Dispersion and Performance of a Nanoclay/Whey Protein Isolate Coating upon its Upscaling as a Novel Ready-to-Use Formulation for Packaging Converters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of WPI-Based Ready-to-Use Formulations by High-Energy Ball-Milling
2.1.2. Coating Preparation, Application, and Drying
Lab-Scale
Pilot-Scale
Semi-Industrial-Scale
2.2. Methods
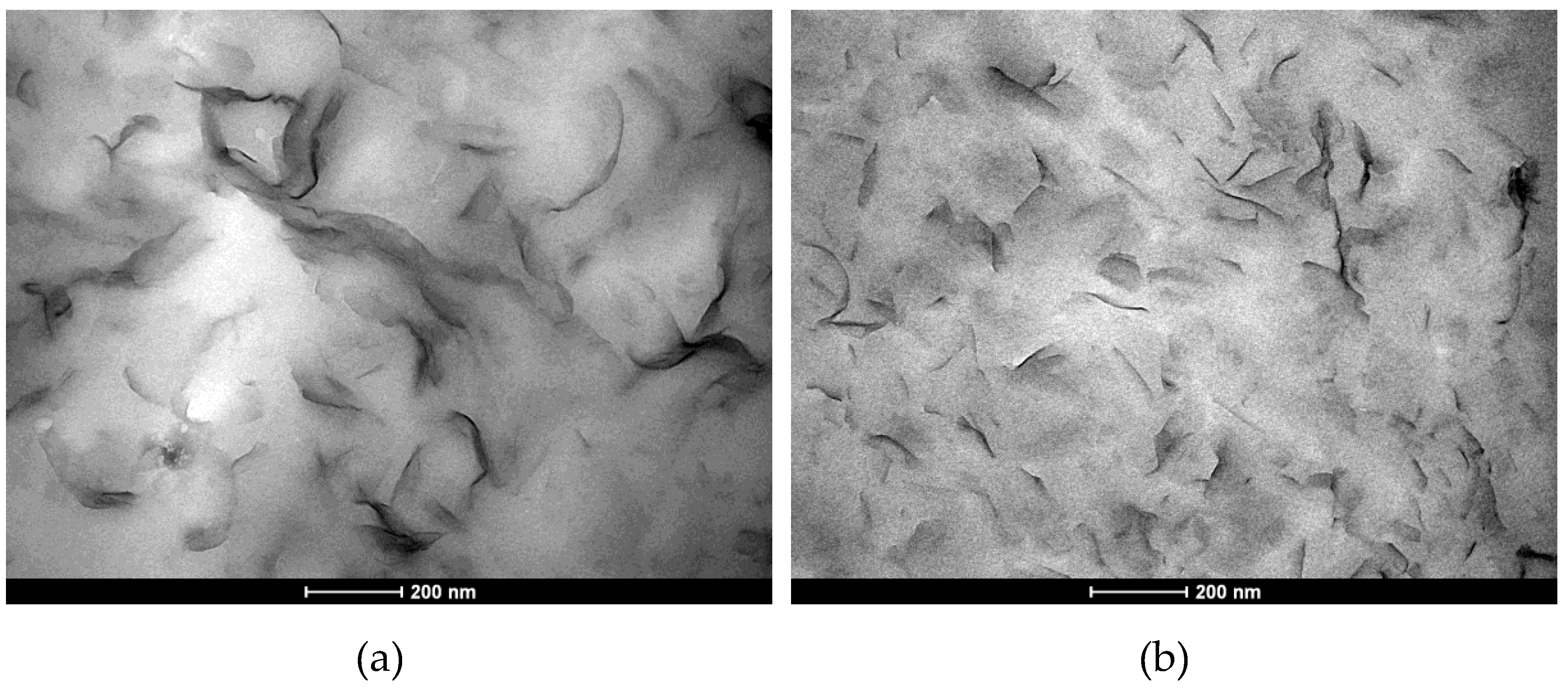
2.2.1. Transmission Electron Microscopy
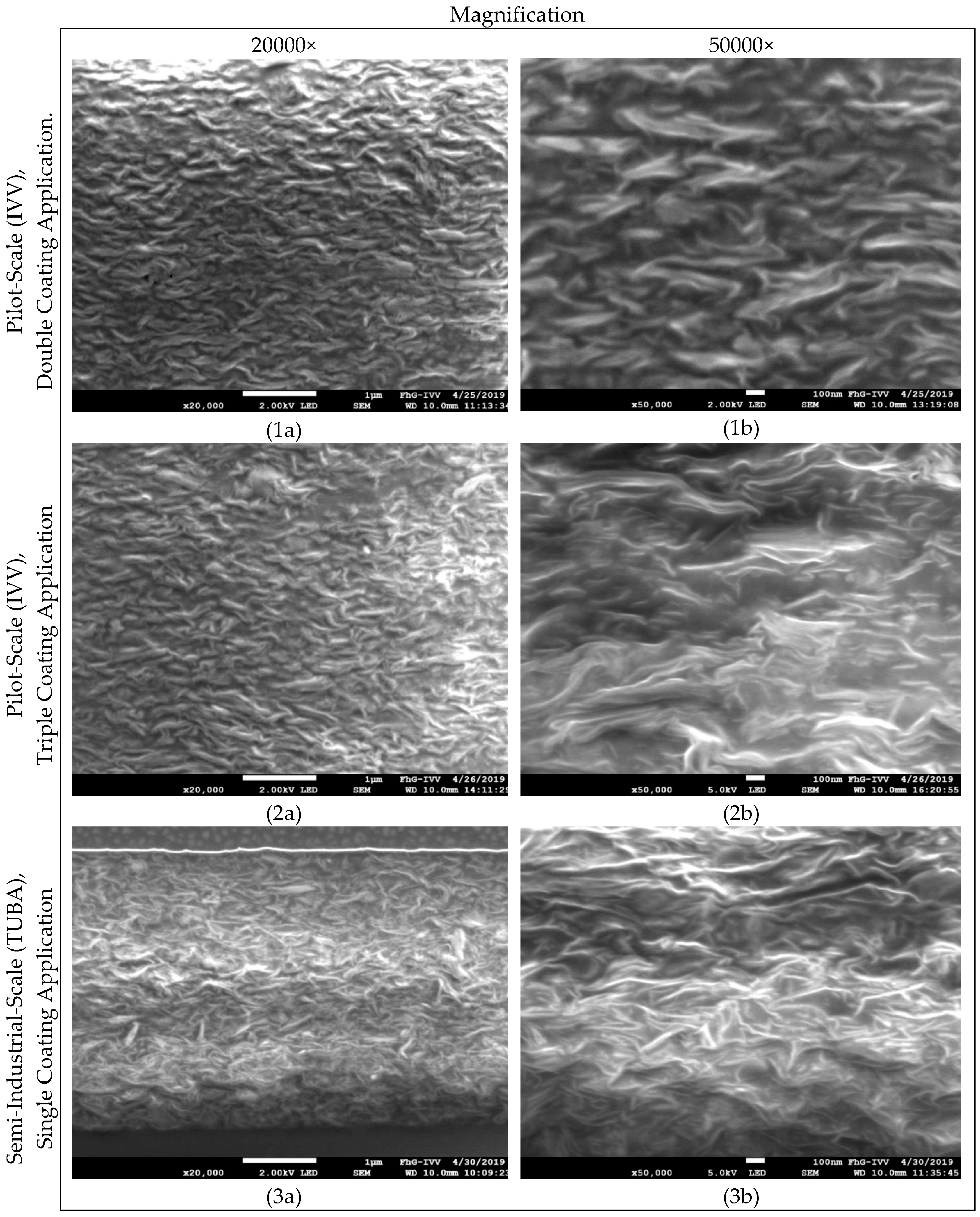
2.2.2. Scanning Electron Microscopy
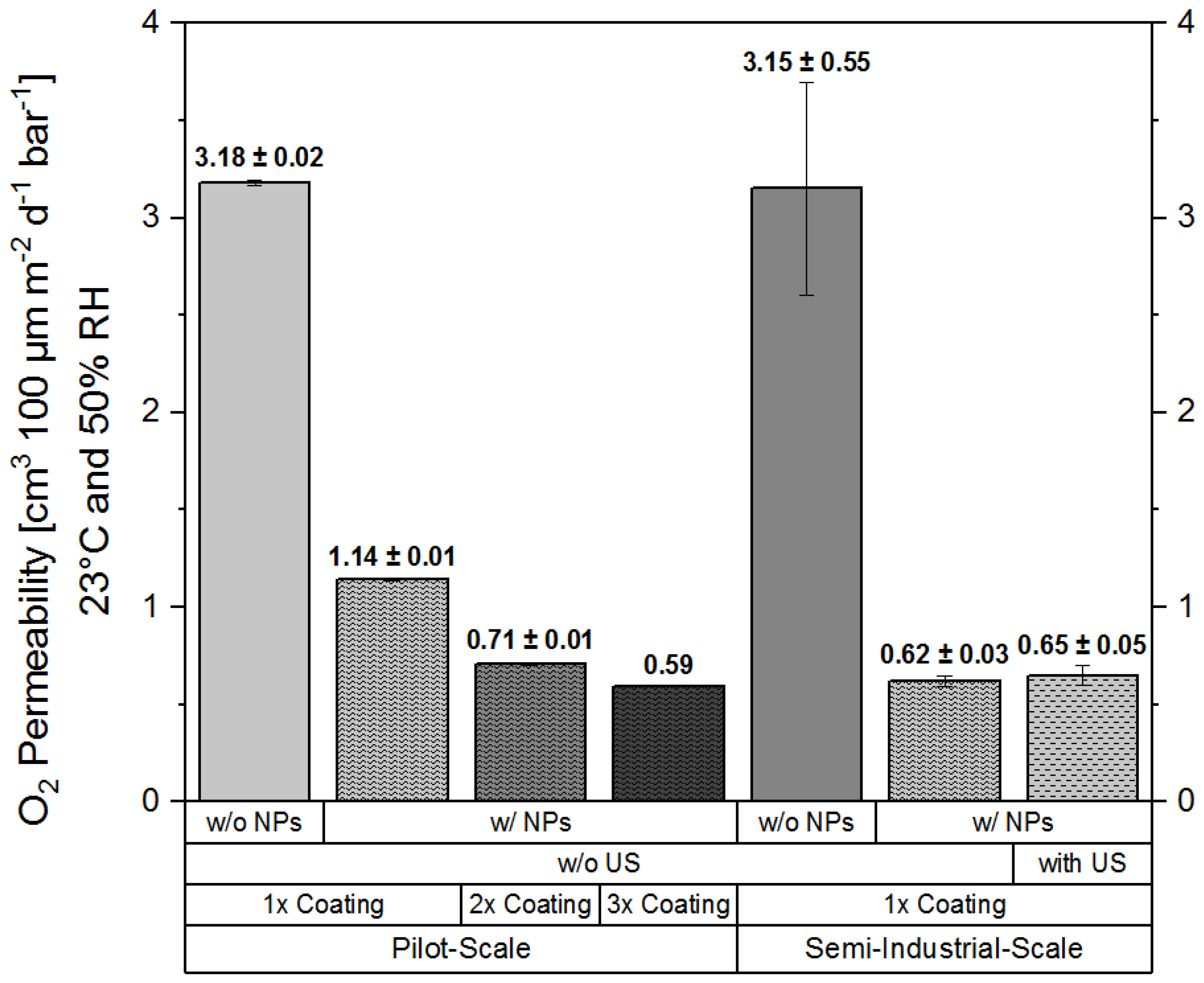
2.2.3. Oxygen permeability
3. Results
3.1. Effect of Ultrasound-Treatment on Lab-Scale Coating Quality Prepared from Liquid Nanoparticles Suspensions
3.2. Oxygen Barrier Performance of Coatings Based on Ready-to-Use Formulations
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Inter. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Perez-gago, M.B.; Krochta, J.M. Denaturation time and temperature effects on solubility, tensile properties, and oxygen permeability of whey protein edible films. J. Food Sci. 2001, 66, 705–710. [Google Scholar] [CrossRef]
- Tunc, S.; Angellier, H.; Cahyana, Y.; Chalier, P.; Gontard, N.; Gastaldi, E. Functional properties of wheat gluten/montmorillonite nanocomposite films processed by casting. J. Mem. Sci. 2007, 289, 159–168. [Google Scholar] [CrossRef]
- Were, L.; Hettiarachchy, N.S.; Coleman, M. Properties of cysteine-added soy protein-wheat gluten films. J. Food Sci. 1999, 64, 514–518. [Google Scholar] [CrossRef]
- Min, S.; Harris, L.J.; Krochta, J.M. Listeria monocytogenes inhibition by whey protein films and coatings incorporating the lactoperoxidase system. J. Food Sci. 2005, 70, m317−m324. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Rauch, D.J.; McCarthy, K.L.; Krochta, J.M. Barrier and tensile properties of whey protein–candelilla wax film/sheet. LWT Food Sci. Technol. 