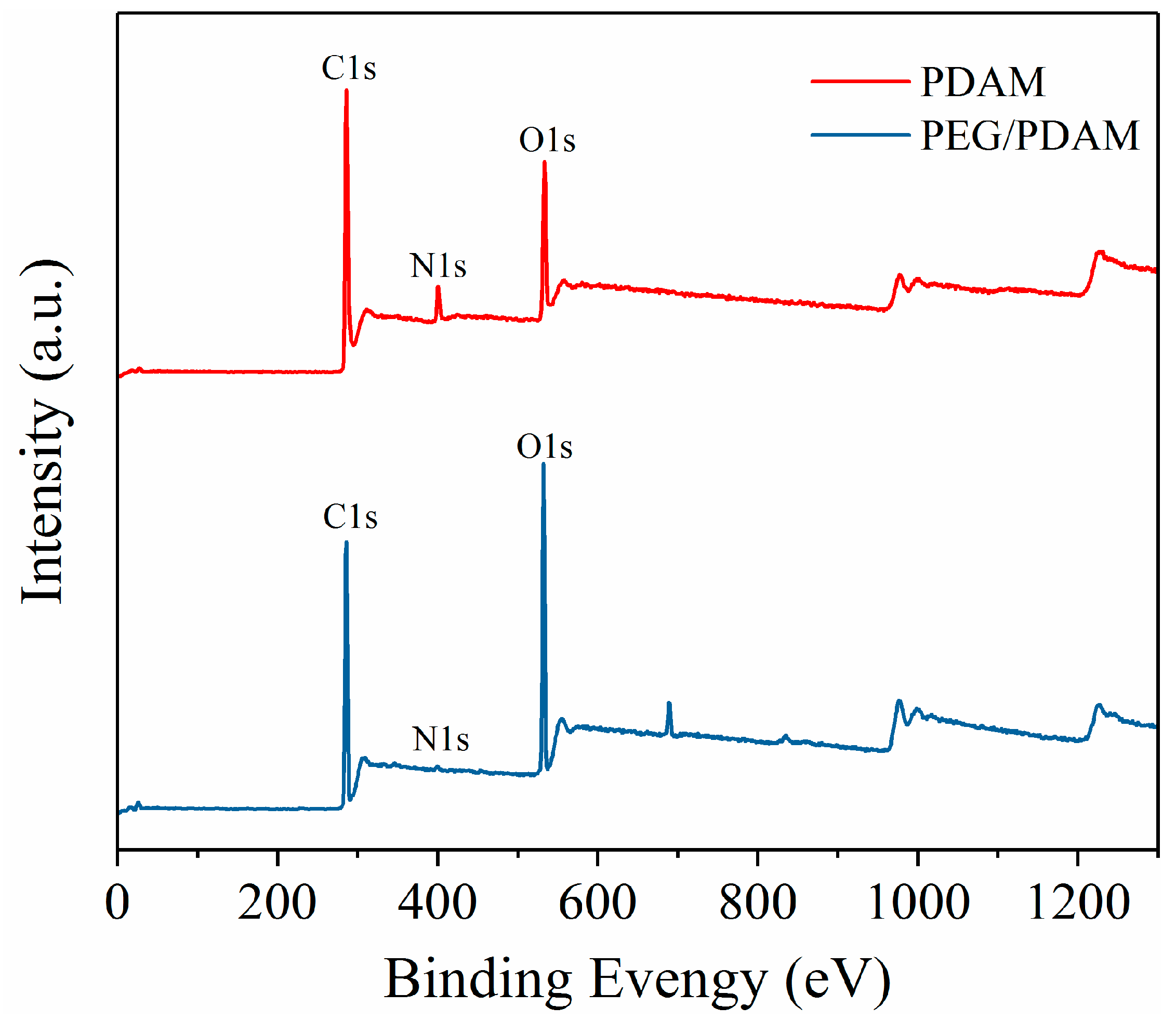
3.5. Chemical Properties
3.6. Latent Heat Storage Analysis
3.7. Thermal Stability of PEG/PDAM Composites
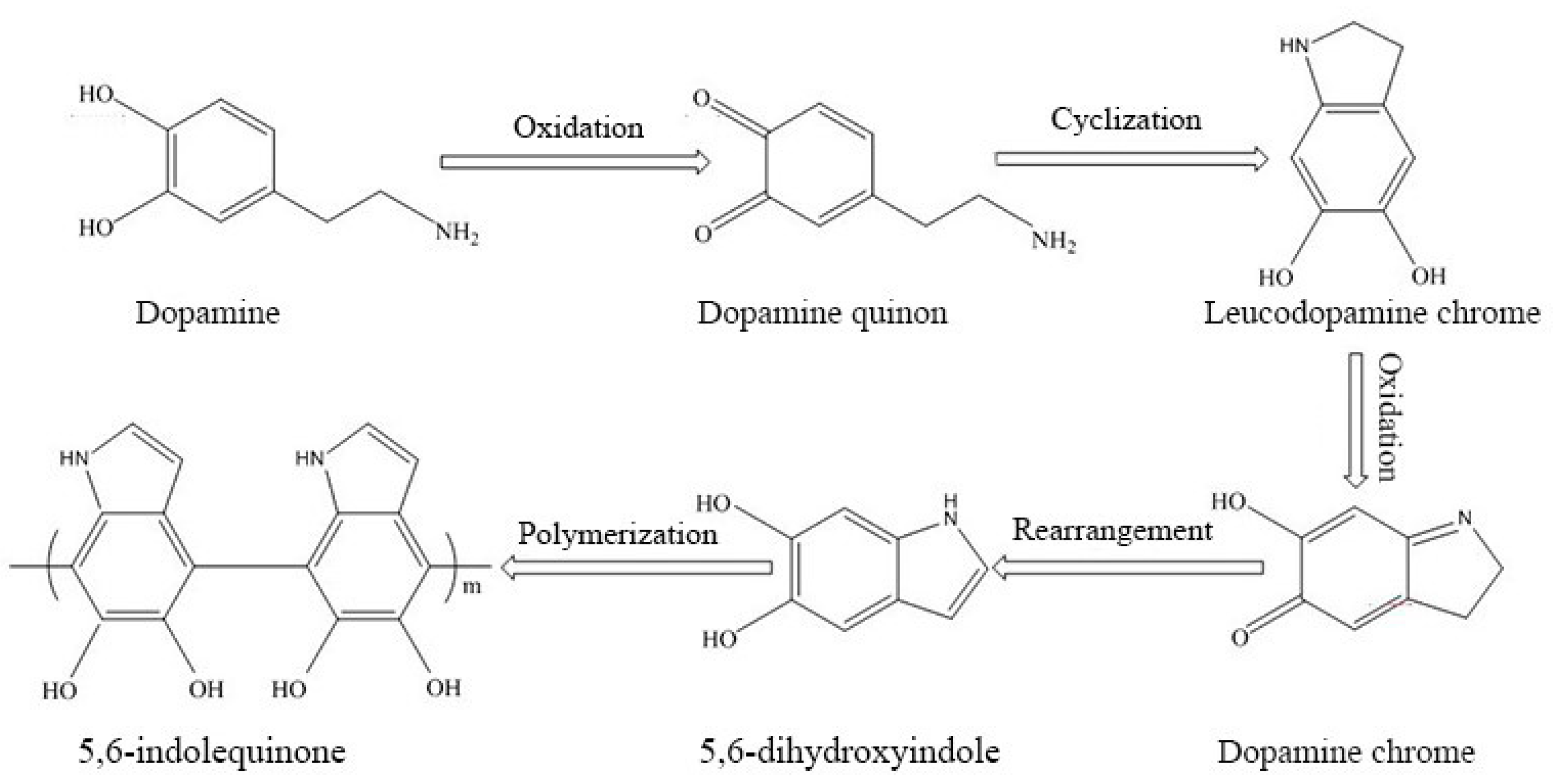
3.8. Structural Evolution of PDAMs and PEG/PDAM Composites
3.9. Comparison of PEG//PDAM-3 with Other Composite Materials
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Chen, Y.; Guo, X.W.; Tao, W.W.; Wang, J.B.; Gao, S.F.; Gao, J.K. Mesoporous silica nanoparticles with wrinkled structure as the matrix of myristic acid for the preparation of a promising new shape-stabilized phase change material via simple method. RSC Adv. 2018, 8, 34224–34231. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Park, J.S. Fabrication of Electrospun Nonwoven Mats of Polyvinylidene Fluoride/Polyethylene Glycol/Fumed Silica for Use as Energy Storage Materials. J. Appl. Polym. Sci. 2011, 121, 3596–3603. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ersoy, O. Preparation and characterization of sepiolite-based phase change material nanocomposites for thermal energy storage. Appl. Therm. Eng. 2016, 107, 575–582. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, H.S.; Liu, L.; Lu, Z.Y.; Sanjayan, J.G.; Duan, W.H. Development of granular expanded perlite/paraffin phase change material composites and prevention of leakage. Sol. Energy 2016, 137, 179–188. [Google Scholar] [CrossRef]
- Chen, J.J.; Ling, Z.Y.; Fang, X.M.; Zhang, Z.G. Experimental and numerical investigation of form-stable dodecane/hydrophobic fumed silica composite phase change materials for cold energy storage. Energy Convers. Manag. 2015, 105, 817–825. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wang, W.; Xin, G.B.; Li, G.L.; Zheng, J.; Tian, W.H.; Li, X.G. Phase change behaviors of PEG on modified graphene oxide mediated by surface functional groups. Eur. Polym. J. 2016, 74, 43–50. [Google Scholar] [CrossRef]
- Shanmugana, S.; Palanib, S.; Janarthananc, B. Productivity enhancement of solar still by PCM and Nanoparticles miscellaneous basin absorbing materials. Desalination 2018, 433, 186–198. [Google Scholar] [CrossRef]
- Pulci, G.; Paglia, L.; Genova, V.; Bartuli, C.; Valente, T.; Marra, F. Low density ablative materials modified by nanoparticles addition: Manufacturing and characterization. Compos. Part A Appl. Sci. Manuf. 2018, 109, 330–337. [Google Scholar] [CrossRef]
