In situ Fabrication of Multi-Walled Carbon Nanotubes/Silica Hybrid Colloidosomes by Pickering Emulsion Templating Using Trialkoxysilanes of Opposite Polarity
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Buffers
2.2. Preparation of Silica Dispersions
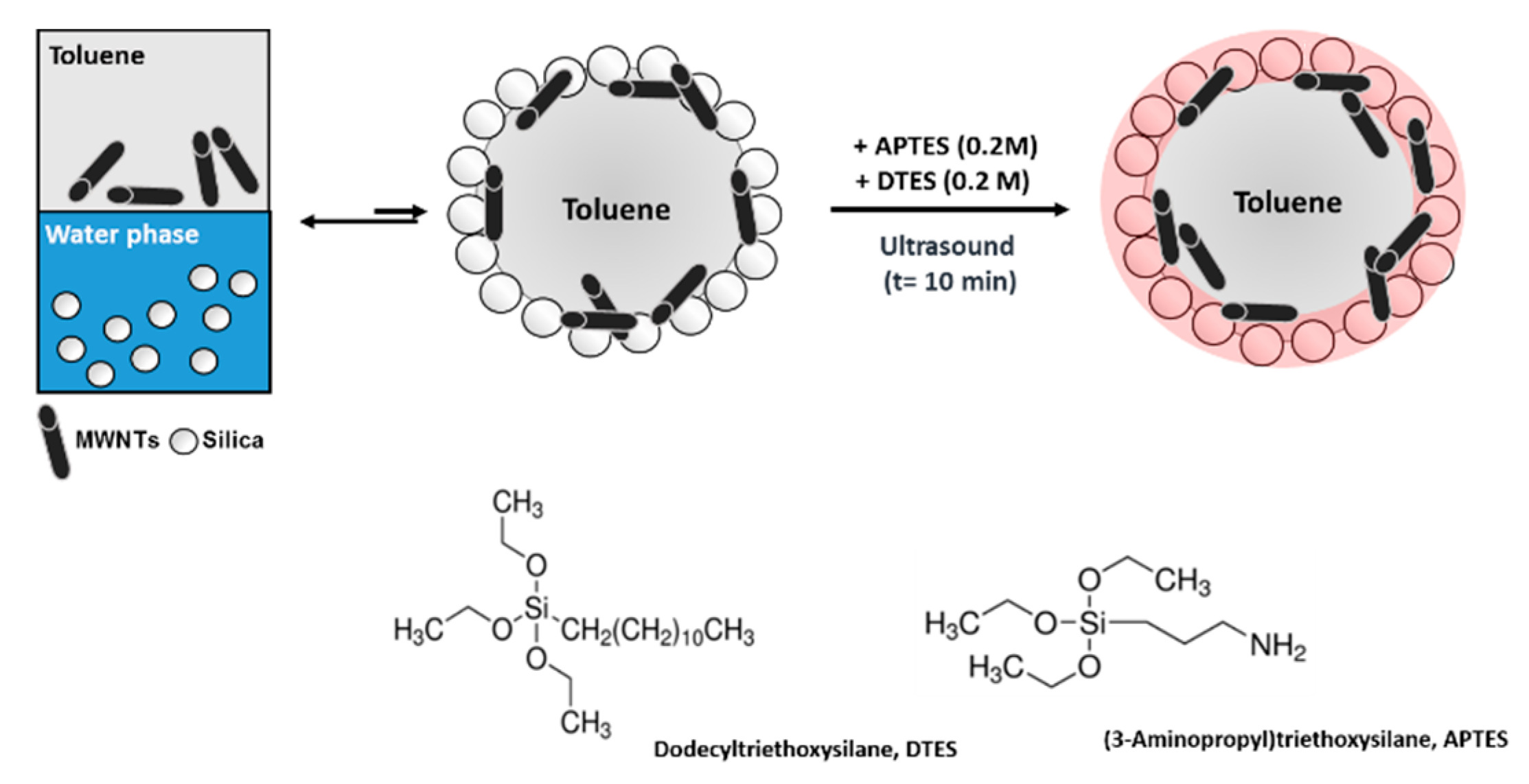
2.3. Pickering Emulsions by In Situ Functionalization of Silica
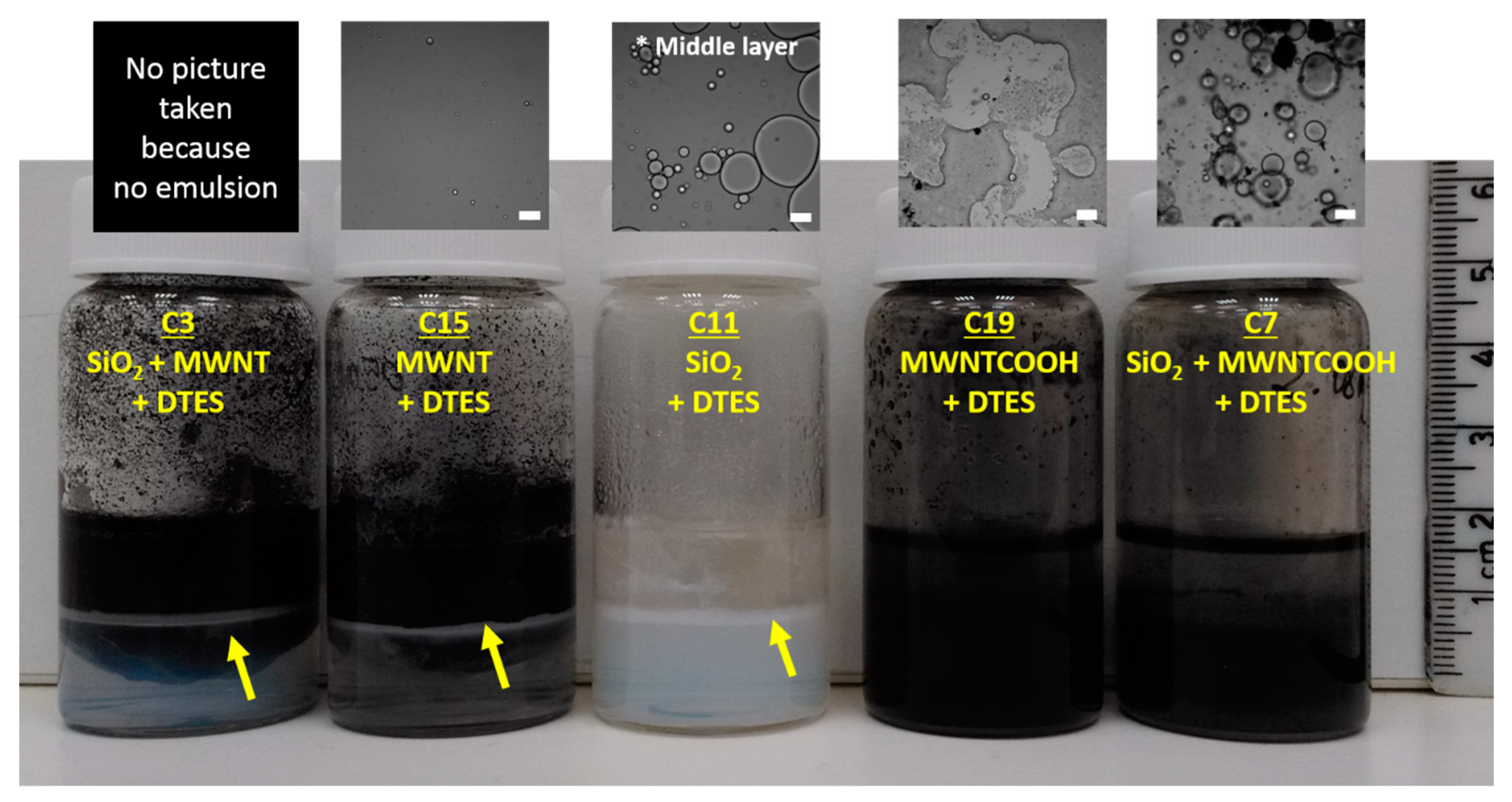
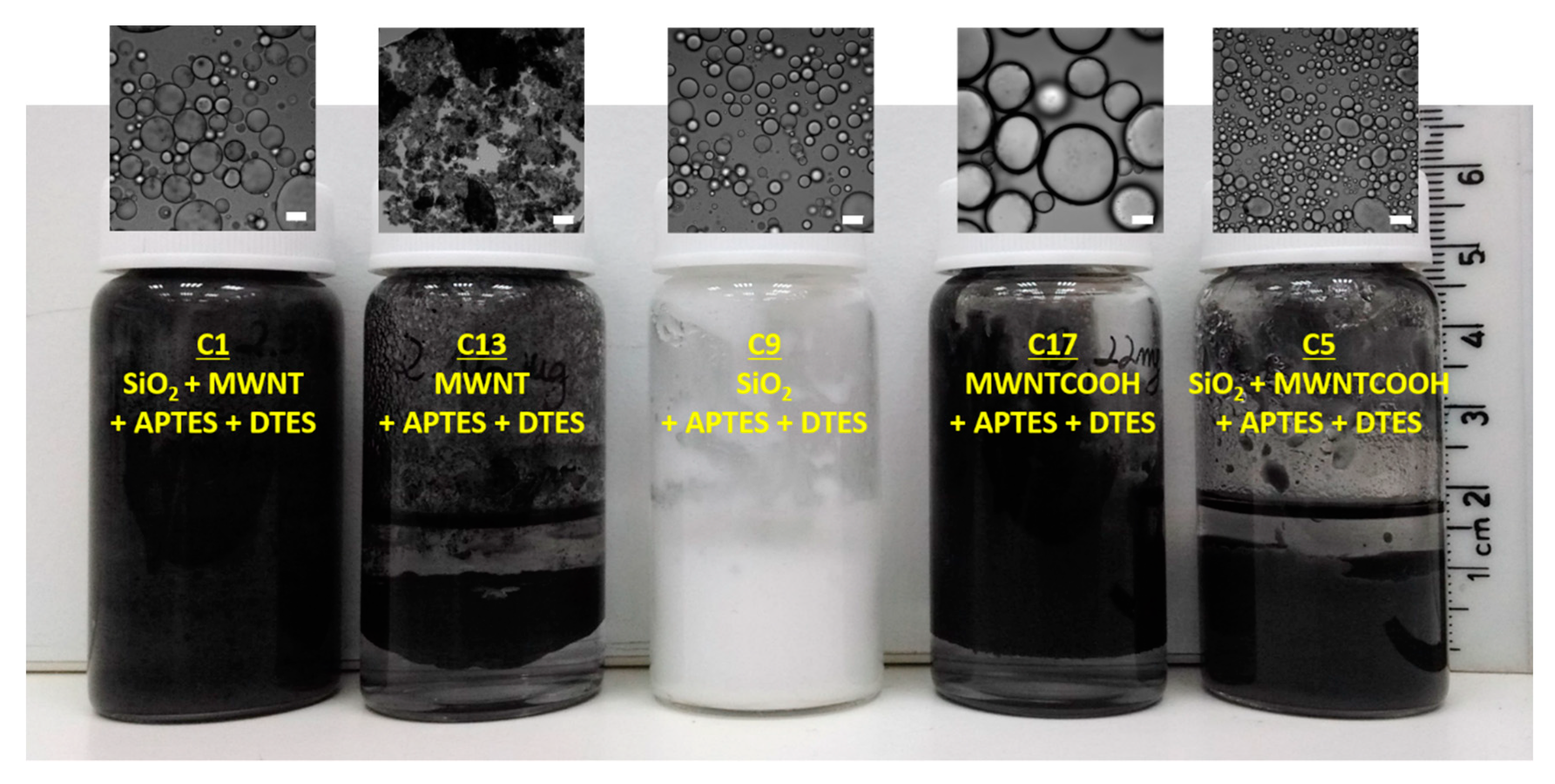
2.4. Preparation of Control Samples
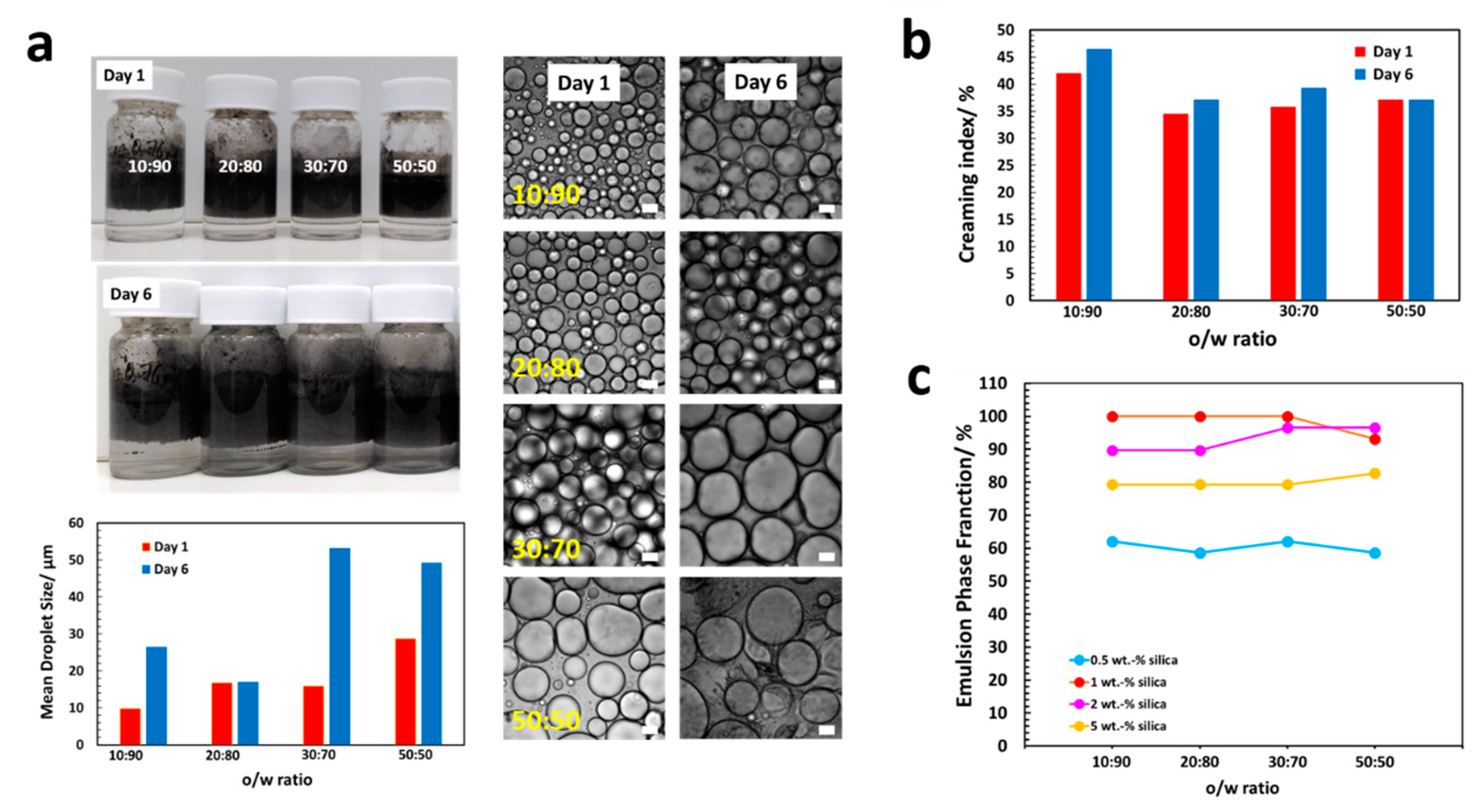
2.5. Determination of Emulsion Stability and Emulsification Ability
2.6. Bright Field Optical Microscopy
2.7. Fluorescence Labelling of MWNT Surface Functional Groups
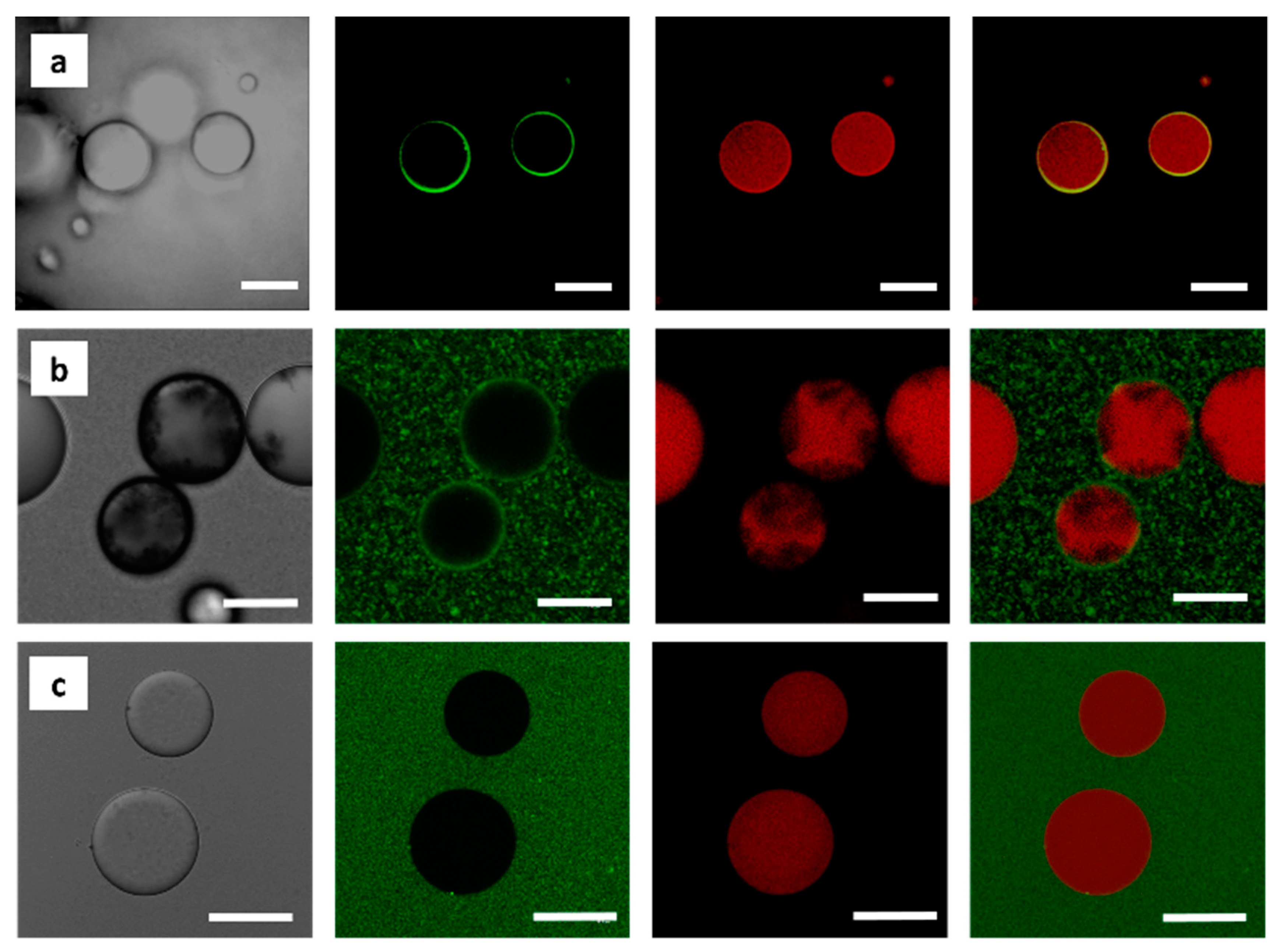
2.8. Confocal Laser Scanning Microscopy (CLSM)
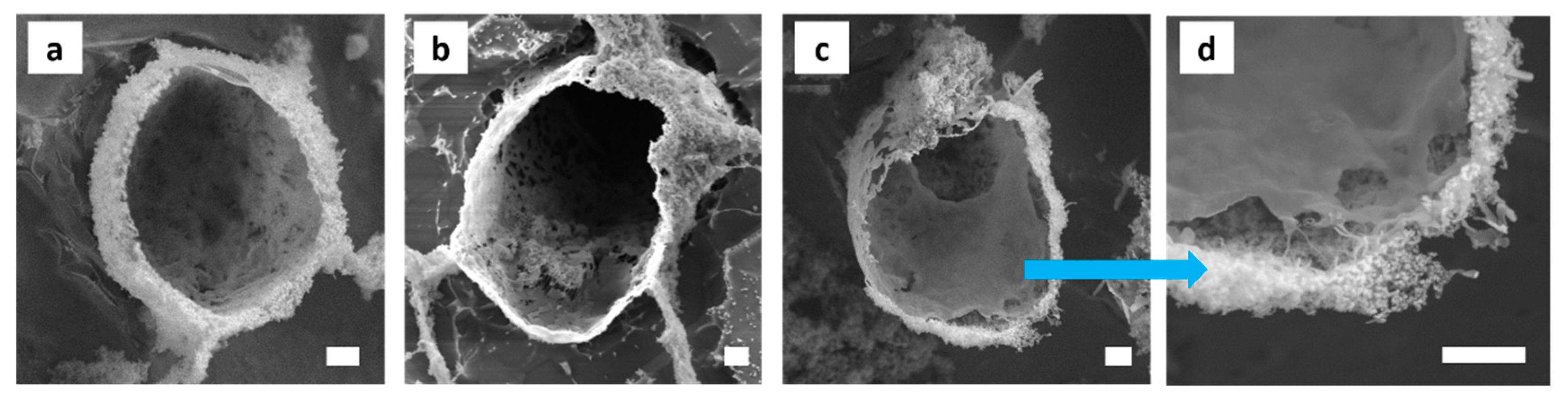
2.9. High-Resolution Scanning Electron Microscopy (HR-SEM)
2.10. Cryogenic-Field Emission Scanning Electron Microscopy
2.11. Electrical Resistance Measurements
3. Results and Discussion
3.1. Preparation of MWNT/Silica Pickering Emulsions By In Situ Functionalization of Silica
3.2. Localization of MWNTs at the O/W Interface as Revealed by CLSM
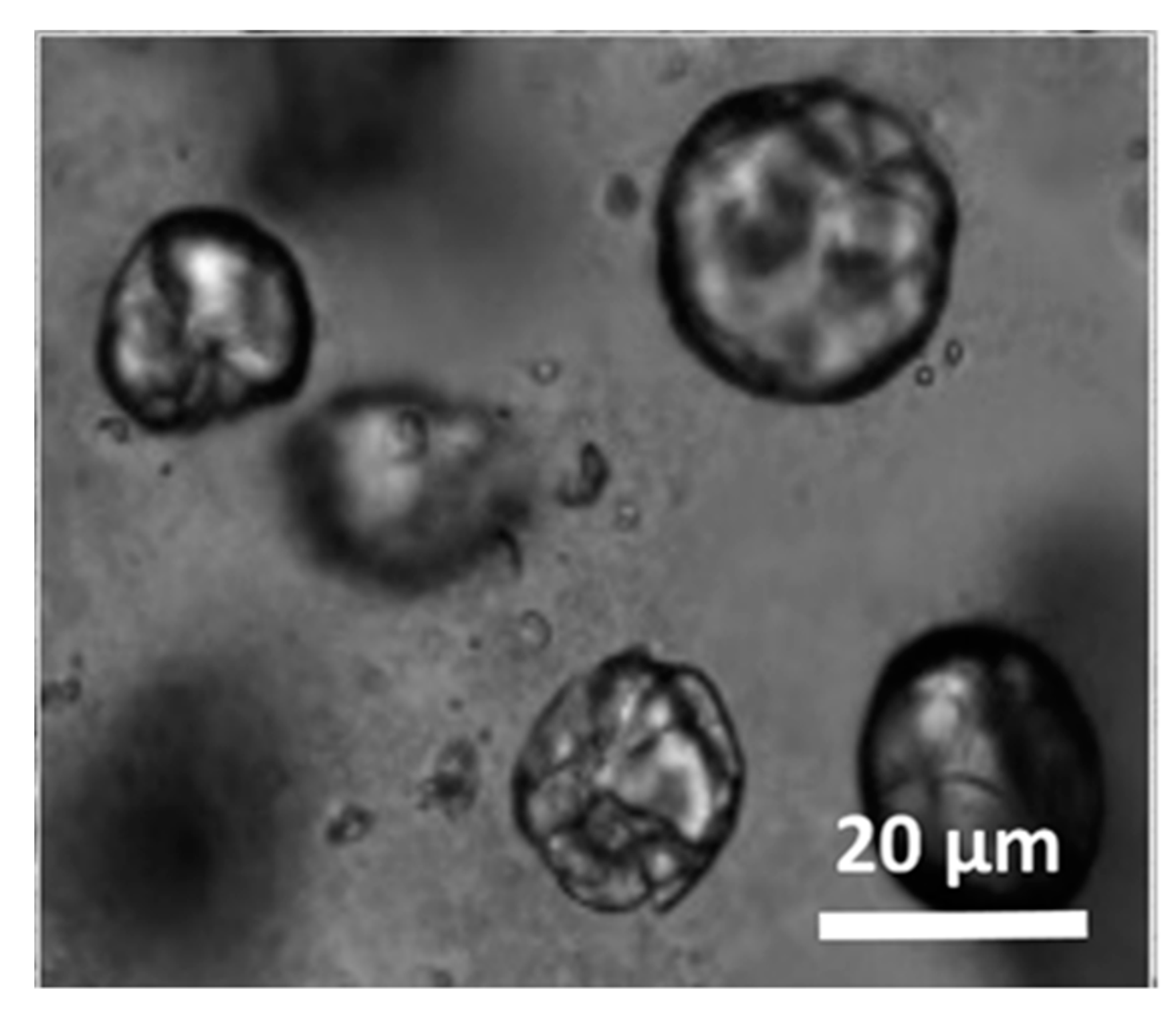
3.3. Morphology and Shell Thickness of the MWNT/Silica Microcapsules
3.4. Highly Interconnected Porous Structures Form in Solid State
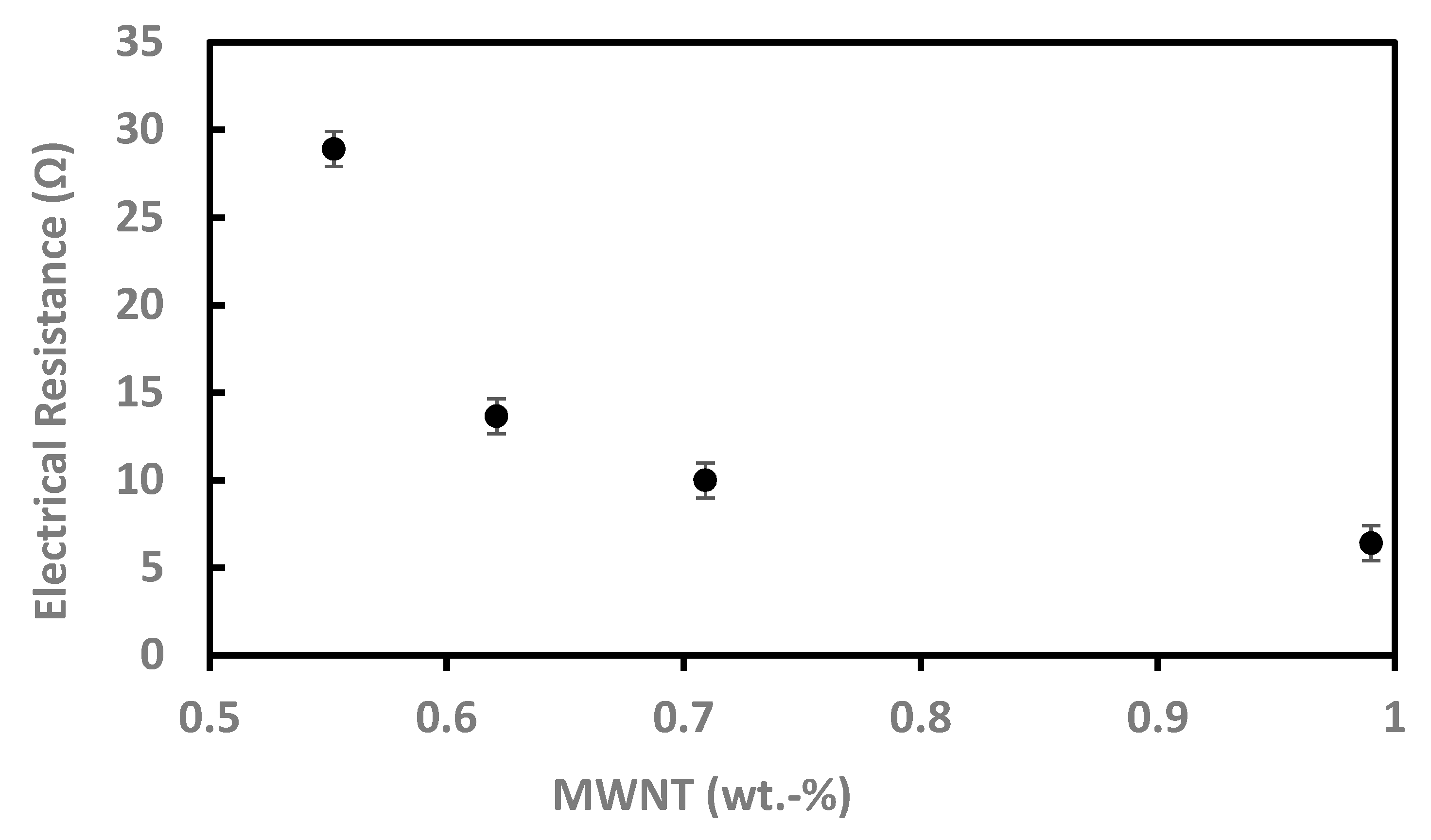
3.5. Electrical Resistance Measurements of the MWNT/Silica Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

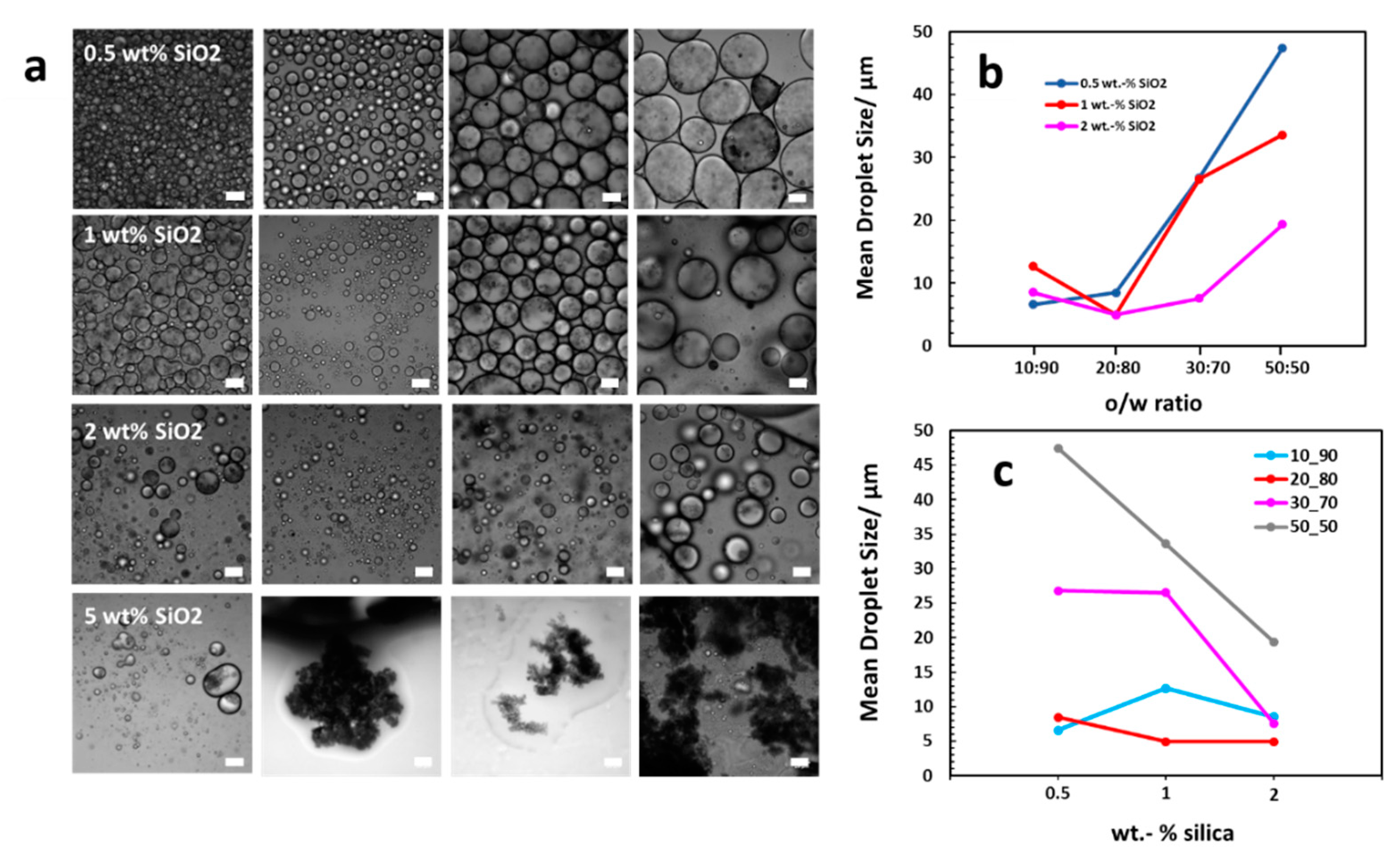



| wt% SiO2 | Mean Droplet Diameter (±Standard Deviation)/μm | ||||
|---|---|---|---|---|---|
| 10 vol% Toluene | 20 vol% Toluene | 30 vol% Toluene | 50 vol% Toluene | ||
| 1 mg MWNT | 0.5 | 9.9 (±6.4) | 16.8 (±8.6) | 15.9 (±13.0) | 28.7 (±20.1) |
| 1 | 5.9 (±2.7) | 13.9 (±6.5) | 18.3 (±6.3) | 26.0 (±14.0) | |
| 2 | 6.2 (±4.4) | 14.0 (±3.4) | 16.5 (±6.7) | 39.2 (±22.0) | |
| 5 | 21.7 (±24.5) | 46.8 (±38.0) | 10.9 (±10.5) | 17.6 (±10.2) | |
| 2 mg MWNT | 0.5 | 9.6 (±4.5) | 18.3 (±9.8) | 20.5 (±11.7) | 53.7 (±17.5) |
| 1 | 5.0 (±2.9) | 12.4 (±6.7) | 21.6 (±9.7) | 38.7 (±23.5) | |
| 2 | 15.5 (±11.5) | 16.3 (±9.1) | 24.1 (±12.5) | 28.9 (±10.6) | |
| 5 | 6.0 (±4.4) | 8.3 (±5.4) | 42.8(±25.0) | 16.4 (±7.1) | |
| 5 mg MWNT | 0.5 | 6. 6 (±2.3) | 8.5 (±3.8) | 26. 8 (±9.8) | 47.5 (±11.3) |
| 1 | 12.7 (±6.3) | 4.9 (±2.5) | 26.5 (±8.3) | 33.6 (±21.0) | |
| 2 | 8.5 (±8.0) | 4.9 (±3.0) | 7.5 (±3.6) | 19.3 (±10.0) | |
| 5 | nd | nd | nd | nd | |
