Functionalized Poly(arylene ether nitrile) Porous Membrane with High Pb(II) Adsorption Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SPEN and Porous Membrane Preparation
2.3. Adsorption Behaviour for Pb(II)
2.4. Characterization
3. Results and Discussion
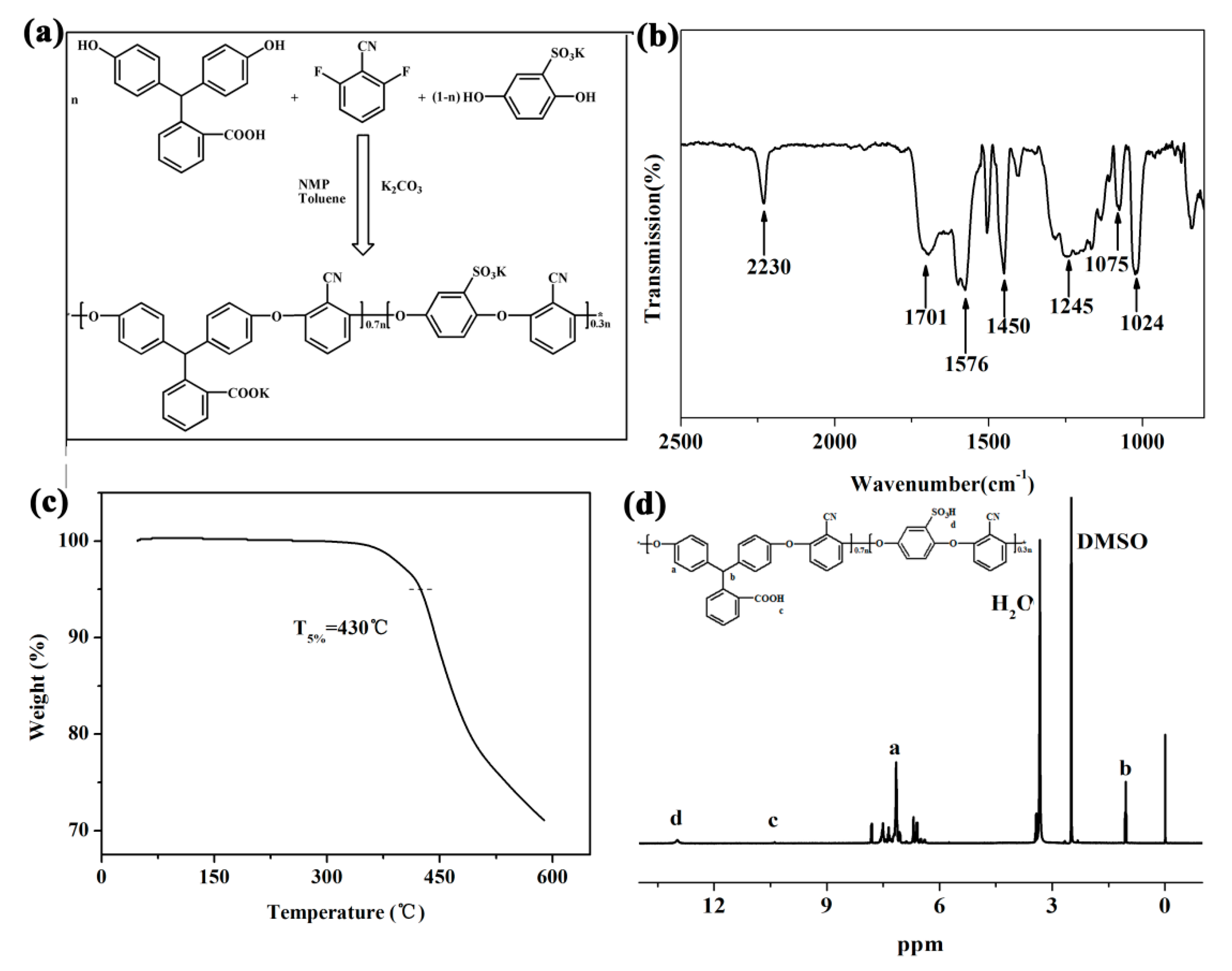
3.1. Characterization of SPEN
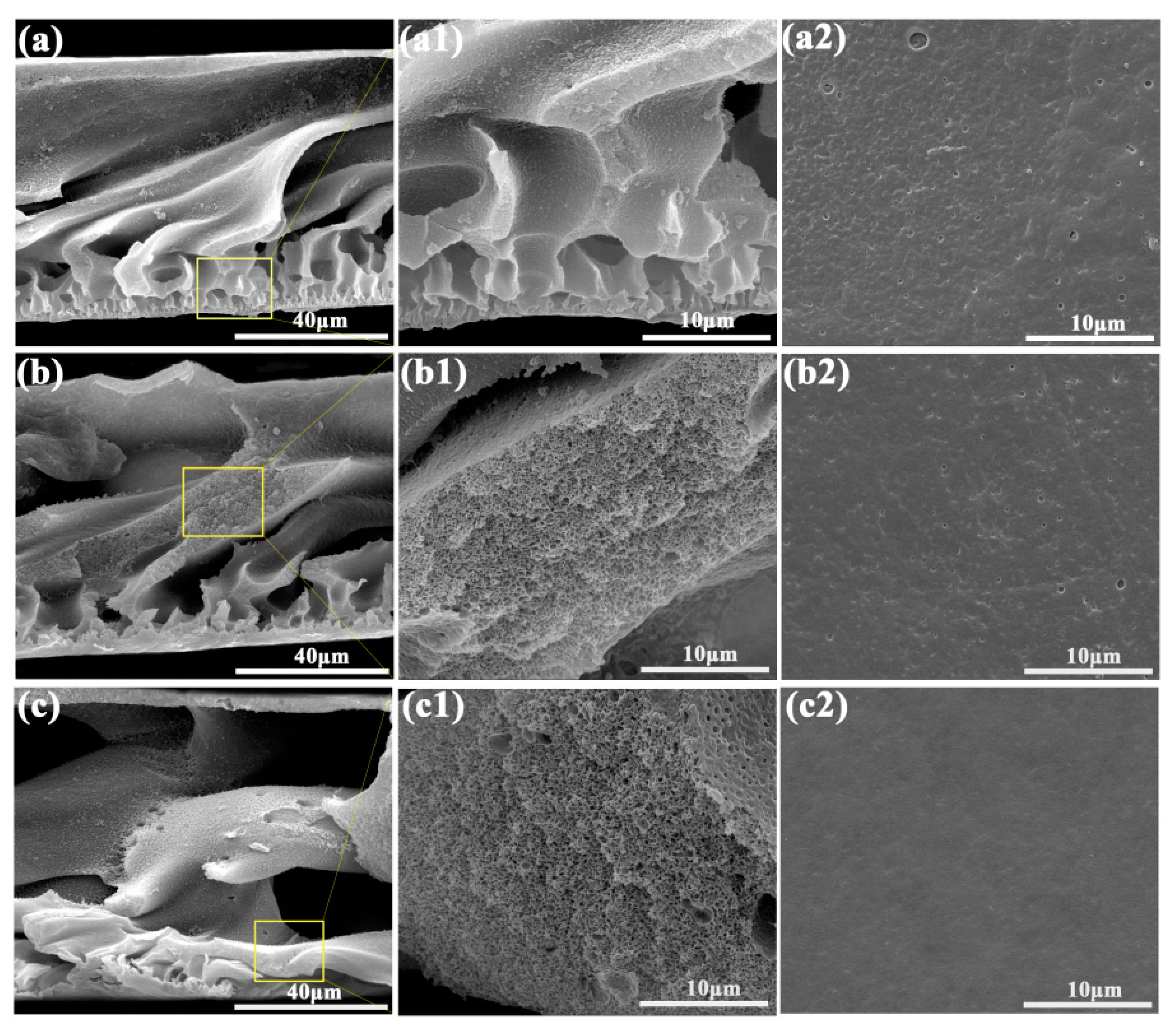
3.2. Structure Characterization of Porous Membrane
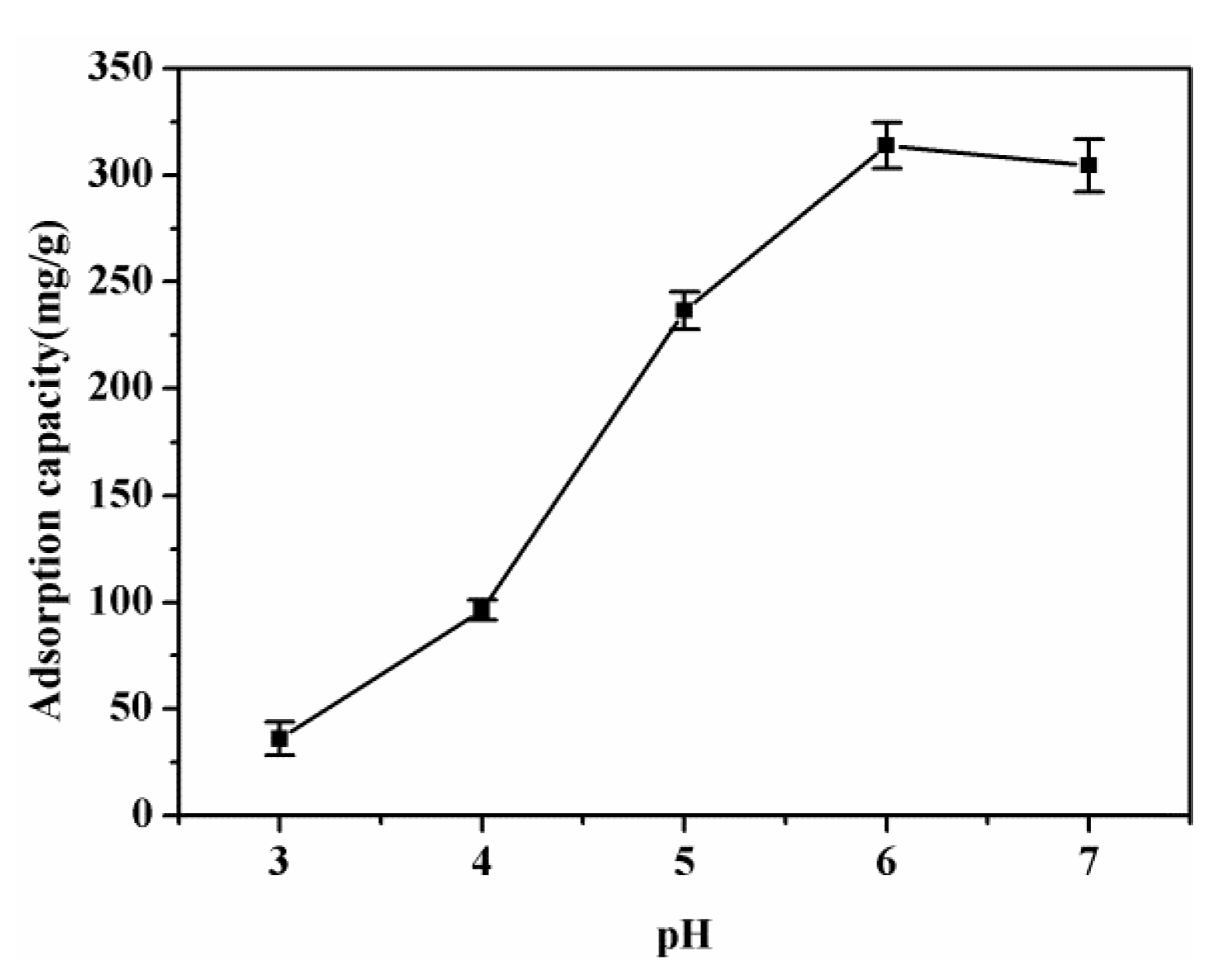
3.3. Effect of Solution pH
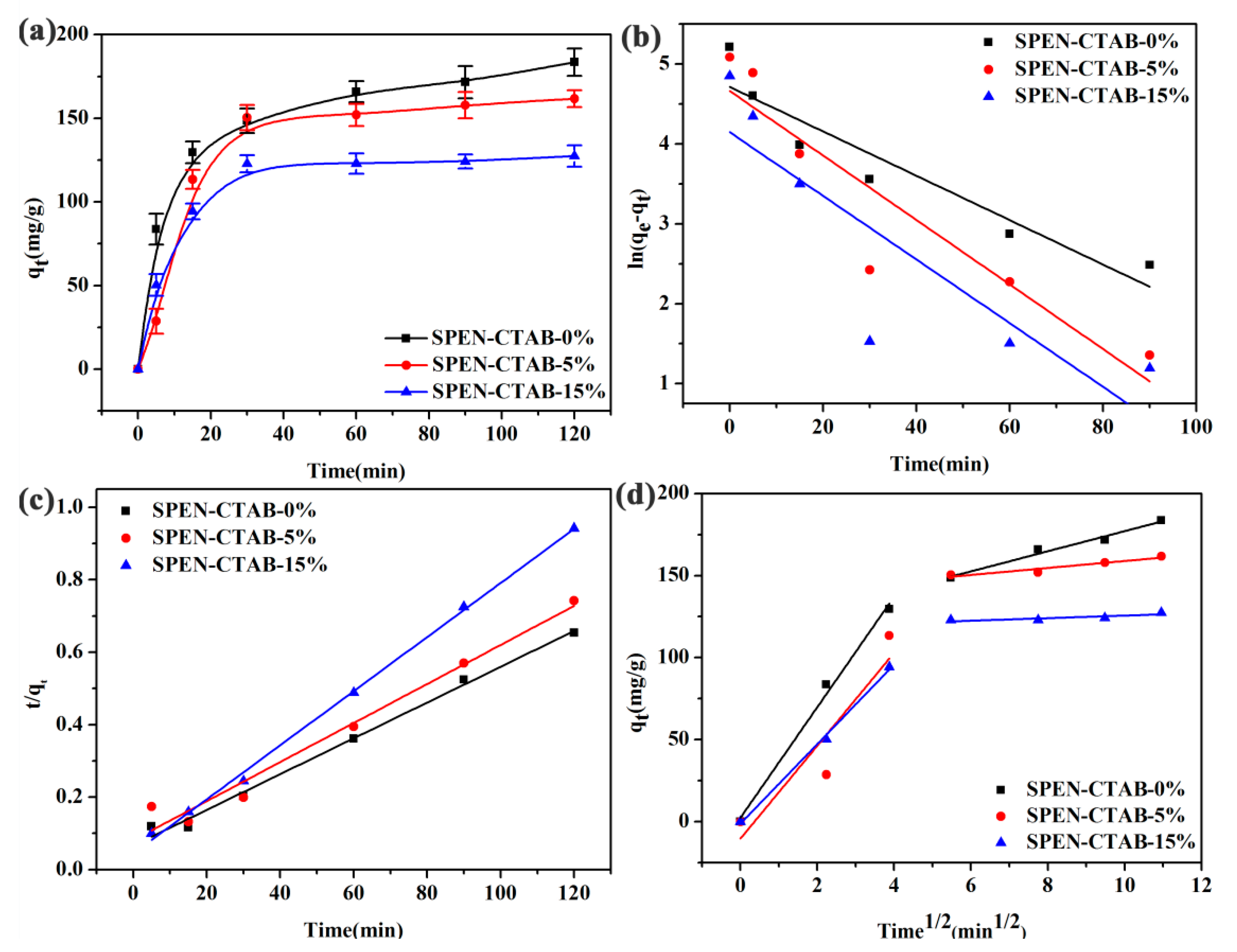
3.4. Effect of CTAB Content in a Porous Membrane
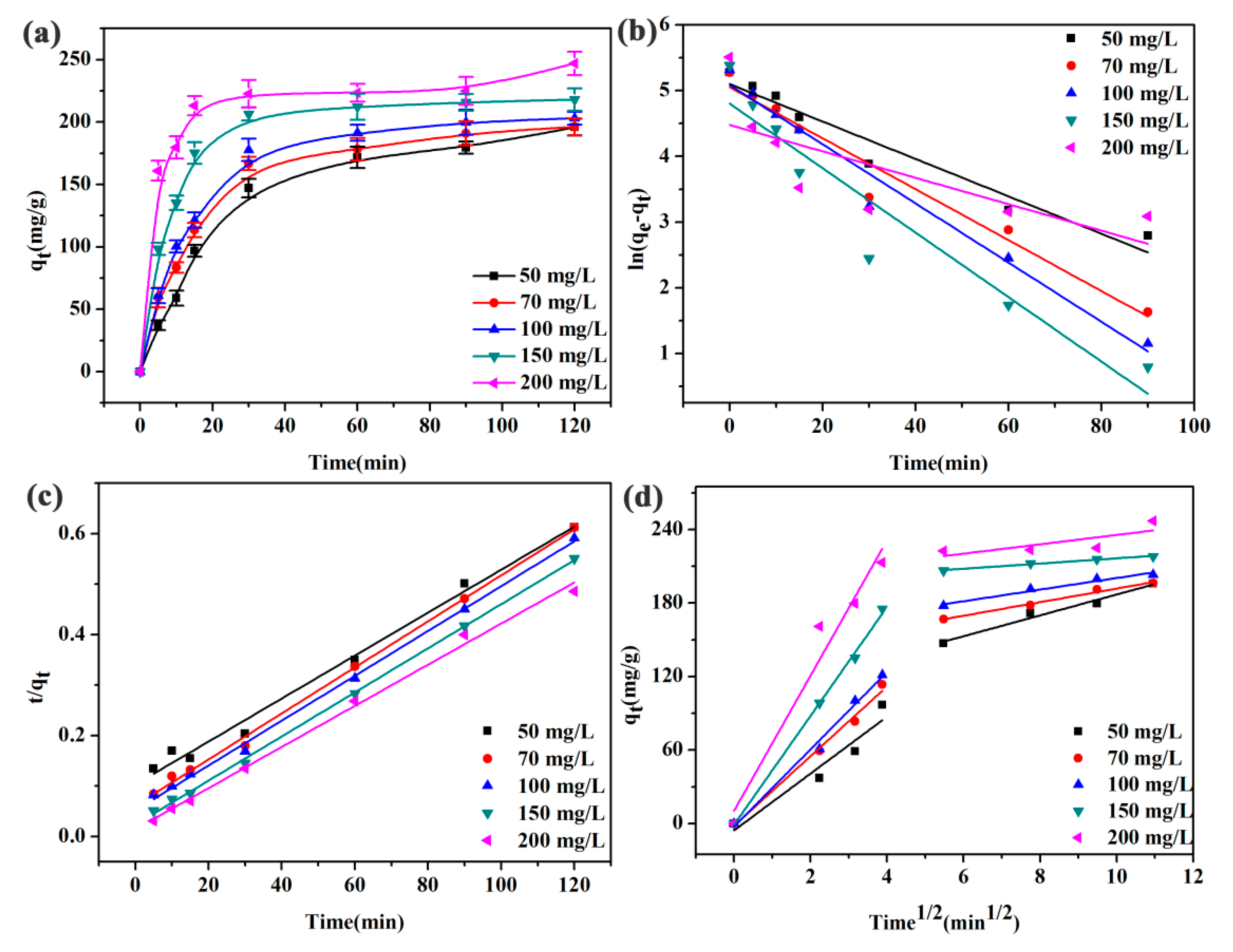
3.5. Effect of Initial Concentrations
3.6. Adsorption Isotherm
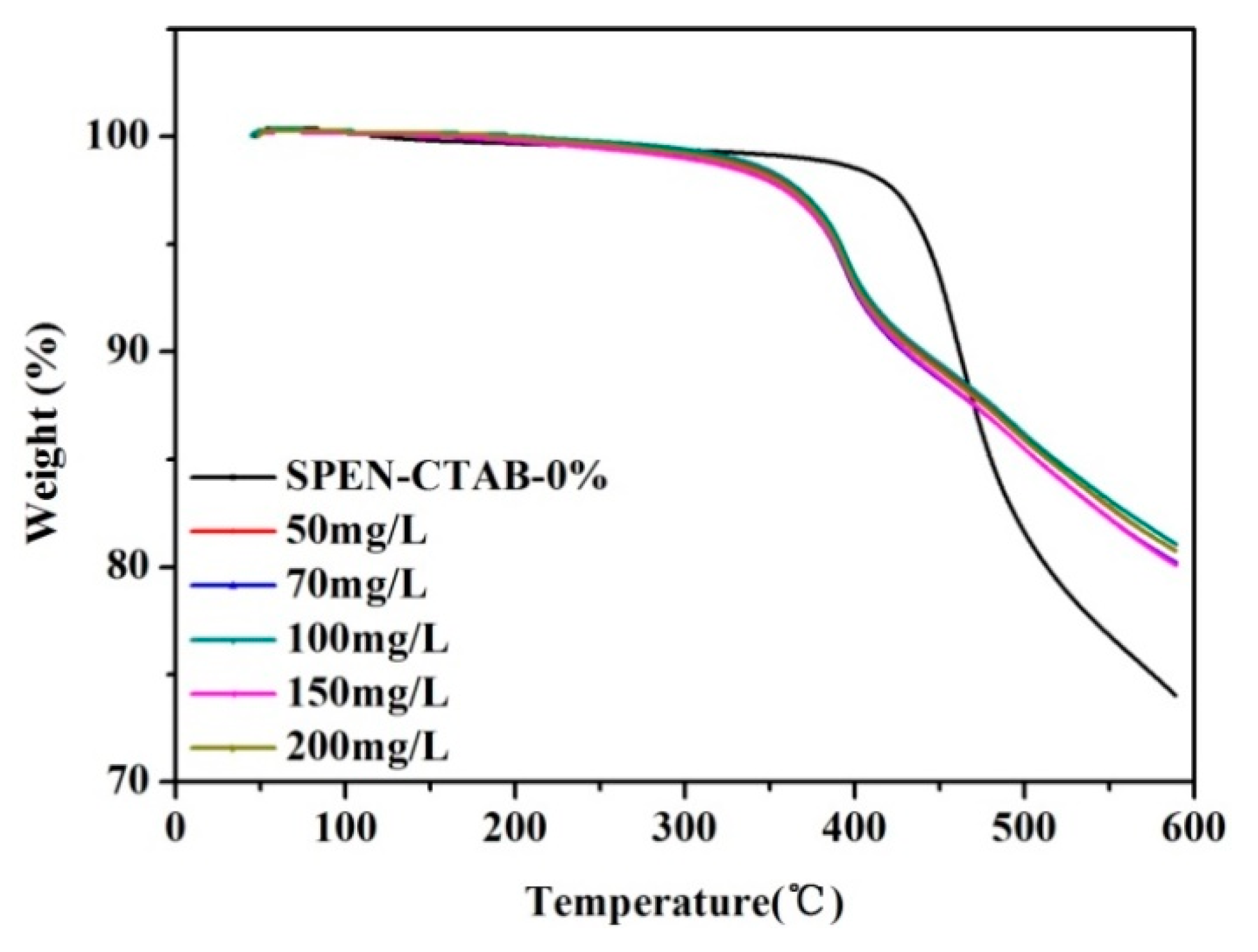
3.7. Thermal Stability Changes before and after Adsorption
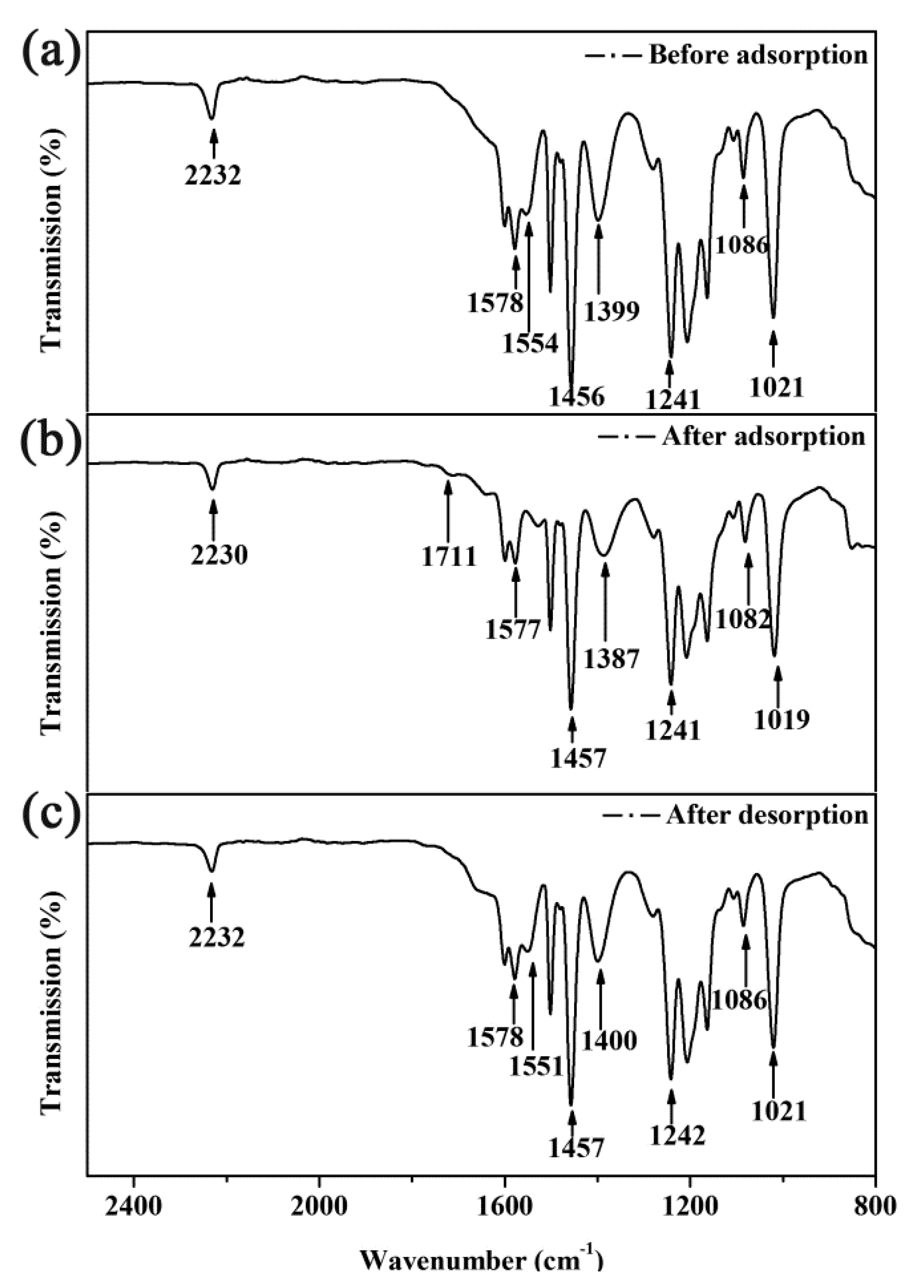
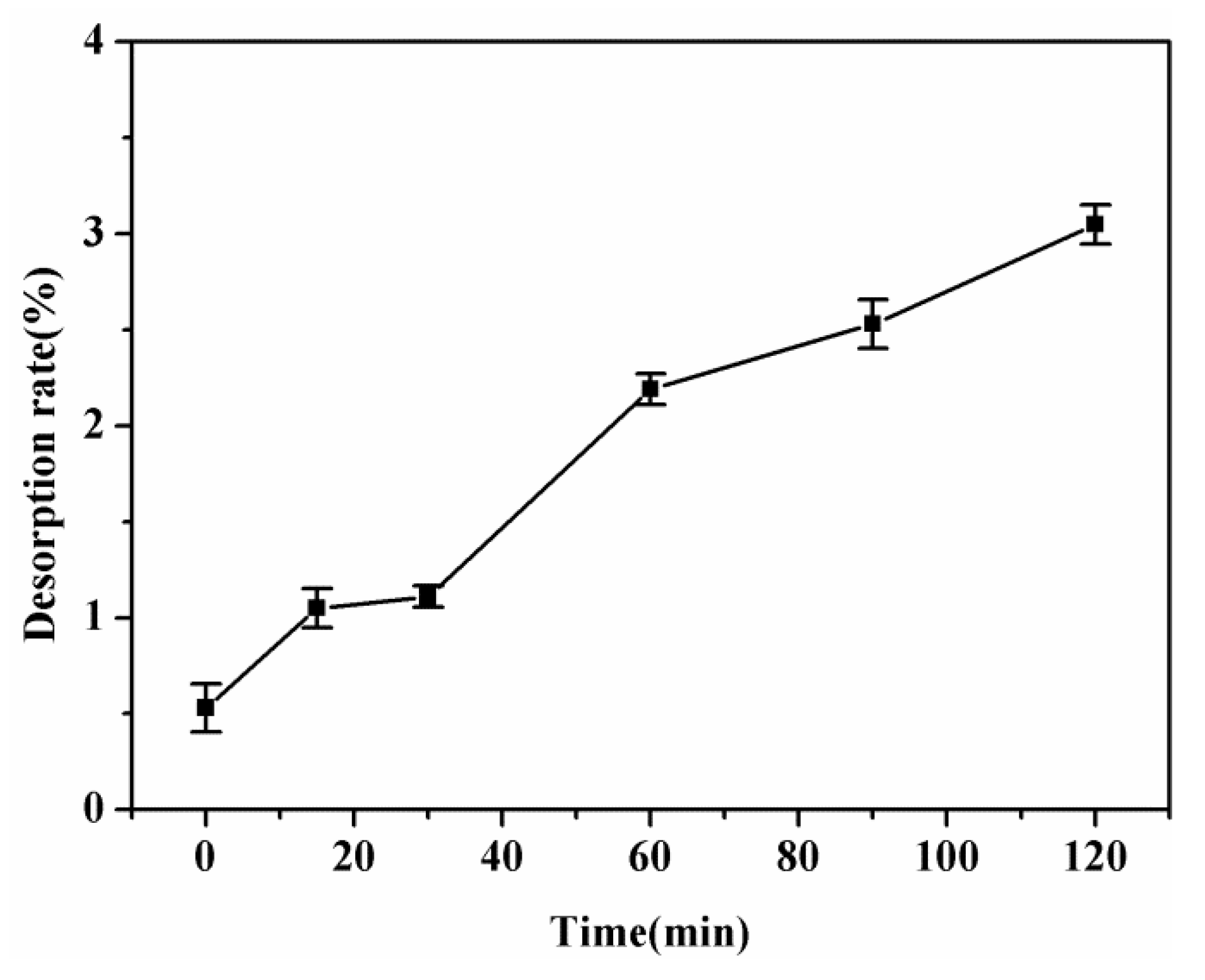
3.8. Adsorption Mechanism
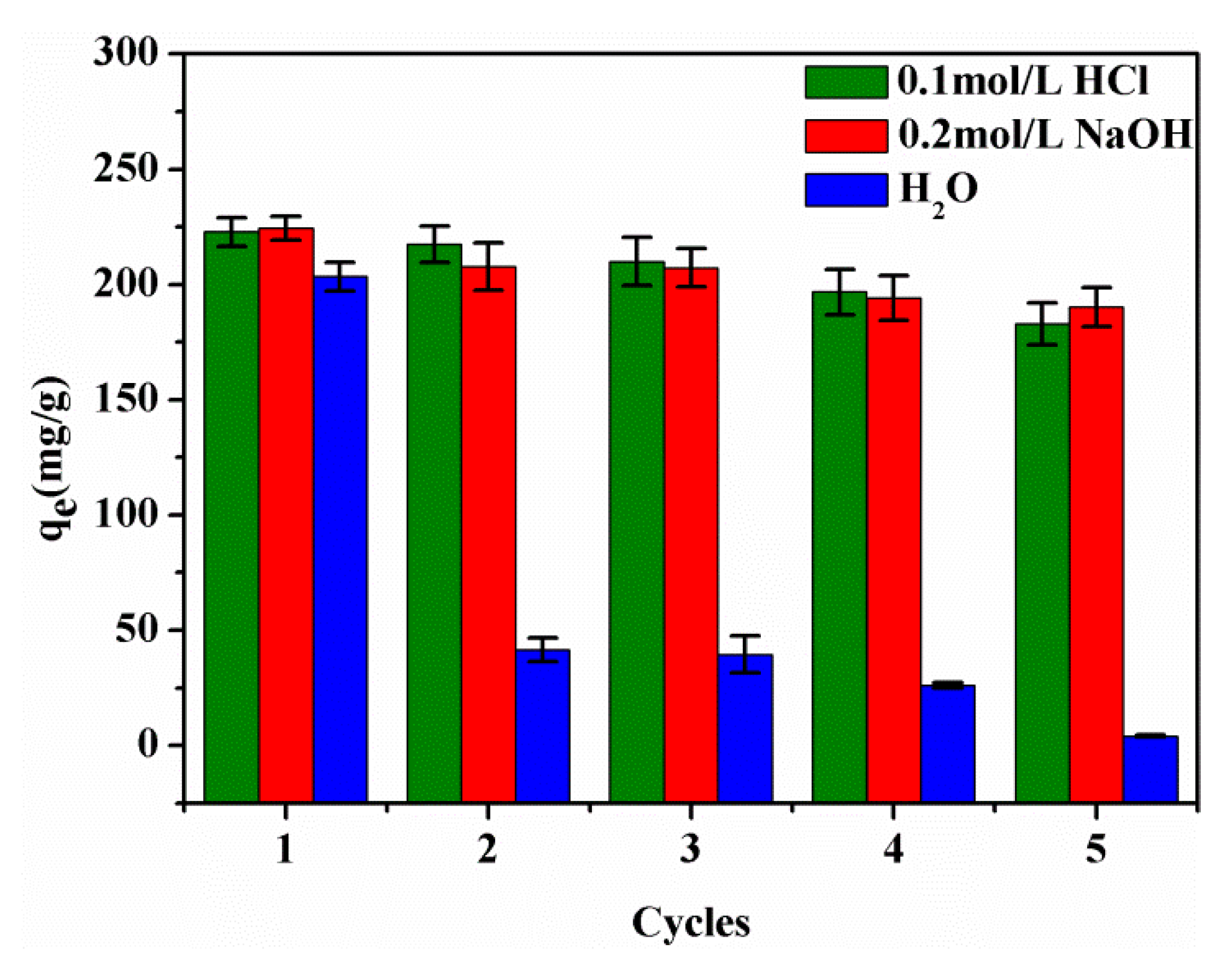
3.9. Recycling of SPEN Porous Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ji, J.; Chen, G.; Zhao, J. Preparation and characterization of amino/thiol bifunctionalized magnetic nanoadsorbent and its application in rapid removal of Pb (II) from aqueous system. J. Hazard. Mater. 2019, 368, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Huang, X.; Wang, J.; Wang, T.; Wu, H.; Yang, J.; Lu, H.; Hao, H. Oil-phase cyclic magnetic adsorption to synthesize Fe3O4@C@TiO2-nanotube composites for simultaneous removal of Pb(II) and Rhodamine B. Chem. Eng. J. 2019, 366, 50–61. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Meknati, A.; Lima, E.C.; Dotto, G.L.; Mendoza-Castillo, D.I.; Anastopoulos, I.; Alakhras, F.; Unuabonah, E.I.; Singh, P.; Hosseini-Bandegharaei, A. A novel route for preparation of chemically activated carbon from pistachio wood for highly efficient Pb(II) sorption. J. Environ. Manag. 