Raspberry-Like Polysilsesquioxane Particles with Hollow-Spheres-on-Sphere Structure: Rational Design, Controllable Synthesis, and Catalytic Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
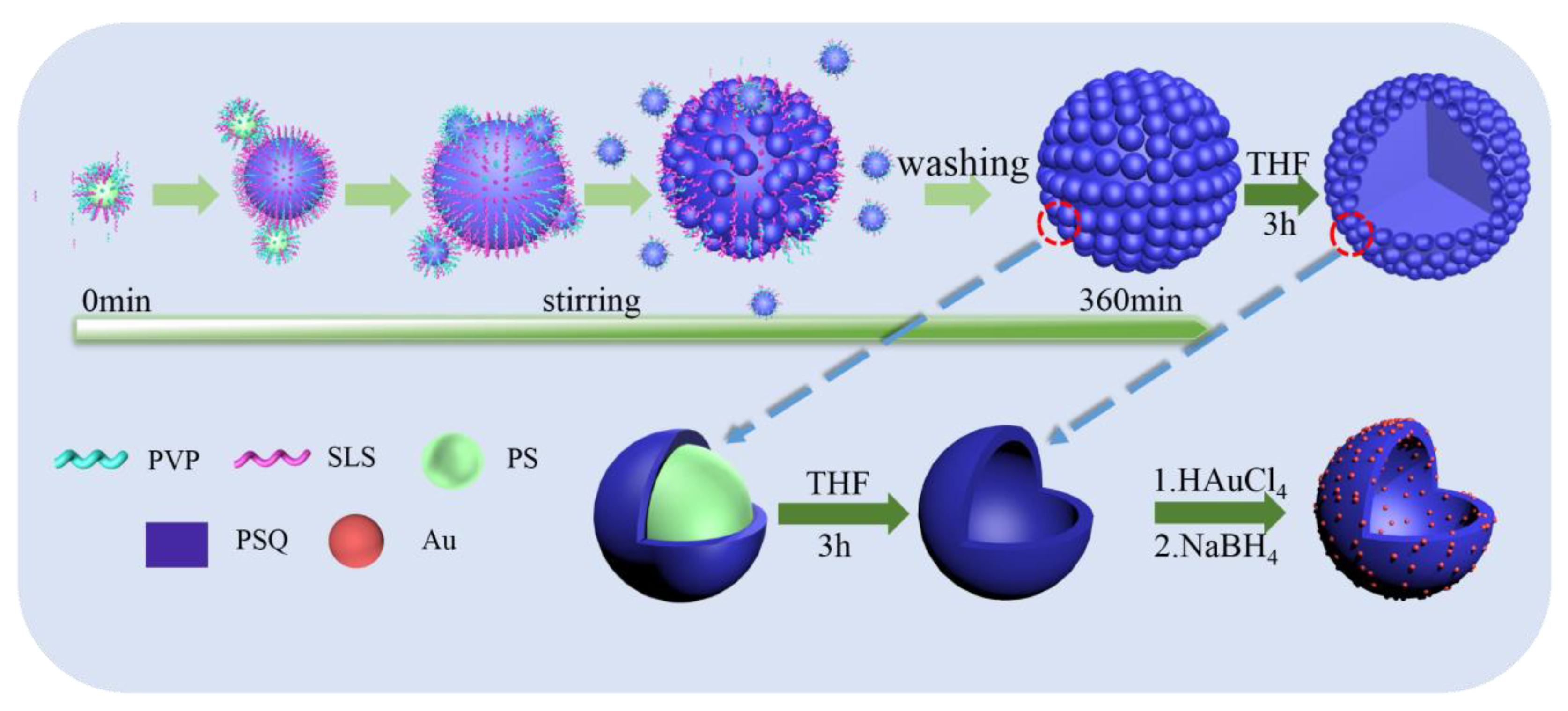
2.2.1. Fabrication of HSOS Particles
2.2.2. Au/HSOS Hybrid Particles
2.2.3. Catalytic Reduction of 4-Nitrophenol
2.3. Characterization
3. Results
3.1. Characterization of HSOS Particles