| Vol% Toluene | Mean Droplet Diameter (±Standard Deviation)/μm | ||
|---|---|---|---|
| Day 1 | Day 2 | ||
| 1 mg MWNT 0.5 wt% silica | 10 | 9.9 (±6.4) | 26.5 (±12.4) |
| 20 | 16.8 (±8.6) | 17.1 (±10.8) | |
| 30 | 15.9 (±13.0) | 53.3 (±11.3) | |
| 50 | 28.7 (±20.1) | 49.3 (±20.3) | |
References
- Hench, L.L.; West, J.K. The sol–gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U.U. Aerogels—Airy Materials: Chemistry, Structure, and Properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Walcarius, A. Electrochemical Applications of Silica-Based Organic-Inorganic Hybrid Materials. Chem. Mater. 2001, 13, 3351–3372. [Google Scholar] [CrossRef]
- Gill, I. Bio-doped Nanocomposite Polymers: Sol–Gel Bioencapsulates. Chem. Mater. 2001, 13, 3404–3421. [Google Scholar] [CrossRef]
- Yang, H.; Shi, Q.; Tian, B.; Xie, S.; Zhang, F.; Yan, Y.; Tu, B.; Zhao, D. A Fast Way for Preparing Crack-Free Mesostructured Silica Monolith. Chem. Mater. 2003, 15, 536–541. [Google Scholar] [CrossRef]
- Tsionsky, M.; Gun, G.; Glezer, V.; Lev, O. Sol-Gel-Derived Ceramic-Carbon Composite Electrodes: Introduction and Scope of Applications. Anal. Chem. 1994, 66, 1747–1753. [Google Scholar] [CrossRef]
- Gill, I.; Ballesteros, A. Encapsulation of Biologicals within Silicate, Siloxane, and Hybrid Sol–Gel Polymers: An Efficient and Generic Approach. J. Am. Chem. Soc. 1998, 120, 8587–8598. [Google Scholar] [CrossRef]
- Wang, B.Q.; Li, B.; Wang, Z.X.; Xu, G.B.; Wang, Q.; Dong, S.J. Sol–Gel Thin-Film Immobilized Soybean Peroxidase Biosensor for the Amperometric Determination of Hydrogen Peroxide in Acid Medium. Anal. Chem. 1999, 71, 1935–1939. [Google Scholar] [CrossRef]
- Walcarius, A.; Mandler, D.; Cox, J.A.; Collinson, M.; Lev, O. Exciting new directions in the intersection of functionalized sol–gel materials with electrochemistry. J. Mater. Chem. 2005, 15, 3663–3689. [Google Scholar] [CrossRef]
- Zhu, J.; Kim, J.D.; Peng, H.Q.; Margrave, J.L.; Khabashesku, V.N.; Barrera, E.V. Improving the Dispersion and Integration of Single-Walled Carbon Nanotubes in Epoxy Composites through Functionalization. Nano Lett. 2003, 3, 1107–1113. [Google Scholar] [CrossRef]
- Velasco-Santos, C.; Martinez-Hernandez, A.L.; Fisher, F.T.; Ruoff, R.; Castano, V.M. Improvement of Thermal and Mechanical Properties of Carbon Nanotube Composites through Chemical Functionalization. Chem. Mater. 2003, 15, 4470–4475. [Google Scholar] [CrossRef]
