Chemical Structure of EVA Films Obtained by Pulsed Electron Beam and Pulse Laser Ablation
Abstract
1. Introduction
2. Materials and Methods
2.1. Film Preparation
2.2. Morphology Study
2.3. Structure Determination
3. Results and Discussion
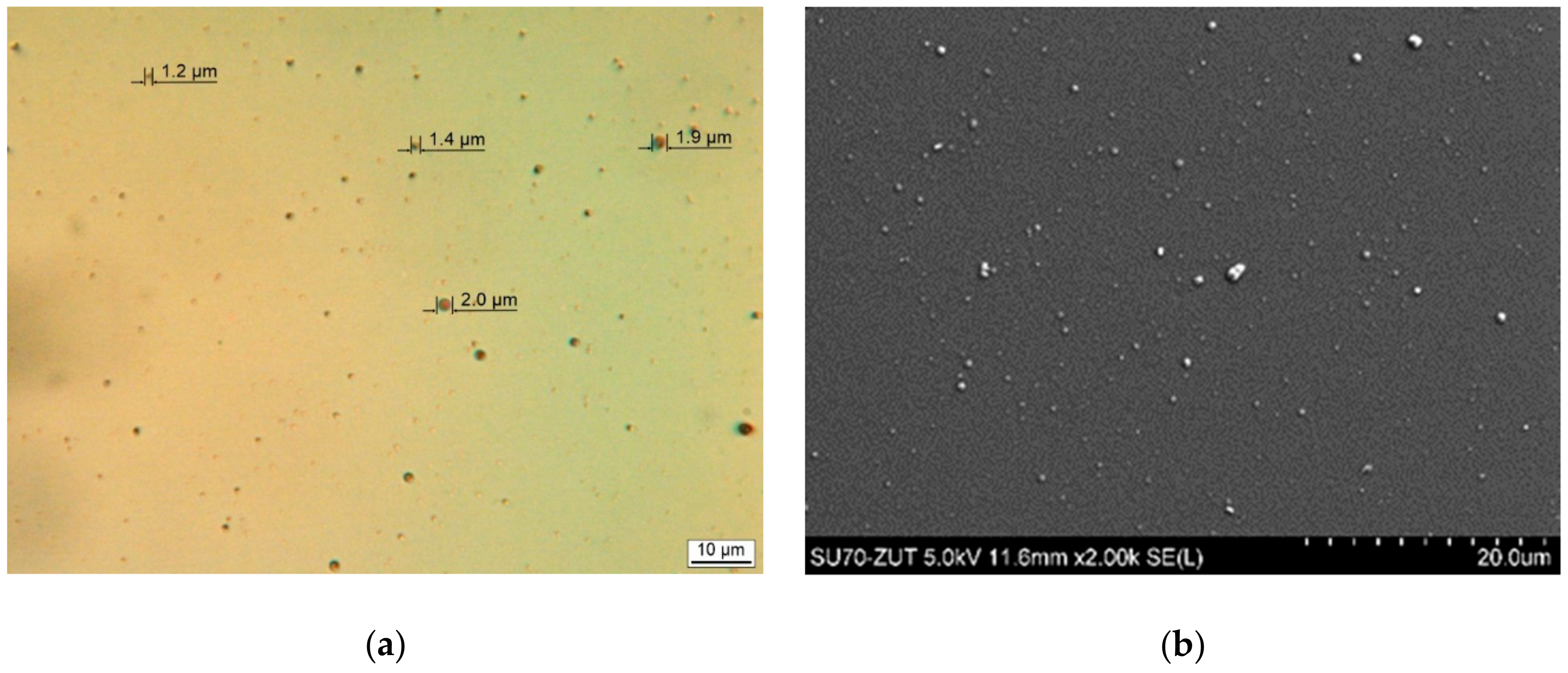
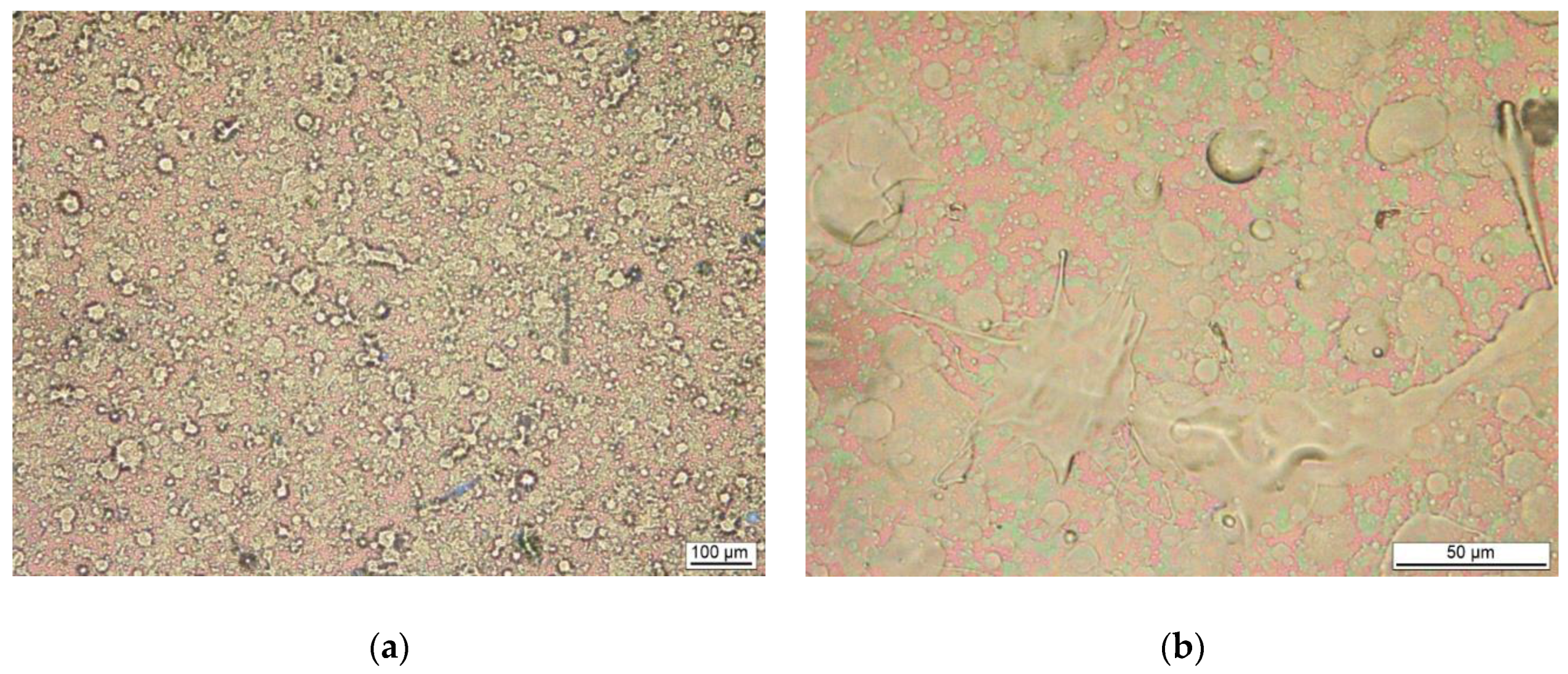
3.1. Film Morphology
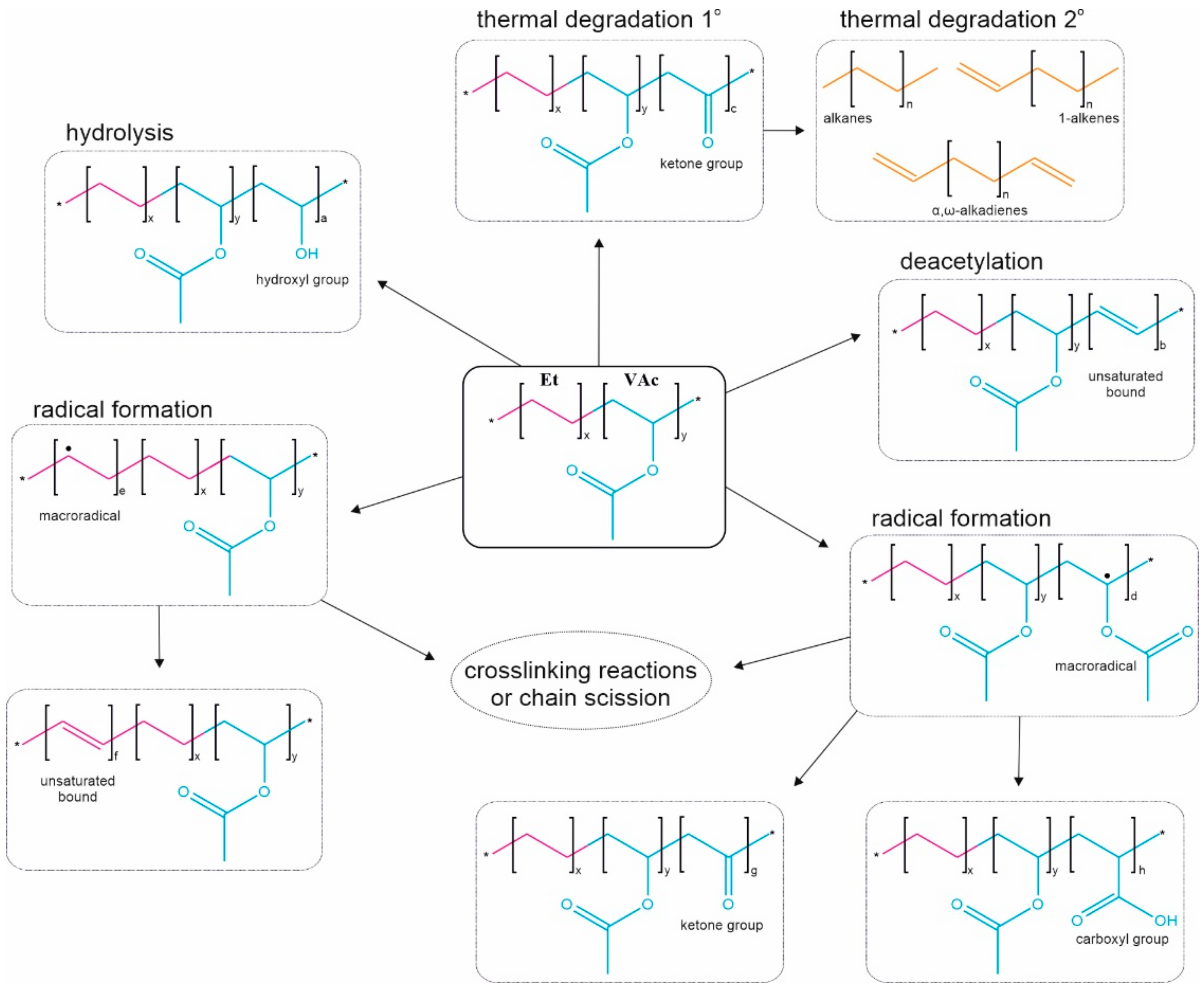
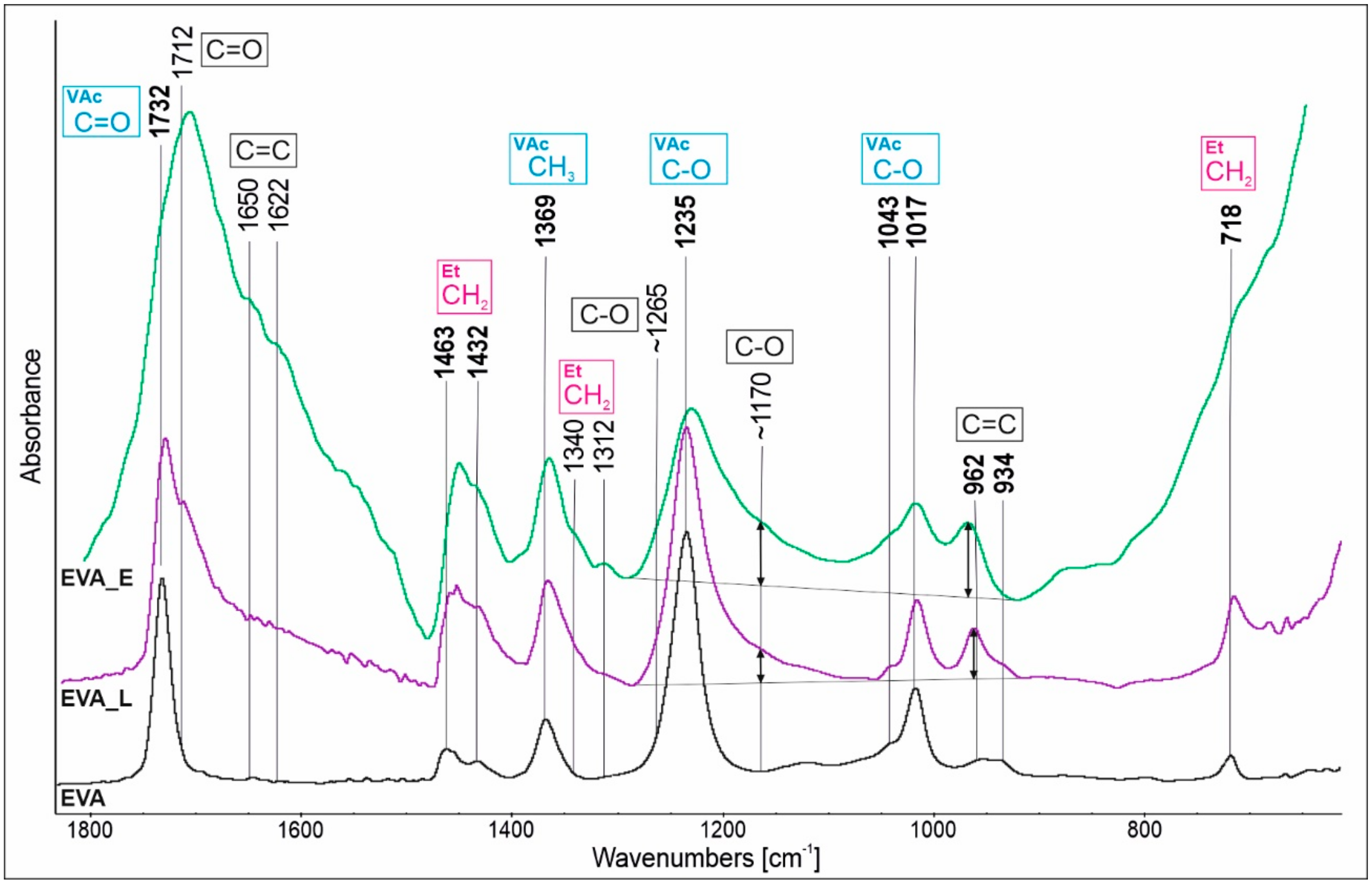
3.2. Chemical Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Jeong, H.; Chowdhury, M.; Arnold, C.B.; Priestley, R.D. Exploiting physical vapor deposition for morphological control in semi-crystalline polymer films. Polym. Cryst. 2018, 1, e10021. [Google Scholar] [CrossRef]
- Gritsenko, K.P.; Krasovsky, A.M. Thin-film deposition of polymers by vacuum degradation. Chem. Rev. 2003, 103, 3607–3650. [Google Scholar] [CrossRef] [PubMed]
- Gritsenko, K.P. Vacuum evaporation-deposited polyterafluoroethylene films: Growth mechanism, properties, and applications. Russ. J. Gen. Chem. 2009, 79, 642–656. [Google Scholar] [CrossRef]
- Blanchet, G.B.; Fincher, C.R.; Jackson, C.L.; Shah, S.I.; Gardner, K.H. Laser ablation and the production of polymer films. Science 1993, 262, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Li, S.T.; Arenholz, E.; Heitz, J.; Bäuerle, D. Pulsed-laser deposition of crystalline Teflon (PTFE) films. Appl. Surf. Sci. 1998, 125, 17–22. [Google Scholar] [CrossRef]
