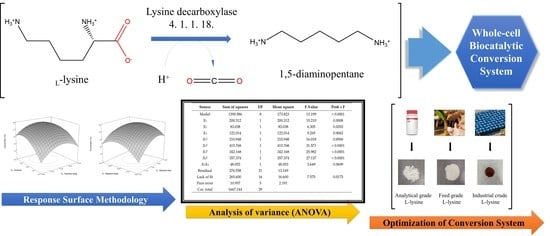
Enhanced l-Lysine into 1,5-Diaminopentane Conversion via Statistical Optimization of Whole-Cell Decarboxylation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Conditions of Whole-Cell Biocatalystic System
2.3. Experimental Design and Statistical Analysis
2.4. Conditions of Whole-Cell Biocatalystic System
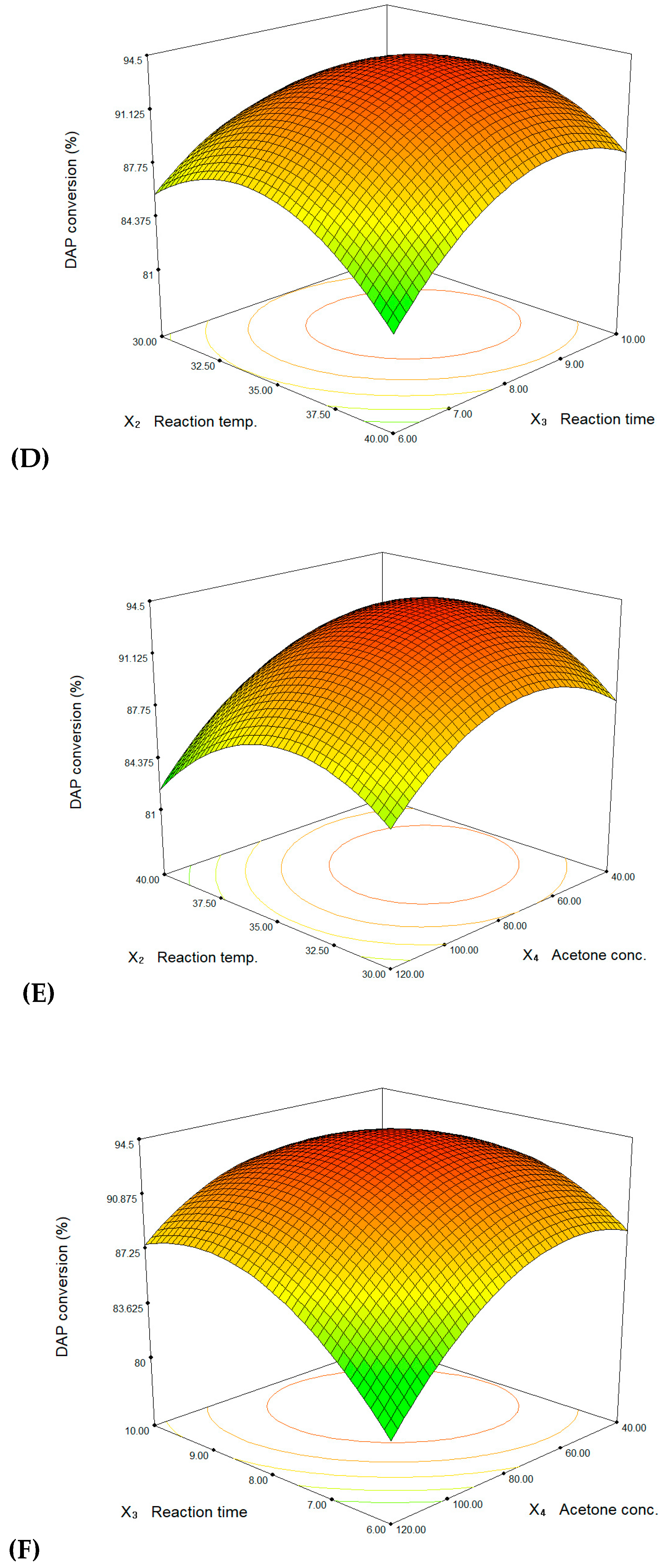
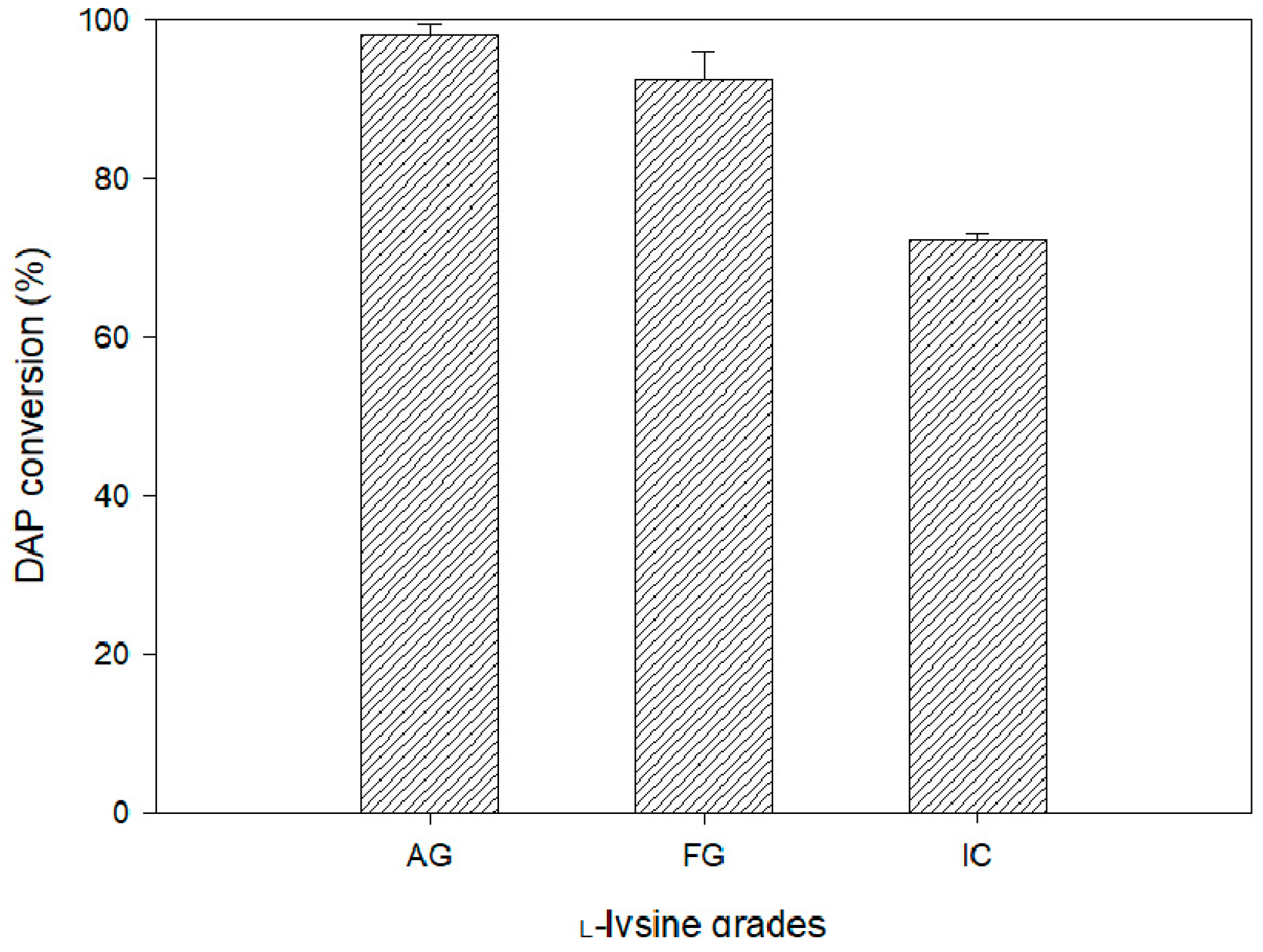
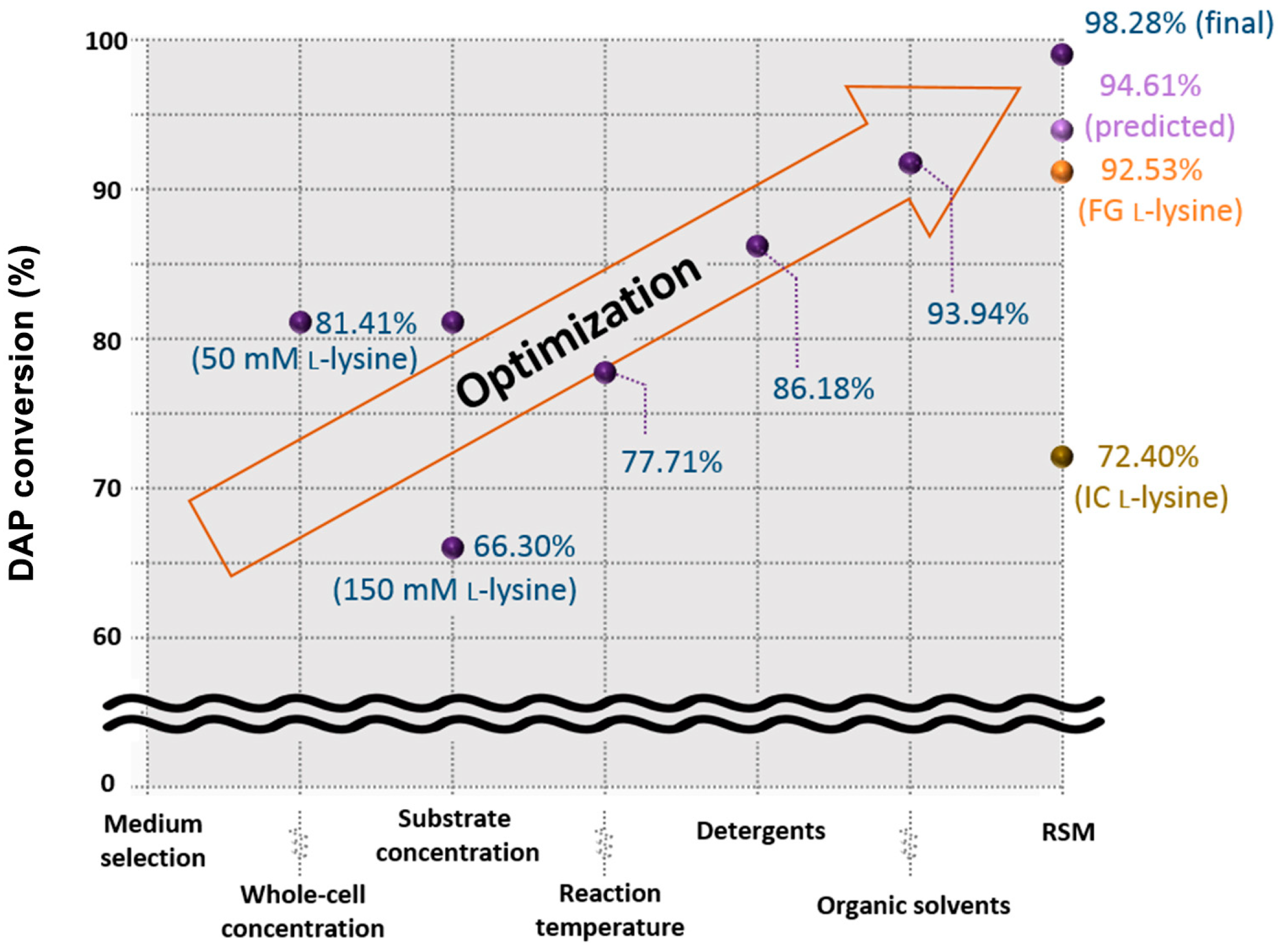
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kelle, R.; Hermann, T.; Bathe, B. l-Lysine production. In Handbook of Corynebacterium glutamicum; CRC Press: Boca Raton, FL, USA, 2005; pp. 465–488. [Google Scholar]
- Leuchtenberger, W.; Huthmacher, K.; Drauz, K. Biotechnological production of amino acids and derivatives: Current status and prospects. Appl. Microbiol. Biotechnol. 2005, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Evans, J. Commercial amino acids, BCC Research: Market Research Reports. 2017. BIO007L. Available online: http://www.bccresearch.com (accessed on 10 August 2019).