- Zhan, G.D.; Kuntz, J.D.; Wan, J.L.; Mukherjee, A.K. Single-wall carbon nanotubes as attractive toughening agents in alumina-based nanocomposites. Nat. Mater. 2003, 2, 38–42. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Xu, W.; Rajagopalan, S.; Wang, C.; Wang, H.; Fan, Y.; Zhang, L.; Jiang, D.; Kapat, J.; Chow, L.; et al. Carbon-Nanotube-Reinforced Polymer-Derived Ceramic Composites. Adv. Mater. 2004, 16, 2036–2040. [Google Scholar] [CrossRef]
- Peigney, A. Tougher ceramics with nanotubes. Nat. Mater. 2003, 2, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes; Academic Press: San Diego, CA, USA, 1996. [Google Scholar]
- Ajayan, P.M. Nanotubes from Carbon. Chem. Rev. 1999, 99, 1787. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Prato, M. Can Carbon Nanotubes be Considered Useful Tools for Biological Applications? Adv. Mater. 2003, 15, 1765–1768. [Google Scholar] [CrossRef]
- Riggs, J.E.; Walker, D.B.; Carroll, D.L.; Sun, Y.P. Optical Limiting Properties of Suspended and Solubilized Carbon Nanotubes. J. Phys. Chem. B 2000, 104, 7071–7076. [Google Scholar] [CrossRef]
- Yang, Y.L.; Gupta, M.C. Novel Carbon Nanotube–Polystyrene Foam Composites for Electromagnetic Interference Shielding. Nano Lett. 2005, 5, 2131–2134. [Google Scholar] [CrossRef]
- Cho, J.; Boccaccini, A.R.; Shaffer, M.S.P. Ceramic Matrix Composites Containing Carbon Nanotubes. J. Mater. Sci. 2009, 44, 1934–1951. [Google Scholar] [CrossRef]
- Tjong, S.C. Carbon Nanotube Reinforced Composites: Metal and Ceramic Matrices; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Colorado, R.; Diosomito, M.E.; Barron, A.R. In-Situ Fabrication of Freestanding Single-Walled Carbon Nanotube–Silicate Composite Hex Nuts. Adv. Mater. 2005, 17, 1634–1637. [Google Scholar] [CrossRef]
- Guo, S.; Sivakumar, R.; Kagawa, Y. Multiwall Carbon Nanotube-SiO2 Nanocomposites: Sintering, Elastic Properties, and Fracture Toughness. Adv. Eng. Mater. 2007, 9, 84–87. [Google Scholar] [CrossRef]
- Berguiga, L.; Bellessa, J.; Vocanson, F.; Bernstein, E.; Plenet, J.C. Carbon nanotube silica glass composites in thin films by the sol–gel technique. Opt. Mater. 2006, 28, 167–171. [Google Scholar] [CrossRef]
- Sivakumar, R.; Guo, S.; Nishimura, T.; Kagawa, Y. Thermal conductivity in multi-wall carbon nanotube/silica-based nanocomposites. Scr. Mater. 2007, 56, 265–268. [Google Scholar] [CrossRef]
- Xiang, C.; Pan, Y.; Liu, X.; Sun, X.; Shi, X.; Guo, J.K. Microwave attenuation of multiwalled carbon nanotube-fused silica composites. Appl. Phys. Lett. 2005, 87, 123103–123106. [Google Scholar] [CrossRef]