2019, 236, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Xu, J.; Yu, C.; Cheng, K.; Wu, Y.; Wang, Y.; Li, F. Graphene oxide wrapped melamine sponge as an efficient and recoverable adsorbent for Pb(II) removal from fly ash leachate. J. Hazard. Mater. 2019, 367, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Janyasuthiwong, S.; Rene, E.R.; Esposito, G.; Lens, P.N.L. Effect of pH on Cu, Ni and Zn removal by biogenic sulfide precipitation in an inversed fluidized bed bioreactor. Hydrometallurgy 2015, 158, 94–100. [Google Scholar] [CrossRef]
- Cronje, K.J.; Chetty, K.; Carsky, M.; Sahu, J.N.; Meikap, B.C. Optimization of chromium(VI) sorption potential using developed activated carbon from sugarcane bagasse with chemical activation by zinc chloride. Desalination 2011, 275, 276–284. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy metal ions removal from metal plating wastewater using electrocoagulation: Kinetic study and process performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Doggaz, A.; Attour, A.; Le Page Mostefa, M.; Tlili, M.; Lapicque, F. Iron removal from waters by electrocoagulation: Investigations of the various physicochemical phenomena involved. Sep. Purif. Technol. 2018, 203, 217–225. [Google Scholar] [CrossRef]
- Devlin, T.R.; Kowalski, M.S.; Pagaduan, E.; Zhang, X.; Wei, V.; Oleszkiewicz, J.A. Electrocoagulation of wastewater using aluminum, iron, and magnesium electrodes. J. Hazard. Mater. 2019, 368, 862–868. [Google Scholar] [CrossRef]
- Wu, H.; Wu, Q.; Zhang, J.; Gu, Q.; Wei, L.; Guo, W.; He, M. Chromium ion removal from raw water by magnetic iron composites and Shewanella oneidensis MR-1. Sci. Rep. 2019, 9, 3687. [Google Scholar] [CrossRef]
- Basu, M.; Guha, A.K.; Ray, L. Adsorption of lead on lentil husk in fixed bed column bioreactor. Bioresour. Technol. 2019, 283, 86–95. [Google Scholar] [CrossRef]