- Elder, M. World markets for fermentation ingredients, BCC Research: Market Research Reports. 2018. FOD020E. Available online: http://www.bccresearch.com (accessed on 10 August 2019).
- Eggeling, L.; Bott, M. A giant market and a powerful metabolism: L-lysine provided by Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 2015, 99, 3387–3394. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, C.; Becker, J. The L-lysine story: From metabolic pathways to industrial production. In Amino Acid Biosynthesis - Pathways, 1st ed.; Wendisch, V.F., Ed.; Springer: Berlin, Germany, 2007; pp. 39–70. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, H.Y.; Ki, Y.H.; Kim, I.-K.; Byun, E.-H.; Yang, Y.-H.; Park, S.J.; Na, J.-G.; Sohn, H.; Lee, T.; et al. Improved reutilization of industrial crude lysine to 1, 5-diaminopentane by enzymatic decarboxylation using various detergents and organic solvents. Korean J. Chem. Eng. 2018, 35, 1854–1859. [Google Scholar] [CrossRef]
- Adkins, J.; Jordan, J.; Nielsen, D.R. Engineering Escherichia coli for renewable production of the 5-carbon polyamide building-blocks 5-aminovalerate and glutarate. Biotechnol. Bioeng. 2013, 110, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Siripong, P.; Doungporn, P.; Yoo, H.Y.; Kim, S.W. Improvement of sugar recovery from Sida acuta (Thailand Weed) by NaOH pretreatment and application to bioethanol production. Korean J. Chem. Eng. 2018, 35, 2413–2420. [Google Scholar] [CrossRef]
- Kim, C.K.; Choi, H.S.; Lee, S.J.; Lee, J.H.; Lee, J.H.; Yoo, H.Y.; Han, S.O.; Kim, S.W. Production of xylanase from a novel engineered Pichia pastoris and application to enzymatic hydrolysis process for biorefinery. Process. Biochem. 2018, 65, 130–135. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.R.; Lee, J.H.; Lee, S.K.; Chun, Y.; Han, S.O.; Yoo, H.Y.; Park, C.; Kim, S.W. Enhanced in-vitro Hemozoin polymerization by optimized process using Histidine-Rich Protein II (HRPII). Polymers 2019, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Minzanova, S.T.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological activity and pharmacological application of pectic polysaccharides: A review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef] [PubMed]
- Kind, S.; Wittmann, C. Bio-based production of the platform chemical 1,5-diaminopentane. Appl. Microbiol. Biotechnol. 2011, 91, 1287–1296. [Google Scholar] [CrossRef]
- Kind, S.; Neubauer, S.; Becker, J.; Yamamoto, M.; Volkert, M.; Abendroth, G.; Zelder, O.; Wittmann, C. From zero to hero–production of bio-based nylon from renewable resources using engineered Corynebacterium glutamicum. Metab. Eng. 2014, 25, 113–123. [Google Scholar] [CrossRef]