- Akeiber, H.; Nejat, P.; Majid, M.; Abd, M.Z.; Wahid, M.A.; Jomehzadeh, F.; Famileh, I.Z.; Calautit, J.K.; Hughes, B.R. A review on phase change material (PCM) for sustainable passive cooling in building envelopes. Renew. Sustain. Energy Rev. 2016, 60, 1470–1497. [Google Scholar] [CrossRef]
- Qian, T.T.; Li, J.H.; Ma, H.W.; Yang, J. The preparation of a green shape-stabilized composite phase change material of polyethylene glycol/SiO2 with enhanced thermal performance based on oil shale ash via temperature-assisted sol-gel method. Sol. Energy Mater. Sol. Cells 2015, 132, 29–39. [Google Scholar] [CrossRef]
- Veerakumar, C.; Sreekumar, A. Phase change material based cold thermal energy storage: Materials, techniques and applications—A review. Int. J. Refrig. 2016, 67, 271–289. [Google Scholar] [CrossRef]
- Wang, J.J.; Yang, M.; Lu, Y.F.; Jin, Z.K.; Tan, L.; Gao, H.Y.; Fan, S.; Dong, W.J.; Wang, G. Surface functionalization engineering driven crystallization behavior of polyethylene glycol confined in mesoporous silica for shape-stabilized phase change materials. Nano Energy 2016, 19, 78–87. [Google Scholar] [CrossRef]
- Cellata, K.; Beyhana, B.; Konuklub, Y.; Dundarc, C.; Karahand, O.; Gungore, C.; Paksoya, H. 2 years of monitoring results from passive solar energy storage in test cabins with phase change materials. Sol. Energy 2019. [Google Scholar] [CrossRef]
- Su, W.G.; Darkwa, J.; Kokogiannakis, G. Review of solid-liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Liu, Y.R.; Xia, Y.P.; An, K.; Huang, C.W.; Cui, W.W.; Wei, S.; Ji, R.; Xu, F.; Zhang, H.Z.; Sun, L.X. Fabrication and characterization of novel meso-porous carbon/n-octadecane as form-stable phase change materials for enhancement of phase-change behavior. J. Mater. Sci. Technol. 2019, 35, 939–945. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.G.; Nutt, S. Carbon nanotube/paraffin/montmorillonite composite phase change material for thermal energy storage. Sol. Energy 2017, 146, 1–7. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.M.; Sanjayan, J.; Petinakis, E.; Wilson, J. Development of thermal energy storage cementitious composites (TESC) containing a novel paraffin/hydrophobic expanded perlite composite phase change material. Sol. Energy 2017, 158, 626–635. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zheng, S.L.; Zhu, S.Q.; Ma, J.N.; Sun, Z.M.; Farid, M. Evaluation of paraffin infiltrated in various porous silica matrices as shape stabilized phase change materials for thermal energy storage. Energy Convers. Manag. 2018, 171, 361–370. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Li, Y.X.; Yang, Q.G.; Chen, H.; Chen, X.Q.; Jiao, T.F.; Peng, Q.M. Distinguished Cr (VI) capture with rapid and superior capability using polydopamine microsphere: Behavior and mechanism. J. Hazard. Mater. 2018, 342, 732–740. [Google Scholar] [CrossRef]