- Chandra, V.; Manoharan, S.S. Pulsed electron beam deposition of highly oriented thin films of polytetrafluoroethylene. Appl. Surf. Sci. 2008, 254, 4063–4066. [Google Scholar] [CrossRef]
- Henda, R.; Wilson, G.; Gray-Munro, J.; Alshekhli, O.; McDonald, A.M. Preparation of polytetrafluoroethylene by pulsed electron ablation: Deposition and wettability aspects. Thin Solid Films 2012, 520, 1885–1889. [Google Scholar] [CrossRef]
- Jȩdrzejewski, R.; Piwowarczyk, J.; Kwiatkowski, K.; Baranowska, J. Polytetrafluoroethylene thin films obtained by the pulsed electron beam deposition method at different gas pressures. Polimery 2017, 62, 743–749. [Google Scholar] [CrossRef]
- Kusiński, J.; Kopia, A.; Kac, S.; Radziszewska, A. Deposition of oxide and intermetallic thin films by pulsed laser (PLD) and electron beam (PED) methods. Arch. Metall. Mater. 2015, 60, 2173–2182. [Google Scholar] [CrossRef][Green Version]
- Jędrzejewski, R.; Piwowarczyk, J.; Jędrzejewska, A.; Kwiatkowski, K.; Baranowska, J. Poly(propylene)/carbon composite thin films obtained by pulsed electron-beam deposition. Plasma Process. Polym. 2018, 15, 1700239. [Google Scholar] [CrossRef]
- Bidsorkhi, H.C.; Soheilmoghaddam, M.; Pour, R.H.; Adelnia, H.; Mohamad, Z. Mechanical, thermal and flammability properties of ethylene-vinyl acetate (EVA)/sepiolite nanocomposites. Polym. Test. 2014, 37, 117–122. [Google Scholar] [CrossRef]