- Tateno, T.; Okada, Y.; Tsuchidate, T.; Tanaka, T.; Fukuda, H.; Kondo, A. Direct production of cadaverine from soluble starch using Corynebacterium glutamicum coexpressing α-amylase and lysine decarboxylase. Appl. Microbiol. Biotechnol. 2009, 82, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Buschke, N.; Becker, J.; Schäfer, R.; Kiefer, P.; Biedendieck, R.; Wittmann, C. Systems metabolic engineering of xylose-utilizing Corynebacterium glutamicum for production of 1,5-diaminopentane. Biotechnol. J. 2013, 8, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seo, H.M.; Sathiyanarayanan, G.; Bhatia, S.K.; Song, H.S.; Kim, J.; Jeon, J.M.; Kim, Y.G.; Park, K.; Yang, Y.H. Development of a continuous l-lysine bioconversion system for cadaverine production. J. Ind. Eng. Chem. 2017, 46, 44–48. [Google Scholar] [CrossRef]
- Matsuura, R.; Kishida, M.; Konishi, R.; Hirata, Y.; Adachi, N.; Segawa, S.; Imao, K.; Tanaka, T.; Kondo, A. Metabolic engineering to improve 1, 5-diaminopentane production from cellobiose using β-glucosidase-secreting Corynebacterium glutamicum. Biotechnol. Bioeng. 2019. [Google Scholar] [CrossRef] [PubMed]
- Buschke, N.; Schröder, H.; Wittmann, C. Metabolic engineering of Corynebacterium glutamicum for production of 1, 5-diaminopentane from hemicellulose. Biotechnol. J. 2011, 6, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Krithika, G.; Arunachalam, J.; Priyanka, H.; Indulekha, K. The two forms of lysine decarboxylase; kinetics and effect of expression in relation to acid tolerance response in E. coli. J. Exp. Sci. 2011, 1, 10–21. [Google Scholar]
- Takatsuka, Y.; Yamaguchi, Y.; Ono, M.; Kamio, Y. Gene cloning and molecular characterization of lysine decarboxylase from Selenomonas ruminantiumdelineate its evolutionary relationship to ornithine decarboxylases from eukaryotes. J. Bacteriol. 2000, 182, 6732–6741. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ju, J.H.; Oh, B.R.; Ryu, S.K.; Heo, S.Y.; Kim, S.Y.; Hong, W.K.; Kim, C.H.; Seo, J.W. Production of Lipid Containing High Levels of Docosahexaenoic Acid by Cultivation of Aurantiochytrium sp. KRS101 Using Jerusalem Artichoke Extract. Biotechnol. Bioprocess. Eng. 2018, 23, 726–732. [Google Scholar] [CrossRef]
- Jung, D.U.; Yoo, H.Y.; Kim, S.B.; Lee, J.H.; Park, C.; Kim, S.W. Optimization of medium composition for enhanced cellulase production by mutant Penicillium brasilianum KUEB15 using statistical method. J. Ind. Eng. Chem. 2015, 25, 145–150. [Google Scholar] [CrossRef]
- Lee, H.; Shin, H.H.; Kim, H.R.; Nam, H.R.; Seo, D.H.; Seo, M.J. Culture Optimization Strategy for 1-Deoxynojirimycin-producing Bacillus methylotrophicus K26 Isolated from Korean Fermented Soybean Paste, Doenjang. Biotech. Bioprocess. Eng. 2018, 23, 424–431. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Lee, J.H.; Kim, D.S.; Lee, J.H.; Lee, S.K.; Lee, S.J.; Park, C.; Kim, S.W. Enhancement of glucose yield from canola agricultural residue by alkali pretreatment based on multi-regression models. J. Ind. Eng. Chem. 2017, 51, 303–3311. [Google Scholar] [CrossRef]