- Cheng, C.; Li, S.; Zhao, J.; Li, X.X.; Liu, Z.Y.; Ma, L.; Zhang, X.; Sun, S.D.; Zhao, C.S. Biomimetic assembly of polydopamine-layer on graphene: Mechanisms, versatile 2D and 3D architectures and pollutant disposal. Chem. Eng. J. 2013, 228, 468–481. [Google Scholar] [CrossRef]
- Zhu, Y.; Chi, Y.; Liang, S.; Luo, X.; Chen, K.; Tian, C.; Wang, J.; Zhang, L. Novel metal coated nanoencapsulated phase change materials with high thermal conductivity for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 176, 212–221. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Peng, H.N.; Li, J.B. Recent developments in dopamine-based materials for cancer diagnosis and therapy. Adv. Colloid Interface Sci. 2018, 252, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.F.; Duan, S.X.; Jiang, W.Q.; Liu, M.; Wang, S.; Sang, M.; Gong, X.L.; Li, J.X.; Xuan, S.H. High performance polydopamine-functionalized mesoporous silica nanospheres for U(VI) removal. Appl. Surf. Sci. 2017, 426, 1121–1132. [Google Scholar] [CrossRef]
- Khalil, F.; Neda, F. Polydopamine nanoparticles as a new nanobiopolymer for the biosorption of L-cysteine from aqueous solutions. J. Iran. Chem. Soc. 2015, 12, 347–357. [Google Scholar]
- Hong, Y.; Wu, W.; Hu, J.J.; Zhang, M.H.; Voevodin, A.A.; Chow, L.; Su, M. Controlling supercooling of encapsulated phase change nanoparticles for enhanced heat transfer. Chem. Phys. Lett. 2011, 504, 180–184. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Xia, T.D.; Zhao, W.J.; Yang, W.H. Effects of fabricated technology on particle size distribution and thermal properties of stearic-eicosanoic acid/polymethylmethacrylate nanocapsules. Sol. Energy Mat. Sol. Cells 2014, 120, 481–490. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Min, X.; Huang, Z.H.; Liu, Y.G.; Wu, X.W.; Fang, M.H. Honeycomb-like structured biological porous carbon encapsulating PEG: A shape-stable phase change material with enhanced thermal conductivity for thermal energy storage. Energy Build. 2018, 158, 1049–1062. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.Y.; Luo, J.P.; Sun, K.Y.; Zhang, J.; Tan, Z.C.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Meng, M.J.; Meng, X.G.; Liu, Y.; Liu, Z.C.; Han, J.; Wang, Y.; Luo, M.; Chen, R.; Ni, L.; Yan, Y.S. An ion-imprinted functionalized SBA-15 adsorbent synthesized by surface imprinting technique via reversible addition-fragmentation chain transfer polymerization for selective removal of Ce (III) from aqueous solution. J. Hazard. Mater. 2014, 278, 134–143. [Google Scholar] [CrossRef]