- Chan, Z.; Miao, F.; Xiao, Z.; Juan, H.; Zhan, H. effect of doping levels on the pore structure of carbon nanotubes/silica xerogels composites. Mater. Lett. 2007, 61, 644–647. [Google Scholar] [CrossRef]
- Zhan, H.; Chan, Z.; Chen, W.; Wang, M. Characterization and nonlinear optical property of a multi-walled carbon nanotube/silica xerogel composite. Chem. Phys. Lett. 2005, 411, 373–377. [Google Scholar]
- Bottini, M.; Tautz, L.; Huynh, H.; Monosov, E.; Bottini, N.; Dawson, M.I.; Bellucci, S.; Mustelin, T. Covalent decoration of multi-walled carbon nanotubes with silica nanoparticles. Chem. Commun. 2005, 6, 758–760. [Google Scholar] [CrossRef]
- Peng, H.; Jain, M.; Peterson, D.S.; Zhu, Y.; Jia, Q. Composite Carbon Nanotube/Silica Fibers with Improved Mechanical Strengths and Electrical Conductivities. Small 2008, 4, 1964–1967. [Google Scholar] [CrossRef]
- Dinsmore, A.D.; Hsu, M.F.; Nikolaides, M.G.; Marquez, M.; Bausch, A.R.; Weitz, D.A. Colloidosomes: Selectively Permeable Capsules Composed of Colloidal Particles. Science 2002, 298, 1006–1009. [Google Scholar] [CrossRef]
- Price, K.E.; Mason, B.P.; Bogdan, A.R.; Broadwater, S.J.; Steinbacher, J.L.; McQuade, D.T. Microencapsulated Linear Polymers: “Soluble” Heterogeneous Catalysts. J. Am. Chem. Soc. 2006, 128, 10376–10377. [Google Scholar] [CrossRef]
- Yow, H.N.; Routh, A.F. Formation of Liquid Core–Polymer Shell Microcapsules. Soft Matter 2006, 2, 940–949. [Google Scholar] [CrossRef]
- Sander, J.S.; Studart, A.R. Nanoparticle-Filled Complex Colloidosomes for Tunable Cargo Release. Langmuir 2013, 29, 15168–15173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; van Oers, M.C.M.; Rutjes, F.P.J.T.; van Hest, J.C.M. Polymersome Colloidosomes for Enzyme Catalysis in a Biphasic System. Angew. Chem. Int. Ed. 2012, 51, 10746–10750. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, J.; Bai, Y.; Zhang, Q.; Yin, Y. Synthesis, Properties, and Applications of Hollow Micro-/Nanostructures. Chem. Rev. 2016, 116, 10983–11060. [Google Scholar] [CrossRef] [PubMed]
- Velev, O.D.; Furusawa, K.; Nagayama, K. Assembly of Latex Particles by Using Emulsion Droplets as Templates. 1. Microstructured Hollow Spheres. Langmuir 1996, 12, 2374–2384. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Zhang, Z.; Vincent, B. Silica-Shell/Oil-Core Microcapsules with Controlled Shell Thickness and Their Breakage Stress. Langmuir 2009, 25, 7962–7966. [Google Scholar] [CrossRef]