2014, 56, 377–382. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocolloids 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Schmid, M.; Sängerlaub, S.; Wege, L.; Stäbler, A. Properties of transglutaminase crosslinked whey protein isolate coatings and cast films. Packag. Technol. Sci. 2014, 27, 799–817. [Google Scholar] [CrossRef]
- Di Pierro, P.; Chico, B.; Villalonga, R.; Mariniello, L.; Damiao, A.E.; Masi, P.; Porta, R. Chitosan−whey protein edible films produced in the absence or presence of transglutaminase: Analysis of their mechanical and barrier properties. Biomacromolecules 2006, 7, 744–749. [Google Scholar] [CrossRef]
- Gennadios, A.; Brandenburg, A.H.; Weller, C.L.; Testin, R.F. Effect of ph on properties of wheat gluten and soy protein isolate films. J. Agric. Food Chem. 1993, 41, 1835–1839. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Water vapor permeability and solubility of films from hydrolyzed whey protein. J. Food Sci. 2000, 65, 700–703. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Schmid, M.; Kainz, D.M.; Lafortune, P.; Rodriguez-Turienzo, L.; Cinelli, P. Simulation and experimental validation of the denaturation of a whey protein-based coating during convection and/or infrared drying on a plastic film and influence on its oxygen barrier properties. Poly. Plast. Technol. Eng. 2016, 55, 1503–1511. [Google Scholar] [CrossRef]
- Schmid, M.; Noller, K.; Wild, F.; Bugnicourt, E. Whey Protein Coated Films. Patent EP2734575A1, 22 July 2011. [Google Scholar]
- Hong, S.-I.; Krochta, J.M. Oxygen barrier properties of whey protein isolate coatings on polypropylene films. J. Food Sci. 2003, 68, 224–228. [Google Scholar] [CrossRef]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Coltelli, M.B.; Lazzeri, A. Recyclability of pet/wpi/pe multilayer films by removal of whey protein isolate-based