- Antoine, F.R.; Wei, C.I.; Littell, R.C.; Marshall, M.R. HPLC method for analysis of free amino acids in fish using o-phthaldialdehyde precolumn derivatization. J. Agric. Food. Chem. 1999, 47, 5100–5107. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Liu, J.; Li, C.; Zong, F.; Xu, W.; Zhang, X.; Fang, Z. A multipath peroxymonosulfate activation process over supported by magnetic CuO-Fe3O4 nanoparticles for efficient degradation of 4-chlorophenol. Korean J. Chem. Eng. 2018, 35, 1662–1672. [Google Scholar] [CrossRef]
- Park, C.; Heo, K.; Oh, S.; Kim, S.B.; Lee, S.H.; Kim, Y.H.; Kim, Y.; Lee, J.; Han, S.O.; Lee, S.W.; et al. Eco-design and evaluation for production of 7-aminocephalosporanic acid from carbohydrate wastes discharged after microalgae-based biodiesel production. J. Clean. Prod. 2016, 133, 511–517. [Google Scholar] [CrossRef]
- Go, A.R.; Ko, J.W.; Lee, S.J.; Kim, S.W.; Han, S.O.; Lee, J.; Woo, H.M.; Um, Y.; Nam, J.; Park, C. Process design and evaluation of value-added chemicals production from biomass. Biotechnol. Bioprocess. Eng. 2012, 17, 1055–1061. [Google Scholar] [CrossRef]
- Park, M.R.; Kim, S.G.; Jeong, G.T. Biosugar Production from Gracilaria verrucosa with Sulfamic Acid Pretreatment and Subsequent Enzymatic Hydrolysis. Biotechnol. Bioprocess. Eng. 2018, 23, 302–310. [Google Scholar] [CrossRef]
- Momin, B.; Chakraborty, S.; Annapure, U. Investigation of the cell disruption methods for maximizing the extraction of arginase from mutant Bacillus licheniformis (M09) using statistical approach. Korean J. Chem. Eng. 2018, 35, 2024–2035. [Google Scholar] [CrossRef]


| Factors | Symbols | Coded Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| l-Lysine concentration (mM) | X1 | 50 | 100 | 150 | 200 | 250 |
| Reaction temperature (°C) | X2 | 25 | 30 | 35 | 40 | 45 |
| Reaction time (h) | X3 | 4 | 6 | 8 | 10 | 12 |
| Acetone concentration (g/L) | X4 | 0 | 40 | 80 | 120 | 160 |
| Std. | l-Lysine Conc. (mM), X1 | Reaction Temp. (°C), X2 | Reaction Time (h), X3 | Acetone Conc. (g/L), X4 | DAP Conversion (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Coded | Actual | Coded | Actual | Coded | Actual | Coded | Actual | ||
| 1 | −1 | 100 | −1 | 30 | −1 | 6 | −1 | 40 | 78.99 |
| 2 | 1 | 200 | −1 | 30 | −1 | 6 | −1 | 40 | 86.13 |
| 3 | −1 | 100 | 1 | 40 | −1 | 6 | −1 | 40 | 84.64 |
| 4 | 1 | 200 | 1 | 40 | −1 | 6 | −1 | 40 | 79.41 |
| 5 | −1 | 100 | −1 | 30 | 1 | 10 | −1 | 40 | 86.63 |
| 6 | 1 | 200 | −1 | 30 | 1 | 10 | −1 | 40 | 75.39 |
| 7 | −1 | 100 | 1 | 40 | 1 | 10 | −1 | 40 | 85.05 |
| 8 | 1 | 200 | 1 | 40 | 1 | 10 | −1 | 40 | 80.85 |
| 9 | −1 | 100 | −1 | 30 | −1 | 6 | 1 | 120 | 80.00 |
| 10 | 1 | 200 | −1 | 30 | −1 | 6 | 1 | 120 | 70.53 |
| 11 | −1 | 100 | 1 | 40 | −1 | 6 | 1 | 120 | 75.65 |
| 12 | 1 | 200 | 1 | 40 | −1 | 6 | 1 | 120 | 63.73 |