- Drabczyk, K.; Panek, P. A comparative study of EVA with and without thermal history for different lamination process parameters. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2012, 177, 1378–1383. [Google Scholar] [CrossRef]
- Ucar, I.O.; Doganci, M.D.; Cansoy, C.E.; Erbil, H.Y.; Avramova, I.; Suzer, S. Combined XPS and contact angle studies of ethylene vinyl acetate and polyvinyl acetate blends. Appl. Surf. Sci. 2011, 257, 9587–9594. [Google Scholar] [CrossRef]
- Biggs, K.B.; Balss, K.M.; Maryanoff, C.A. Pore networks and polymer rearrangement on a drug-eluting stent as revealed by correlated confocal Raman and atomic force microscopy. Langmuir 2012, 28, 8238–8243. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Kim, D.; Lee, K.D.; Seo, J.; Lee, H.J. The effect of coating process and additives on EVA coated Tyvek® for gas sterilizable medical packaging applications. Packag. Technol. Sci. 2017, 30, 195–208. [Google Scholar] [CrossRef]
- Tambe, S.P.; Singh, S.K.; Patri, M.; Kumar, D. Studies on anticorrosive properties of thermally sprayable EVA and EVAl coating. Prog. Org. Coatings 2010, 67, 239–245. [Google Scholar] [CrossRef]