coatings with enzymatic detergents. Materials (Basel) 2016, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, P.; Schmid, M.; Bugnicourt, E.; Wildner, J.; Bazzichi, A.; Anguillesi, I.; Lazzeri, A. Whey protein layer applied on biodegradable packaging film to improve barrier properties while maintaining biodegradability. Polym. Degrad. Stab. 2014, 108, 151–157. [Google Scholar] [CrossRef]
- Commission. A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Bugnicourt, E.; Schmid, M.; Nerney, O.M.; Wildner, J.; Smykala, L.; Lazzeri, A.; Cinelli, P. Processing and validation of whey-protein-coated films and laminates at semi-industrial scale as novel recyclable food packaging materials with excellent barrier properties. Adv. Mater. Sci. Eng. 2013, 2013, 10. [Google Scholar] [CrossRef]
- Ray, S.; Quek Siew, Y.; Easteal, A.; Chen Xiao, D. The potential use of polymer-clay nanocomposites in food packaging. Inter. J. Food Eng. 2006, 2, 1556–3758. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Hedenqvist, M.S.; Backman, A.; Gällstedt, M.; Boyd, R.H.; Gedde, U.W. Morphology and diffusion properties of whey/montmorillonite nanocomposites. Comp. Sci. Technol. 2006, 66, 2350–2359. [Google Scholar] [CrossRef]
- Zhou, J.J.; Wang, S.Y.; Gunasekaran, S. Preparation and characterization of whey protein film incorporated with tio2 nanoparticles. J. Food Sci. 2009, 74, N50−N56. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R.; Hong, S.-I.; An, D.J.; Rhim, J.-W. Effect of clay content on the physical and antimicrobial properties of whey protein isolate/organo-clay composite films. LWT Food Sci. Technol. 2010, 43, 279–284. [Google Scholar] [CrossRef]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. The improvement of characteristics of biodegradable films made from kefiran–whey protein by nanoparticle incorporation. Carbohydr. Polym. 