- Golitsyna, Y.; Pulstb, M.; Samiullahb, M.H.; Busseb, K.; Kresslerb, J.; Reicherta, D. Crystallization in PEG networks: The importance of network topology and chain tilt in crystals. Polymer 2019, 165, 72–82. [Google Scholar] [CrossRef]
- Wang, C.Y.; Feng, L.L.; Li, W.; Zheng, J.; Tian, W.H.; Li, X.G. Shape-stabilized phase change materials based on polyethylene glycol/porous carbon composite: The influence of the pore structure of the carbon materials. Sol. Energy Mat. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Sulaiman, F.A.; Karaipekli, A.; Tyagi, V.V. Diatomite/CNTs/PEG composite PCMs with shape-stabilized and improved thermal conductivity: Preparation and thermal energy storage properties. Energy Build. 2018, 164, 166–175. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.H.; Qian, T.T.; Guan, W.M.; Li, Y.L.; Yin, X.P. Thermal conductivity enhancement of polyethylene glycol/expanded vermiculite shape-stabilized composite phase change materials with silver nanowire for thermal energy storage. Chem. Eng. J. 2016, 295, 427–443. [Google Scholar] [CrossRef]
- Li, C.E.; Yu, H.; Song, Y.; Zhao, M. Synthesis and characterization of PEG/ZSM-5 composite phase change materials for latent heat storage. Renew. Energy 2018, 121, 45–52. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wen, R.L.; Tang, C.; Wu, B.G.; Huang, Z.H.; Min, X.; Huang, Y.T.; Liu, Y.G.; Fang, M.H.; Wu, X.W. Thermal conductivity enhancement of polyethylene glycol/expanded perlite with carbon layer for heat storage application. Energy Build. 2016, 130, 113–121. [Google Scholar] [CrossRef]
- Liu, X.S.; Gao, J.M.; Li, H.; Li, J.Y.; Jin, Q.; Ren, K.F.; Ji, J. Mussel-Inspired Polydopamine: A biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Rudner, M.S.; Jeremic, S.; Petterson, K.A.; Kent, D.R.; Brown, K.A.; Drake, M.D.; Goddard, W.A.; Roberts, J.D. Intramolecular Hydrogen Bonding in Disubstituted Ethanes. A Comparison of NH…O- and OH…O- Hydrogen Bonding through Conformational Analysis of 4-Amino-4-oxobutanoate (succinamate) and Monohydrogen 1,4-Butanoate (monohydrogen succinate) Anions. J. Phys. Chem. A. 2015, 109, 9076–9082. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Vecchia, N.F.D.; Avolio, R.; Alfè, M.; Errico, M.E.; Napolitano, A.; D’Ischia, M. Building-Block Diversity in Polydopamine Underpins a Multifunctional Eumelanin-Type Platform Tunable Through a Quinone Control Point. Adv. Funct. Mater. 2013, 23, 1331–1340. [Google Scholar] [CrossRef]