| 13 | −1 | 100 | −1 | 30 | 1 | 10 | 1 | 120 | 78.14 |
| 14 | 1 | 200 | −1 | 30 | 1 | 10 | 1 | 120 | 79.15 |
| 15 | −1 | 100 | 1 | 40 | 1 | 10 | 1 | 120 | 82.12 |
| 16 | 1 | 200 | 1 | 40 | 1 | 10 | 1 | 120 | 76.99 |
| 17 | −2 | 50 | 0 | 35 | 0 | 8 | 0 | 80 | 91.32 |
| 18 | 2 | 250 | 0 | 35 | 0 | 8 | 0 | 80 | 76.18 |
| 19 | 0 | 150 | −2 | 25 | 0 | 8 | 0 | 80 | 81.19 |
| 20 | 0 | 150 | 2 | 45 | 0 | 8 | 0 | 80 | 77.35 |
| 21 | 0 | 150 | 0 | 35 | −2 | 4 | 0 | 80 | 75.87 |
| 22 | 0 | 150 | 0 | 35 | 2 | 12 | 0 | 80 | 85.56 |
| 23 | 0 | 150 | 0 | 35 | 0 | 8 | −2 | 0 | 81.24 |
| 24 | 0 | 150 | 0 | 35 | 0 | 8 | 2 | 160 | 79.57 |
| 25 | 0 | 150 | 0 | 35 | 0 | 8 | 0 | 80 | 92.86 |
| 26 | 0 | 150 | 0 | 35 | 0 | 8 | 0 | 80 | 91.39 |
| 27 | 0 | 150 | 0 | 35 | 0 | 8 | 0 | 80 | 94.79 |
| 28 | 0 | 150 | 0 | 35 | 0 | 8 | 0 | 80 | 95.20 |
| 29 | 0 | 150 | 0 | 35 | 0 | 8 | 0 | 80 | 92.45 |
| 30 | 0 | 150 | 0 | 35 | 0 | 8 | 0 | 80 | 94.01 |
| Source | Sum of Squares | DF | Mean Square | F-Value | P-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 1390.586 | 8 | 173.823 | 13.199 | <0.0001 |
| X1 | 200.312 | 1 | 200.312 | 15.210 | 0.0008 |
| X3 | 83.038 | 1 | 83.038 | 6.305 | 0.0203 |
| X4 | 122.014 | 1 | 122.014 | 9.265 | 0.0062 |
| X12 | 210.948 | 1 | 210.948 | 16.018 | 0.0006 |
| X22 | 415.768 | 1 | 415.768 | 31.571 | <0.0001 |
| X32 | 342.168 | 1 | 342.168 | 25.982 | <0.0001 |
| X42 | 357.374 | 1 | 357.374 | 27.137 | <0.0001 |
| X3X4 | 48.053 | 1 | 48.053 | 3.649 | 0.0699 |
| Residual | 276.558 | 21 | 13.169 | ||
| Lack of fit | 265.600 | 16 | 16.600 | 7.575 | 0.0173 |
| Pure error | 10.957 | 5 | 2.191 | ||
| Cor. total | 1667.144 | 29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Yoo, H.Y.; Park, N.; Kim, H.; Lee, J.; Baek, Y.; Lee, T.; Oh, J.-M.; Cho, J.; Park, C. Enhanced l-Lysine into 1,5-Diaminopentane Conversion via Statistical Optimization of Whole-Cell Decarboxylation System. Polymers 2019, 11, 1372. https://doi.org/10.3390/polym11081372
Kim H, Yoo HY, Park N, Kim H, Lee J, Baek Y, Lee T, Oh J-M, Cho J, Park C. Enhanced l-Lysine into 1,5-Diaminopentane Conversion via Statistical Optimization of Whole-Cell Decarboxylation System. Polymers. 2019; 11(8):1372. https://doi.org/10.3390/polym11081372
Chicago/Turabian StyleKim, Hanyong, Hah Young Yoo, Nohseong Park, Haeun Kim, Jonghwa Lee, Yesol Baek, Taek Lee, Jong-Min Oh, Jaehoon Cho, and Chulhwan Park. 2019. "Enhanced l-Lysine into 1,5-Diaminopentane Conversion via Statistical Optimization of Whole-Cell Decarboxylation System" Polymers 11, no. 8: 1372. https://doi.org/10.3390/polym11081372
APA StyleKim, H., Yoo, H. Y., Park, N., Kim, H., Lee, J., Baek, Y., Lee, T., Oh, J.-M., Cho, J., & Park, C. (2019). Enhanced l-Lysine into 1,5-Diaminopentane Conversion via Statistical Optimization of Whole-Cell Decarboxylation System. Polymers, 11(8), 1372. https://doi.org/10.3390/polym11081372