2014, 109, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, V.M.; Dias, M.V.; Borges, S.V.; Costa, A.L.R.; Silva, E.K.; Medeiros, É.A.A.; Soares, N.d.F.F. Development of whey protein isolate bio-nanocomposites: Effect of montmorillonite and citric acid on structural, thermal, morphological and mechanical properties. Food Hydrocolloids 2015, 48, 179–188. [Google Scholar] [CrossRef]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Pourahmad, R.; Shahabi-Ghahfarrokhi, I. Development of ecofriendly bionanocomposite: Whey protein isolate/pullulan films with nano-sio2. Inter. J. Biol. Macromol. 2016, 86, 139–144. [Google Scholar] [CrossRef]
- Langowski, H.C. Permeation of gases and condensable substances through monolayer and multilayer structures. In Plastic Packaging; Piringer, O.G., Baner, A.L., Eds.; Wiley-VCH: Weinheim, Germany, 2008; p. 297. [Google Scholar]
- Yang, Y.; Zhu, Z.K.; Yin, J.; Wang, X.Y.; Qi, Z.E. Preparation and properties of hybrids of organo-soluble polyimide and montmorillonite with various chemical surface modification methods. Polymer 1999, 40, 4407–4414. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Müller, K.; Jesdinszki, M.; Schmid, M. Modification of functional properties of whey protein isolate nanocomposite films and coatings with nanoclays. J. Nanomater. 2017, 2017, 10. [Google Scholar] [CrossRef]
- Schmid, M.; Merzbacher, S.; Brzoska, N.; Müller, K.; Jesdinszki, M. Improvement of food packaging-related properties of whey protein isolate-based nanocomposite films and coatings by addition of montmorillonite nanoplatelets. Front. Mater. 2017, 4. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch–cmc–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, E.R.; Gilbert, S.G. Gas permeation of collagen films as affected by cross-linkage, moisture, and plasticizer content. J. Polym. Sci. 1973, 41, 33–43. [Google Scholar] [CrossRef]