- Dong, Q.; Guo, X.; Huang, X.; Liu, L.; Tallon, R.; Taylor, B.; Chen, J. Selective removal of lead ions through capacitive deionization: Role of ion-exchange membrane. Chem. Eng. J. 2019, 361, 1535–1542. [Google Scholar] [CrossRef]
- Zhang, Q.; Du, Q.; Hua, M.; Jiao, T.; Gao, F.; Pan, B. Sorption enhancement of lead ions from water by surface charged polystyrene-supported nano-zirconium oxide composites. Environ. Sci. Technol. 2013, 47, 6536–6544. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Adsorptive removal of heavy metals from water using sodium titanate nanofibres loaded onto GAC in fixed-bed columns. J. Hazard. Mater. 2015, 287, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, K.; Ma, Z.; Lu, X.; Wang, L.; Wang, Z.; Gao, X. Preparation and characterization of novel polyvinylidene fluoride/2-aminobenzothiazole modified ultrafiltration membrane for the removal of Cr(VI) in wastewater. Polymers (Basel) 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, Y.; Fan, Y.; Zeng, G.; Zhang, L. Nature-inspired polyphenol chemistry to fabricate halloysite nanotubes decorated PVDF membrane for the removal of wastewater. Sep. Purif. Technol. 2019, 212, 326–336. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Peng, Y.; Min, X.; Yin, D.; Shao, L. Mgal-layered-double-hydroxide/sepiolite composite membrane for high-performance water treatment based on layer-by-layer hierarchical architectures. Polymers (Basel) 2019, 11, 525. [Google Scholar] [CrossRef]
- Chen, S.; Lv, C.; Hao, K.; Jin, L.; Xie, Y.; Zhao, W.; Sun, S.; Zhang, X.; Zhao, C. Multifunctional negatively-charged poly (ether sulfone) nanofibrous membrane for water remediation. J. Colloid. Interface Sci. 2019, 538, 648–659. [Google Scholar] [CrossRef]