- Tomić, N.Z.; Veljović, Đ.; Trifković, K.; Međo, B.; Rakin, M.; Radojević, V.; Jančić-Heinemann, R. Numerical and experimental approach to testing the adhesive properties of modified polymer blend based on EVA/PMMA as coatings for optical fibers. Int. J. Adhes. Adhes. 2017, 73, 80–91. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Rodriguez, M.; Liauw, C.M.; Fontan, E. Aspects of the thermal oxidation, yellowing and stabilization of ethylene vinyl acetate copolymer. Polym. Degrad. Stab. 2000, 71, 1–14. [Google Scholar] [CrossRef]
- Sharif, J.; Dahlan, K.Z.M.; Yunus, W.M.Z.W. Electron beam crosslinking of poly(ethylene-co-vinyl acetate)/clay nanocomposites. Radiat. Phys. Chem. 2007, 76, 1698–1702. [Google Scholar] [CrossRef]
- Datta, S.K.; Bhowmick, A.K.; Chaki, T.K.; Majali, A.B.; Despande, R.S. Electron beam initiated modification of ethylene vinyl acetate using trimethylolpropane trimethacrylate. Polymer 1996, 37, 45–55. [Google Scholar] [CrossRef]
- Gaston, F.; Dupuy, N.; Marque, S.R.A.; Barbaroux, M.; Dorey, S. FTIR study of ageing of γ-irradiated biopharmaceutical EVA based film. Polym. Degrad. Stab. 2016, 129, 19–25. [Google Scholar] [CrossRef]
- Dutta, S.K.; Bhowmick, A.K.; Mukunda, P.G.; Chaki, T.K. Thermal degradation studies of electron beam cured ethylene-vinyl acetate copolymer. Polym. Degrad. Stab. 1995, 50, 75–82. [Google Scholar] [CrossRef]