- Iuczak, T. Preparation and characterization of the dopamine film electrochemically deposited on a gold template and its applications for dopamine sensing in aqueous solution. Electrochim. Acta 2008, 53, 5725–5731. [Google Scholar]
- Chen, Y.; Ding, H.; Wang, B.F.; Shi, Q.; Gao, J.K.; Cui, Z.X.; Wan, Y.C. Dopamine functionalization for improving crystallization behavior of polyethylene glycol in shape-stable phase change material with silica fume as the matrix. J. Clean. Prod. 2019, 208, 951–959. [Google Scholar] [CrossRef]
- Clark, M.B.; Gardella, J.A.; Schultz, T.M.; Patil, D.G.; Salvati, L. Solid-state Analysis of Eumelanin Biopolymers by Electron Spectroscopy for Chemical Analysis. Anal. Chem. 1990, 62, 949–956. [Google Scholar] [CrossRef]
- Kaminska, I.; Das, M.R.; Coffinier, Y.; Jonsson, J.N.; Sobczak, J.; Woisel, P.; Lyskawa, J.; Opallo, M.; Boukherroub, R.; Szunerits, S. Reduction and Functionalization of Graphene Oxide Sheets Using Biomimetic Dopamine Derivatives in One Step. ACS Appl. Mater. Interfaces 2012, 4, 1016–1020. [Google Scholar] [CrossRef]
- Xi, Z.Y.; Xu, Y.Y.; Zhu, L.P.; Wang, Y.; Zhu, B.K. A facile method of surface modification for hydrophobic polymer membranes based on the adhesive behavior of poly (DOPA) and poly(dopamine). J. Membr. Sci. 2009, 327, 244–253. [Google Scholar] [CrossRef]
- Mackie, N.M.; Castner, D.G.; Fisher, E.R. Characterization of Pulsed-Plasma-Polymerized Aromatic Films. Langmuir 1998, 14, 1227–1235. [Google Scholar] [CrossRef]
- Feng, L.L.; Zhao, W.; Zheng, J.; Frisco, S.; Song, P.; Li, X.G. The shape-stabilized phase change materials composed of polyethylene glycol and various mesoporous matrices (AC, SBA-15 and MCM-41). Sol. Energy Mat. Sol. Cells 2011, 95, 3550–3556. [Google Scholar] [CrossRef]
- Min, X.; Fang, M.H.; Huang, Z.H.; Liu, Y.G.; Huang, Y.T.; Wen, R.L.; Qian, T.T.; Wu, X.W. Enhanced thermal properties of novel shape-stabilized PEG composite phase change materials with radial mesoporous silica sphere for thermal energy storage. Sci. Rep. 2015, 5, 12964. [Google Scholar] [CrossRef]








| Samples | Tmo (°C) | Tmp (°C) | Tme (°C) | ΔHm (J/g) | Tco (°C) | Tcp (°C) | Tce (°C) | ΔHc (J/g) |