- Min, M.; Shen, L.; Hong, G.; Zhu, M.; Zhang, Y.; Wang, X.; Chen, Y.; Hsiao, B.S. Micro-nano structure poly(ether sulfones)/poly(ethyleneimine) nanofibrous affinity membranes for adsorption of anionic dyes and heavy metal ions in aqueous solution. Chem. Eng. J. 2012, 197, 88–100. [Google Scholar] [CrossRef]
- Hossaini Zahed, S.S.; Khanlari, S.; Mohammadi, T. Hydrous metal oxide incorporated polyacrylonitrile-based nanocomposite membranes for Cu(II) ions removal. Sep. Purif. Technol. 2019, 213, 151–161. [Google Scholar] [CrossRef]
- Sarker, A.K.; Cashin, P.J.; Balakrishnan, V.K.; Exall, K.; Chung, H.K.; Buncel, E.; Brown, R.S. Determining binding of sulfonamide antibiotics to CTABr micelles using semi-equilibrium dialysis. Sep. Purif. Technol. 2016, 162, 134–141. [Google Scholar] [CrossRef]
- Daniş, Ü.; Keskinler, B. Chromate removal from wastewater using micellar enhanced crossflow filtration: Effect of transmembrane pressure and crossflow velocity. Desalination 2009, 249, 1356–1364. [Google Scholar] [CrossRef]
- Zhan, Y.; Wan, X.; He, S.; He, Y. Sulfonated poly(arylene ether nitrile)/polypyrrole core/shell nanofibrous mat: an efficient absorbent for the removal of hexavalent chromium from aqueous solution. J. Chem. Technol. Biot. 2018, 93, 1432–1442. [Google Scholar] [CrossRef]
- You, Y.; Liu, S.; Tu, L.; Wang, Y.; Zhan, C.; Du, X.; Wei, R.; Liu, X. Controllable fabrication of poly(arylene ether nitrile) dielectrics for thermal-resistant film capacitors. Macromolecules 2019, 52, 5850–5859. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, P.; Zhang, W.; Xu, M.; Jia, K.; Liu, X. Detection of Cu2+ metals by luminescent sensor based on sulfonated poly(arylene ether nitrile)/ metal-organic frameworks. Mater. Today Commun. 2018, 16, 258–263. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, P.; Wang, L.; Liu, X. Preparation of sulfonated poly(arylene ether nitrile)-based adsorbent as a highly selective and efficient adsorbent for cationic dyes. Polymers (Basel) 2018, 11, 32. [Google Scholar] [CrossRef]