- Choi, S.S.; Kim, E. Analysis of pyrolysis products of ethylene-vinyl acetate coploymer (EVA) using pre-deacetylation. J. Anal. Appl. Pyrolysis 2017, 127, 1–7. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Zhang, J. UV aging behaviour of ethylene-vinyl acetate copolymers (EVA) with different vinyl acetate contents. Polym. Degrad. Stab. 2010, 95, 725–732. [Google Scholar] [CrossRef]
- Şen, M.; Çopuroǧlu, M. A comparative study of gamma irradiation of poly(ethylene-co-vinyl acetate) and poly(ethylene-co-vinyl acetate)/carbon black mixture. Mater. Chem. Phys. 2005, 93, 154–158. [Google Scholar] [CrossRef]
- Soto Puente, J.A.; Fatyeyeva, K.; Marais, S.; Dargent, E. Multifunctional hydrolyzed EVA membranes with tunable microstructure and water barrier properties. J. Memb. Sci. 2015, 480, 93–103. [Google Scholar] [CrossRef]
- Klemchuk, P.; Ezrin, M.; Lavigne, G.; Holley, W.; Galica, J.; Agro, S. Investigation of the degradation and stabilization of EVA-based encapsulant in field-aged solar energy modules. Polym. Degrad. Stab. 1997, 55, 347–365. [Google Scholar] [CrossRef]
- Datta, S.K.; Chaki, T.K.; Bhowmick, A.K. Electron beam initiated grafting and crosslinking of ethylene vinyl acetate copolymer. Part-I: Structural characterization. Rubber Chem. Technol. 2011, 69, 120–129. [Google Scholar] [CrossRef]
- Briggs, D.; Grant, J.T. Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; IM Publications: Chichester, UK, 2003; ISBN 1901019047. [Google Scholar]



| Sample | Elements | C 1s Components with BE [eV] | |||||
|---|---|---|---|---|---|---|---|