- Wakai, M.; Almenar, E. Effect of the presence of montmorillonite on the solubility of whey protein isolate films in food model systems with different compositions and pH. Food Hydrocolloids 2015, 43, 612–621. [Google Scholar] [CrossRef]
- Cooper, P.; Loughlin, A.; Carnaghan, E.; Read, S. Processing and Control of Novel Nanomaterials in Packaging, Automotive and Solar Panel Processing Lines-optinanopro, Deliverable 9.10, Nanosafety Assessment Report; OptiNanoProGrant Agreement No: 686116; European Commission: Brussels, Belgium, 2018. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugnicourt, E.; Brzoska, N.; Kucukpinar, E.; Philippe, S.; Forlin, E.; Bianchin, A.; Schmid, M. Dispersion and Performance of a Nanoclay/Whey Protein Isolate Coating upon its Upscaling as a Novel Ready-to-Use Formulation for Packaging Converters. Polymers 2019, 11, 1410. https://doi.org/10.3390/polym11091410
Bugnicourt E, Brzoska N, Kucukpinar E, Philippe S, Forlin E, Bianchin A, Schmid M. Dispersion and Performance of a Nanoclay/Whey Protein Isolate Coating upon its Upscaling as a Novel Ready-to-Use Formulation for Packaging Converters. Polymers. 2019; 11(9):1410. https://doi.org/10.3390/polym11091410
Chicago/Turabian StyleBugnicourt, Elodie, Nicola Brzoska, Esra Kucukpinar, Severine Philippe, Enrico Forlin, Alvise Bianchin, and Markus Schmid. 2019. "Dispersion and Performance of a Nanoclay/Whey Protein Isolate Coating upon its Upscaling as a Novel Ready-to-Use Formulation for Packaging Converters" Polymers 11, no. 9: 1410. https://doi.org/10.3390/polym11091410
APA StyleBugnicourt, E., Brzoska, N., Kucukpinar, E., Philippe, S., Forlin, E., Bianchin, A., & Schmid, M. (2019). Dispersion and Performance of a Nanoclay/Whey Protein Isolate Coating upon its Upscaling as a Novel Ready-to-Use Formulation for Packaging Converters. Polymers, 11(9), 1410. https://doi.org/10.3390/polym11091410