- Abdullah, N.; Gohari, R.J.; Yusof, N.; Ismail, A.F.; Juhana, J.; Lau, W.J.; Matsuura, T. Polysulfone/hydrous ferric oxide ultrafiltration mixed matrix membrane: Preparation, characterization and its adsorptive removal of lead (II) from aqueous solution. Chem. Eng. J. 2016, 289, 28–37. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Pan, S.; Zhang, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Internal pore decoration with polydopamine nanoparticle on polymeric ultrafiltration membrane for enhanced heavy metal removal. Chem. Eng. J. 2017, 314, 38–49. [Google Scholar] [CrossRef]
- Koushkbaghi, S.; Zakialamdari, A.; Pishnamazi, M.; Ramandi, H.F.; Aliabadi, M.; Irani, M. Aminated-Fe3O4 nanoparticles filled chitosan/PVA/PES dual layers nanofibrous membrane for the removal of Cr(VI) and Pb(II) ions from aqueous solutions in adsorption and membrane processes. Chem. Eng. J. 2018, 337, 169–182. [Google Scholar] [CrossRef]
- Wei, G.; Qi, J.; Lin, P.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Polyethersulfone enwrapped hydrous zirconium oxide nanoparticles for efficient removal of Pb(II) from aqueous solution. Chem. Eng. J. 2018, 349, 500–508. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari Rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, N.; Zereshki, S.; Heidari, S. Removal of lead ions from water using PES-based nanocomposite membrane incorporated with polyaniline modified GO nanoparticles: Performance optimization by central composite design. Process. Saf. Environ. 2017, 111, 475–490. [Google Scholar] [CrossRef]