- Bean, K.; Black, C.F.; Govan, N.; Reynolds, P.; Sambrook, M.R. Preparation of Aqueous Core/Silica Shell Microcapsules. J. Colloid Interface Sci. 2012, 366, 16–22. [Google Scholar] [CrossRef]
- Chen, T.; Colver, P.J.; Bon, S.A.F. Organic–Inorganic Hybrid Hollow Spheres Prepared from TiO2-Stabilized Pickering Emulsion Polymerization. Adv. Mater. 2007, 19, 2286–2289. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, X.; Tsarkova, L.; Pich, A.; Möller, M. All-Silica Colloidosomes with a Particle-Bilayer Shell. ACS Nano 2011, 5, 3937–3942. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Ishimura, K. In Situ Synthesis and Photoresponsive Rupture of Organosilica Nanocapsules. Chem. Commun. 2011, 47, 1518–1520. [Google Scholar] [CrossRef]
- Wibowo, D.; Zhao, C.X.; Middelberg, A.P.J. Emulsion-Templated Silica Nanocapsules Formed Using Bio-Inspired Silicification. Chem. Commun. 2014, 50, 11325–11328. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Nole, M.; Jang, S.; Ko, S.; Daigle, H.; Pope, G.A.; Huh, C. Highly porous CO2 hydrate generation aided by silica nanoparticles for potential secure storage of CO2 and desalination. RSC Adv. 2017, 7, 9545–9550. [Google Scholar] [CrossRef]
- Imhof, A.; Pine, D.J. Ordered macroporous materials by emulsion templating. Nature 1987, 389, 948–951. [Google Scholar] [CrossRef]
- Zoldesi, C.I.; Imhof, A. Synthesis of Monodisperse Colloidal Spheres, Capsules, and Microballoons by Emulsion Templating. Adv. Mater. 2005, 17, 924–928. [Google Scholar] [CrossRef]
- Thompson, K.L.; Williams, M.; Armes, S.P. Colloidosomes: Synthesis, Properties and Applications. J. Colloid Interface Sci. 2015, 447, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.U. CXCVI.—Emulsions. J. Chem. Soc. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Ramsden, W. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation).—Preliminary Account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar]
- Pieranski, P. Two-Dimensional Interfacial Colloidal Crystals. Phys. Rev. Lett. 1980, 45, 569–572. [Google Scholar] [CrossRef]
- Finkle, P.; Draper, H.D.; Hildebrand, J.H. The Theory of Emulsification. J. Am. Chem. Soc. 1923, 45, 2780–2788. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions Stabilised Solely by Colloidal Particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Binks, B.P.; Lumsdon, S.O. Catastrophic Phase Inversion of Water-in-Oil Emulsions Stabilized by Hydrophobic Silica. Langmuir 2000, 16, 2539–2547. [Google Scholar] [CrossRef]