|---|---|---|---|---|---|---|---|---|
| PEG/PDAM-1 | 56.00 ± 0.00 | 60.9 ± 0.15 | 65.45 ± 1.05 | 82.57 ± 4.27 | 23.70 ± 1.40 | 28.05 ± 1.05 | 34.75 ± 0.05 | 66.10 ± 4.16 |
| PEG/PDAM-2 | 54.55 ± 0.95 | 62.00 ± 0.00 | 66.10 ± 0.70 | 108.65 ± 1.85 | 25.10 ± 1.10 | 29.80 ± 0.80 | 34.50 ± 2.00 | 91.17 ± 2.44 |
| PEG/PDAM-3 | 57.35 ± 0.05 | 62.20 ± 0.00 | 68.00 ± 0.50 | 133.20 ± 2.50 | 26.80 ± 0.80 | 31.10 ± 0.60 | 35.90 ± 0.80 | 107.55 ± 4.45 |
| PEG | 59.05 ± 0.15 | 63.30 ± 0.60 | 66.05 ± 0.85 | 227.40 ± 2.00 | 32.30 ± 0.10 | 35.95 ± 0.35 | 40.25 ± 0.65 | 207.10 ± 0.00 |
| Samples | Melting | Freezing | References | ||
|---|---|---|---|---|---|
| ΔHm (J/g) | Tmp (°C) | ΔHc (J/g) | Tcp (°C) | ||
| PEG/PDAM-3 | 133.20 ± 2.50 | 62.20 ± 0.00 | 107.55 ± 4.45 | 31.10 ± 0.60 | Present study |
| PEG/AC a | 81.30 | 49.00 | 72.80 | 27.80 | [48] |
| PEG/Dop-SF-3 b | 73.80 | 53.00 | 69.10 | 44.80 | [43] |
| PEG/RMS c | 129.60 | 57.22 | 118.30 | 39.02 | [49] |
| PEG/SiO2 d | 151.80 | 58.09 | 141.00 | 42.34 | [10] |
| PEG/ZSM-5c e | 76.40 | 60.50 | 64.30 | 44.90 | [35] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Tang, X.; Chen, Z.; Ding, H.; Liu, Y.; Li, X.; Chen, Y. A Facile and Simple Method for Preparation of Novel High-Efficient Form-Stable Phase Change Materials Using Biomimetic–Synthetic Polydopamine Microspheres as a Matrix for Thermal Energy Storage. Polymers 2019, 11, 1503. https://doi.org/10.3390/polym11091503
Gao J, Tang X, Chen Z, Ding H, Liu Y, Li X, Chen Y. A Facile and Simple Method for Preparation of Novel High-Efficient Form-Stable Phase Change Materials Using Biomimetic–Synthetic Polydopamine Microspheres as a Matrix for Thermal Energy Storage. Polymers. 2019; 11(9):1503. https://doi.org/10.3390/polym11091503
Chicago/Turabian StyleGao, Junkai, Xi Tang, Zhengshou Chen, Han Ding, Yi Liu, Xuebin Li, and Yan Chen. 2019. "A Facile and Simple Method for Preparation of Novel High-Efficient Form-Stable Phase Change Materials Using Biomimetic–Synthetic Polydopamine Microspheres as a Matrix for Thermal Energy Storage" Polymers 11, no. 9: 1503. https://doi.org/10.3390/polym11091503
APA StyleGao, J., Tang, X., Chen, Z., Ding, H., Liu, Y., Li, X., & Chen, Y. (2019). A Facile and Simple Method for Preparation of Novel High-Efficient Form-Stable Phase Change Materials Using Biomimetic–Synthetic Polydopamine Microspheres as a Matrix for Thermal Energy Storage. Polymers, 11(9), 1503. https://doi.org/10.3390/polym11091503