- Jamshidi Gohari, R.; Lau, W.J.; Matsuura, T.; Halakoo, E.; Ismail, A.F. Adsorptive removal of Pb(II) from aqueous solution by novel PES/HMO ultrafiltration mixed matrix membrane. Sep. Purif. Technol. 2013, 120, 59–68. [Google Scholar] [CrossRef]
- Zheng, P.; Xu, M.; Liu, X.; Jia, K. Sulfonated poly(arylene ether nitrile)s containing cross-linkable nitrile groups for proton exchange membranes. Solid State Ion. 2018, 316, 110–117. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, K.; He, X.; Wei, S.; Wang, P.; Liu, X. Microemulsion self-assembling of novel amphiphilic block co-polyarylene ether nitriles and photosensitizer ZnPc towards hybrid superparticles for photocatalytic degradation of Rhodamine B. Mater. Chem. Phys. 2018, 207, 212–220. [Google Scholar] [CrossRef]
- Sinha, M.K.; Purkait, M.K. Enhancement of hydrophilicity of poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) membrane using various alcohols as nonsolvent additives. Desalination 2014, 338, 106–114. [Google Scholar] [CrossRef]
- Lin, Y.F.; Chen, H.W.; Chien, P.S.; Chiou, C.S.; Liu, C.C. Application of bifunctional magnetic adsorbent to adsorb metal cations and anionic dyes in aqueous solution. J. Hazard. Mater. 2011, 185, 1124–1130. [Google Scholar] [CrossRef]
- Sarker, A.K.; Brown, R.S. Determining binding of polycyclic aromatic hydrocarbons to CTABr micelles using semi-equilibrium dialysis techniques. Ecotoxicol. Environ. Saf. 2019, 172, 114–119. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; Teva, F. Removal of emerging contaminants from secondary effluents by micellar-enhanced ultrafiltration. Sep. Purif. Technol. 2017, 181, 123–131. [Google Scholar] [CrossRef]
- Moreira, G.F.; Peçanha, E.R.; Monte, M.B.M.; Leal Filho, L.S.; Stavale, F. XPS study on the mechanism of starch-hematite surface chemical complexation. Miner. Eng. 2017, 110, 96–103. [Google Scholar] [CrossRef]