- Briggs, N.M.; Westin, J.S.; Li, B.; Venkataramani, D.; Aichele, C.P.; Harwell, J.H.; Crossley, S.P. Multiwalled Carbon Nanotubes at the Interface of Pickering Emulsions. Langmuir 2015, 31, 13077–13084. [Google Scholar] [CrossRef] [PubMed]
- Hernant, M.C.; Klumperman, B.; Koning, C.E. Conductive Pickering-poly(high internal phase emulsion) composite foams prepared with low loadings of single-walled carbon nanotubes. Chem. Commun. 2009, 2738–2740. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawaguchi, K. Synthesis of composite polymer particles with carbon nanotubes and evaluation of their mechanical properties. Colloids Surf. A 2017, 529, 765–770. [Google Scholar] [CrossRef]
- Bornaee, A.H.; Manteghian, M.; Rashidi, A.; Alaei, M.; Ershadi, M. Oil-in-water Pickering emulsions stabilized with functionalized multi-walled carbon nanotube/silica nanohybrids in the presence of high concentrations of cations in water. J. Ind. Eng. Chem. 2014, 20, 1720–1726. [Google Scholar] [CrossRef]
- Briggs, N.; Raman, A.K.Y.; Barrett, L.; Brown, C.; Li, B.; Leavitt, D.; Aichele, C.P.; Crossley, S. Stable pickering emulsions using multi-walled carbon nanotubes of varying wettability. Colloids Surf. A 2018, 537, 227–235. [Google Scholar] [CrossRef]
- Binks, B.P. Macroporous Silica from Solid-Stabilized Emulsion Templates. Adv. Mater. 2002, 14, 1824–1827. [Google Scholar] [CrossRef]
- In Het Panhuis, M.; Paunov, V.N. Assembling Carbon Nanotubosomes Using an Emulsion-Inversion Technique. Chem. Commun. 2005, 1726–1728. [Google Scholar] [CrossRef]
- Paunov, V.N.; In Het Panhuis, M. Fabrication of Carbon Nanotube-Based Microcapsules by a Colloid Templating Technique. Nanotechnology 2005, 16, 1522. [Google Scholar] [CrossRef]
- Sano, M.; Kamino, A.; Okamura, J.; Shinkai, S. Noncovalent Self-Assembly of Carbon Nanotubes for Construction of “Cages”. Nano Lett. 2002, 2, 531–533. [Google Scholar] [CrossRef]
- Shi, J.; Chen, Z.; Qin, Y.; Guo, Z.X. Multiwalled Carbon Nanotube Microspheres from Layer-by-Layer Assembly and Calcination. J. Phys. Chem. C 2008, 112, 11617–11622. [Google Scholar] [CrossRef]
- Yi, H.; Song, H.; Chen, X. Carbon Nanotube Capsules Self-Assembled by W/O Emulsion Technique. Langmuir 2007, 23, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Van Wijk, J.; Heunis, T.; Harmzen, E.; Dicks, L.M.T.; Meuldijk, J.; Klumperman, B. Compartmentalization of Bacteria in Microcapsules. Chem. Commun. 2014, 50, 15427–15430. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Vincent, B. Aqueous Dispersions of Silica Shell/Water-Core Microcapsules. J. Colloid Interface Sci. 2010, 343, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hobbie, E.K. Amphiphobic Carbon Nanotubes as Macroemulsion Surfactants. Langmuir 2003, 19, 3091–3093. [Google Scholar] [CrossRef]