| C Total | O Total | C–C 285.0 eV | C*–CO 285.5 eV | C–OH C–O–C 286.3 eV | C=O 287.4 eV | O–C=O 289.2 eV | |
| at. % | C Total = 100 | ||||||
| EVA | 89 | 11 | 59 | 22 | 11 | 3 | 5 |
| EVA_E | 82 | 18 | 53 | 21 | 9 | 12 | 5 |
| EVA_L | 82 | 18 | 49 | 22 | 12 | 9 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemczyk, A.; Moszyński, D.; Jędrzejewski, R.; Kwiatkowski, K.; Piwowarczyk, J.; Baranowska, J. Chemical Structure of EVA Films Obtained by Pulsed Electron Beam and Pulse Laser Ablation. Polymers 2019, 11, 1419. https://doi.org/10.3390/polym11091419
Niemczyk A, Moszyński D, Jędrzejewski R, Kwiatkowski K, Piwowarczyk J, Baranowska J. Chemical Structure of EVA Films Obtained by Pulsed Electron Beam and Pulse Laser Ablation. Polymers. 2019; 11(9):1419. https://doi.org/10.3390/polym11091419
Chicago/Turabian StyleNiemczyk, Agata, Dariusz Moszyński, Roman Jędrzejewski, Konrad Kwiatkowski, Joanna Piwowarczyk, and Jolanta Baranowska. 2019. "Chemical Structure of EVA Films Obtained by Pulsed Electron Beam and Pulse Laser Ablation" Polymers 11, no. 9: 1419. https://doi.org/10.3390/polym11091419
APA StyleNiemczyk, A., Moszyński, D., Jędrzejewski, R., Kwiatkowski, K., Piwowarczyk, J., & Baranowska, J. (2019). Chemical Structure of EVA Films Obtained by Pulsed Electron Beam and Pulse Laser Ablation. Polymers, 11(9), 1419. https://doi.org/10.3390/polym11091419