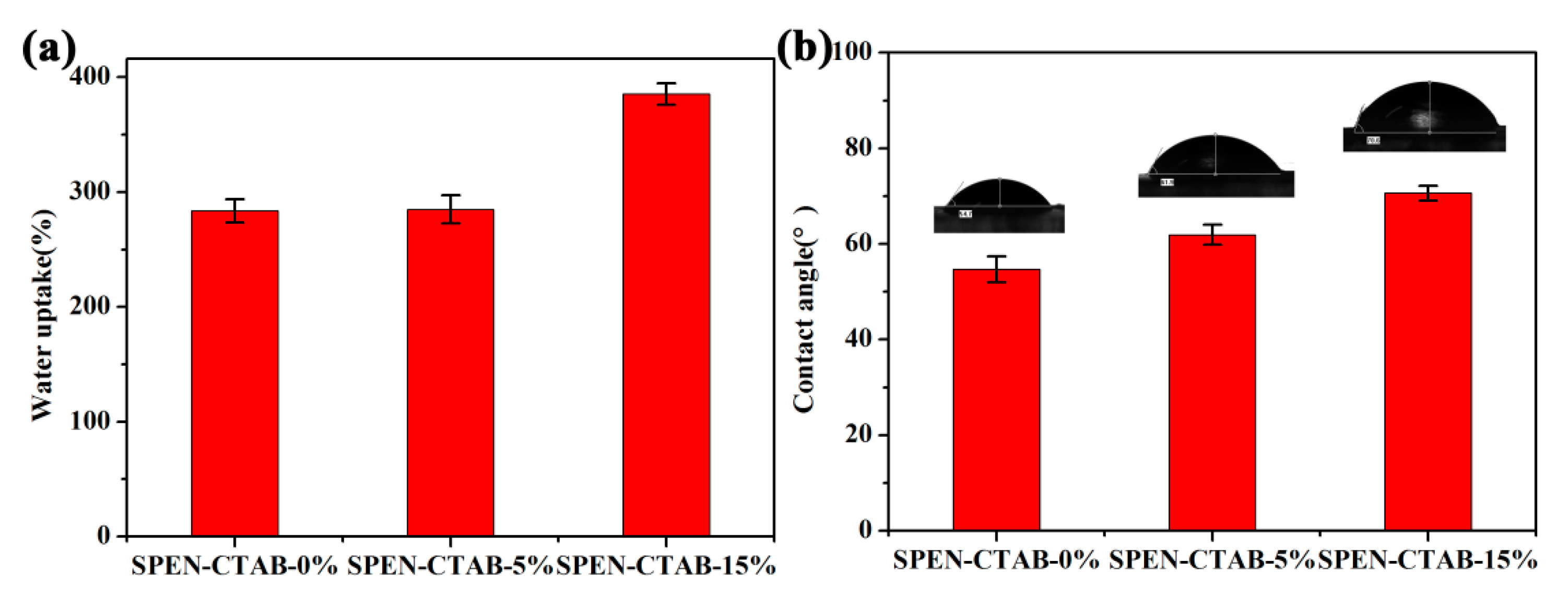

| Membrane | EWC (%) | Porosity (%) | Contact Angle (°) |
|---|---|---|---|
| SPEN-CTAB-0% | 73.93 | 78.95 | 54.7 |
| SPEN-CTAB-5% | 74.02 | 79.03 | 61.9 |
| SPEN-CTAB-15% | 79.40 | 83.60 | 70.6 |
| Membrane | Parameters | SPEN-CTAB-0% | SPEN-CTAB-5% | SPEN-CTAB-15% |
|---|---|---|---|---|
| Pseudo-first-order | k1 (min−1) | 0.0278 | 0.0404 | 0.0398 |
| qe (cal.) (mg/g) | 12.811 | 12.678 | 11.274 | |
| qe (exp.) (mg/g) | 183.60 | 161.73 | 127.43 | |
| R2 | 0.8703 | 0.8320 | 0.6967 | |
| Pseudo-second-order | k2 (g/mg·min) | 0.00037 | 0.00036 | 0.0012 |
| qe (cal.) (mg/g) | 202.43 | 185.53 | 134.05 | |
| qe (exp.) (mg/g) | 183.60 | 161.73 | 127.43 | |
| R2 | 0.9911 | 0.9693 | 0.9978 | |
| Intraparticle diffusion | ki1 | 33.697 | 28.316 | 24.236 |
| C | 2.4810 | −10.281 | −1.170 | |
| R12 | 0.9879 | 0.7428 | 0.9948 | |
| ki2 | 6.1267 | 2.1267 | 0.7810 | |
| C | 115.84 | 137.61 | 117.76 | |
| R22 | 0.9682 | 0.8814 | 0.5995 |
| Adsorbent | pH | Pb(II) Adsorption Capacity (mg/g) | Reference |
|---|---|---|---|
| PSF/HFO NPs MMMs | 6.5–7 | 13.2 | [27] |
| PES/PDA-R UFAMs | 5.4 | 20.23 | [28] |
| PES/chitosan/PVA/A-Fe3O4 MMMs | 6 | 40 | [29] |
| PES/HHZO nanoparticles | 5 | 104 | [30] |
| PAN/chitosan/UiO-66-NH2 MOF nanofibers | 6 | 115 | [31] |
| PES/PANI@GO MMMs | 6 | 145 | [32] |
| PES/HMO MMMs | 7–8 | 204.1 | [33] |
| SPEN porous membranes | 5 | 246.96 | This study |
| C0 (mg/L) | Parameters | 50 | 70 | 100 | 150 | 200 |
|---|---|---|---|---|---|---|
| Pseudo-first-order | k1 (min−1) | 0.0284 | 0.0387 | 0.0450 | 0.0490 | 0.0201 |
| qe (cal.) (mg/g) | 13.861 | 13.730 | 13.821 | 13.052 | 12.174 | |
| qe (exp.) (mg/g) | 195.82 | 196.09 | 202.99 | 217.92 | 246.96 | |
| R2 | 0.9370 | 0.9588 | 0.9712 | 0.8994 | 0.4650 | |