- Wang, R.K.; Reeves, R.D.; Ziegler, K.J. Interfacial Trapping of Single-Walled Carbon Nanotube Bundles. J. Am. Chem. Soc. 2007, 129, 15124–15125. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next Generation of Scientific Image Data. BMC Bioinform. 2017, 18, 529–555. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji—An Open Source Platform for Biological Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.A.; Chevalier, Y. Effects of solid particle content on properties of o/w Pickering emulsions. J. Colloid Interface Sci. 2010, 351, 348–356. [Google Scholar] [CrossRef]
- Witucki, G.L. A Silane Primer: Chemistry and Applications of AIkoxy Silanes. J. Coat. Technol. Res. 1993, 65, 57–60. [Google Scholar]
- Fiorilli, S.; Rivolo, P.; Descrovi, E.; Ricciardi, C.; Pasquardini, L.; Lunelli, L.; Vanzetti, L.; Pederzolli, C.; Onida, B.; Garrone, E. Vapor-Phase Self-Assembled Monolayers of Aminosilane on Plasma-Activated Silicon Substrates. J. Colloid Interface Sci. 2008, 321, 235–241. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Optimization of Silica Silanization by 3-Aminopropyltriethoxysilane. Langmuir 2006, 22, 11142–11147. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of Chemical Oxidation on the Structure of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Nakayama-Ratchford, N.; Bangsaruntip, S.; Sun, X.; Welsher, K.; Dai, H. Noncovalent Functionalization of Carbon Nanotubes by Fluorescein–Polyethylene Glycol: Supramolecular Conjugates with pH-Dependent Absorbance and Fluorescence. J. Am. Chem. Soc. 2007, 129, 2448–2449. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Solvas, E.; Gómez-García, D.; Domínguez-Rodríguez, A. Towards Physical Properties Tailoring of Carbon Nanotubes-Reinforced Ceramic Matrix Composites. J. Eur. Ceram. Soc. 2012, 32, 3001–3020. [Google Scholar] [CrossRef]
- Pulko, I.; Krajnc, P. High Internal Phase Emulsion Templating—A Path To Hierarchically Porous Functional Polymers. Macromol. Rapid Commun. 2012, 33, 1731–1746. [Google Scholar] [CrossRef]







| wt% SiO2 | vol% Toluene | ||||
|---|---|---|---|---|---|
| 1 mg MWNT | 0.5 | 10 | 20 | 30 | 50 |
| 1 | 10 | 20 | 30 | 50 | |
| 2 | 10 | 20 | 30 | 50 | |
| 5 | 10 | 20 | 30 | 50 | |
| 2 mg MWNT | 0.5 | 10 | 20 | 30 | 50 |
| 1 | 10 | 20 | 30 | 50 | |
| 2 | 10 | 20 | 30 | 50 | |
| 5 | 10 | 20 | 30 | 50 | |
| 5 mg MWNT | 0.5 | 10 | 20 | 30 | 50 |
| 1 | 10 | 20 | 30 | 50 | |
| 2 | 10 | 20 | 30 | 50 | |
| 5 | 10 | 20 | 30 | 50 | |
| Emulsion No. | Particle 1 | Particle 2 | Silane 1 | Silane 2 |
|---|---|---|---|---|
| C1 | SiO2 | MWNT | APTES | DTES |
| C2 | SiO2 | MWNT | APTES | - |
| C3 | SiO2 | MWNT | - | DTES |
| C4 | SiO2 | MWNT | - | - |
| C5 | SiO2 | MWNT-COOH | APTES | DTES |
| C6 | SiO2 | MWNT-COOH | APTES | - |
| C7 | SiO2 | MWNT-COOH | - | DTES |
| C8 | SiO2 | MWNT-COOH | - | - |
| C9 | SiO2 | - | APTES | DTES |
| C10 | SiO2 | - | APTES | - |
| C11 | SiO2 | - | - | DTES |
| C12 | SiO2 | - | - | - |
| C13 | - | MWNT | APTES | DTES |
| C14 | - | MWNT | APTES | - |
| C15 | - | MWNT | - | DTES |
| C16 | - | MWNT | - | - |
| C17 | - | MWNT-COOH | APTES | DTES |
| C18 | - | MWNT-COOH | APTES | - |
| C19 | - | MWNT-COOH | - | DTES |
| C20 | - | MWNT-COOH | - | - |
| C21 | - | - | APTES | DTES |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzegorzewski, F.; Benhaim, A.; Itzhaik Alkotzer, Y.; Zelinger, E.; Yaakov, N.; Mechrez, G. In situ Fabrication of Multi-Walled Carbon Nanotubes/Silica Hybrid Colloidosomes by Pickering Emulsion Templating Using Trialkoxysilanes of Opposite Polarity. Polymers 2019, 11, 1480. https://doi.org/10.3390/polym11091480
Grzegorzewski F, Benhaim A, Itzhaik Alkotzer Y, Zelinger E, Yaakov N, Mechrez G. In situ Fabrication of Multi-Walled Carbon Nanotubes/Silica Hybrid Colloidosomes by Pickering Emulsion Templating Using Trialkoxysilanes of Opposite Polarity. Polymers. 2019; 11(9):1480. https://doi.org/10.3390/polym11091480
Chicago/Turabian StyleGrzegorzewski, Franziska, Avital Benhaim, Yafit Itzhaik Alkotzer, Einat Zelinger, Noga Yaakov, and Guy Mechrez. 2019. "In situ Fabrication of Multi-Walled Carbon Nanotubes/Silica Hybrid Colloidosomes by Pickering Emulsion Templating Using Trialkoxysilanes of Opposite Polarity" Polymers 11, no. 9: 1480. https://doi.org/10.3390/polym11091480
APA StyleGrzegorzewski, F., Benhaim, A., Itzhaik Alkotzer, Y., Zelinger, E., Yaakov, N., & Mechrez, G. (2019). In situ Fabrication of Multi-Walled Carbon Nanotubes/Silica Hybrid Colloidosomes by Pickering Emulsion Templating Using Trialkoxysilanes of Opposite Polarity. Polymers, 11(9), 1480. https://doi.org/10.3390/polym11091480