| Pseudo-second-order | k2 (g/mg·min) | 1.8 × 10−4 | 3.4 × 10−4 | 3.8 × 10−4 | 8.1 × 10−4 | 1.1 × 10−3 |
| qe (cal.) (mg/g) | 234.74 | 219.30 | 225.22 | 228.83 | 245.70 | |
| qe (exp.) (mg/g) | 195.82 | 196.09 | 202.99 | 217.92 | 246.96 | |
| R2 | 0.9896 | 0.9975 | 0.9978 | 0.9990 | 0.9951 | |
| Intraparticle diffusion | ki1 | 23.188 | 28.463 | 31.556 | 44.485 | 55.225 |
| C | −5.5100 | −1.8826 | −2.4937 | −0.9429 | 10.448 | |
| R12 | 0.8930 | 0.9875 | 0.9877 | 0.9967 | 0.9446 | |
| ki2 | 8.5084 | 5.5405 | 4.7350 | 2.1192 | 2.2246 | |
| C | 101.91 | 136.41 | 153.10 | 195.22 | 197.36 | |
| R22 | 0.9668 | 0.9819 | 0.9545 | 0.9806 | 0.3939 |
| Isotherms | Parameters (Temperature = 298.15 K) | |
|---|---|---|
| Langmuir | qm (mg/g) | 281.69 |
| KL (L/mg) | 0.1117 | |
| RL | 0.08998 | |
| R2 | 0.9807 | |
| Freundlich | KF ((mg/g) (L/mg)1/n) | 12.861 |
| n−1 | 0.1861 | |
| R2 | 0.8046 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhou, M.; Zhou, X.; Wang, L.; Liu, X. Functionalized Poly(arylene ether nitrile) Porous Membrane with High Pb(II) Adsorption Performance. Polymers 2019, 11, 1412. https://doi.org/10.3390/polym11091412
Liu X, Zhou M, Zhou X, Wang L, Liu X. Functionalized Poly(arylene ether nitrile) Porous Membrane with High Pb(II) Adsorption Performance. Polymers. 2019; 11(9):1412. https://doi.org/10.3390/polym11091412
Chicago/Turabian StyleLiu, Xiaocan, Meirong Zhou, Xuefei Zhou, Lingling Wang, and Xiaobo Liu. 2019. "Functionalized Poly(arylene ether nitrile) Porous Membrane with High Pb(II) Adsorption Performance" Polymers 11, no. 9: 1412. https://doi.org/10.3390/polym11091412
APA StyleLiu, X., Zhou, M., Zhou, X., Wang, L., & Liu, X. (2019). Functionalized Poly(arylene ether nitrile) Porous Membrane with High Pb(II) Adsorption Performance. Polymers, 11(9), 1412. https://doi.org/10.3390/polym11091412