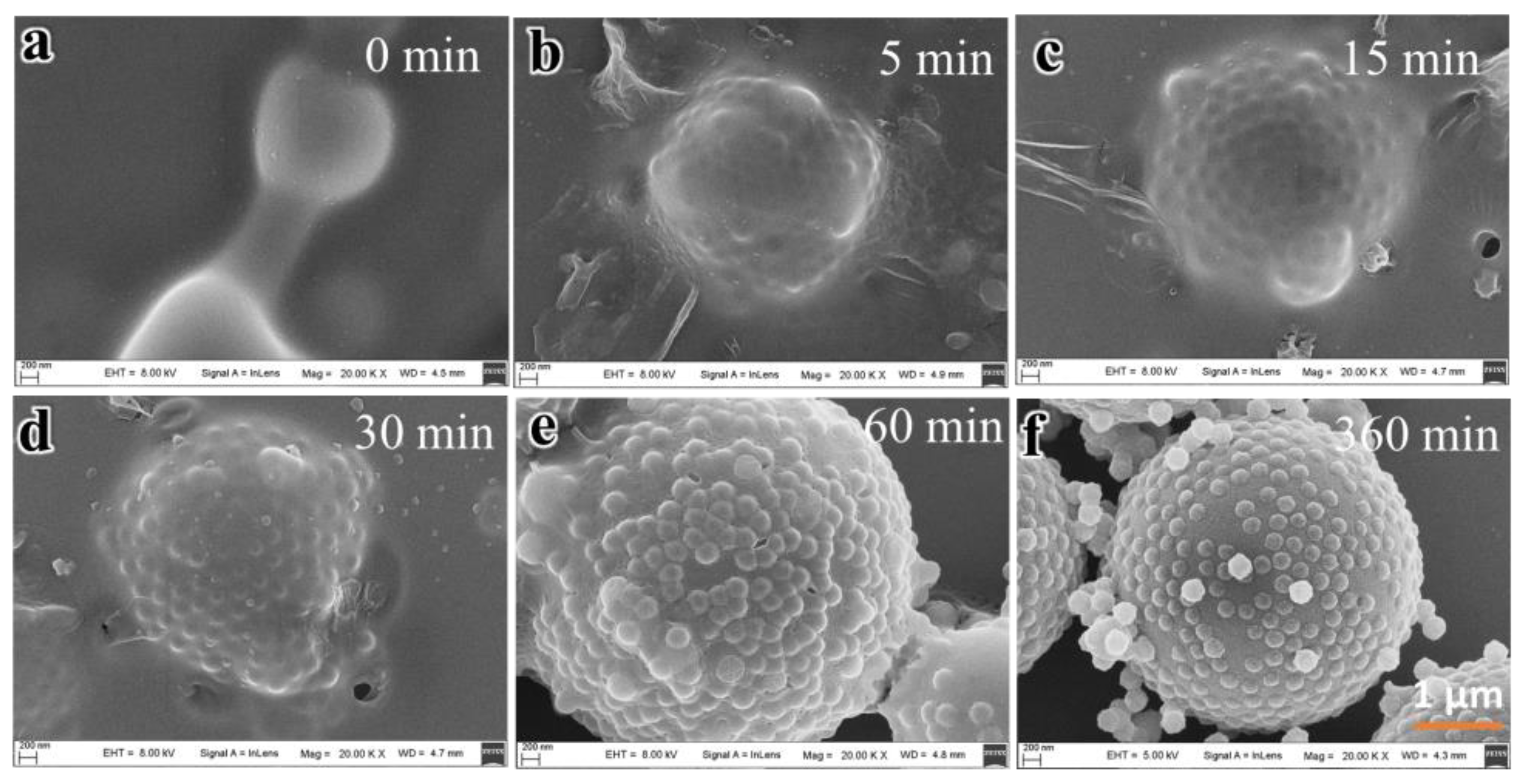
3.2. Formation Mechanism of the HSOS Particles
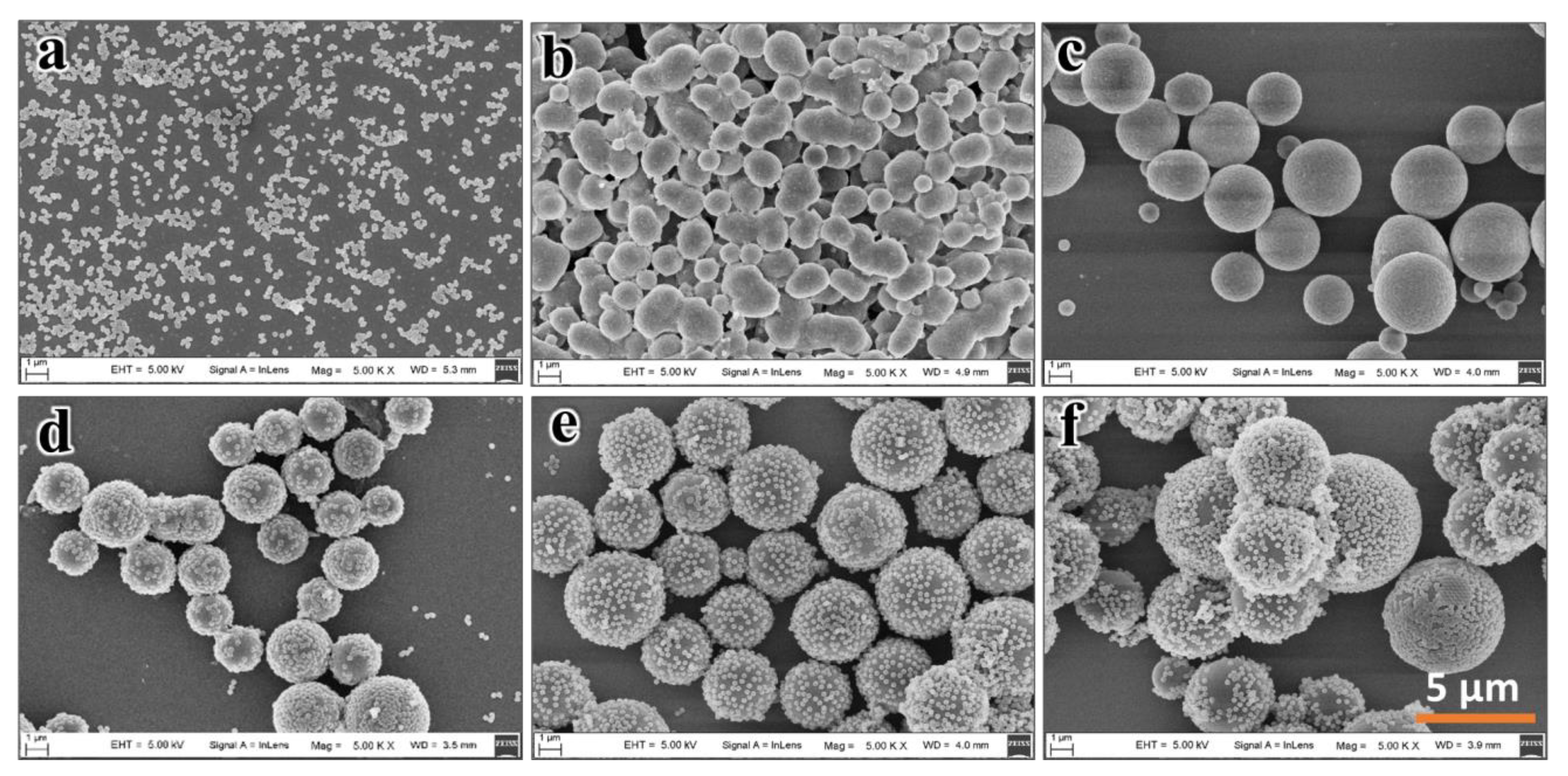
3.3. Effect of Silane Precursor
3.4. Effect of Catalyst
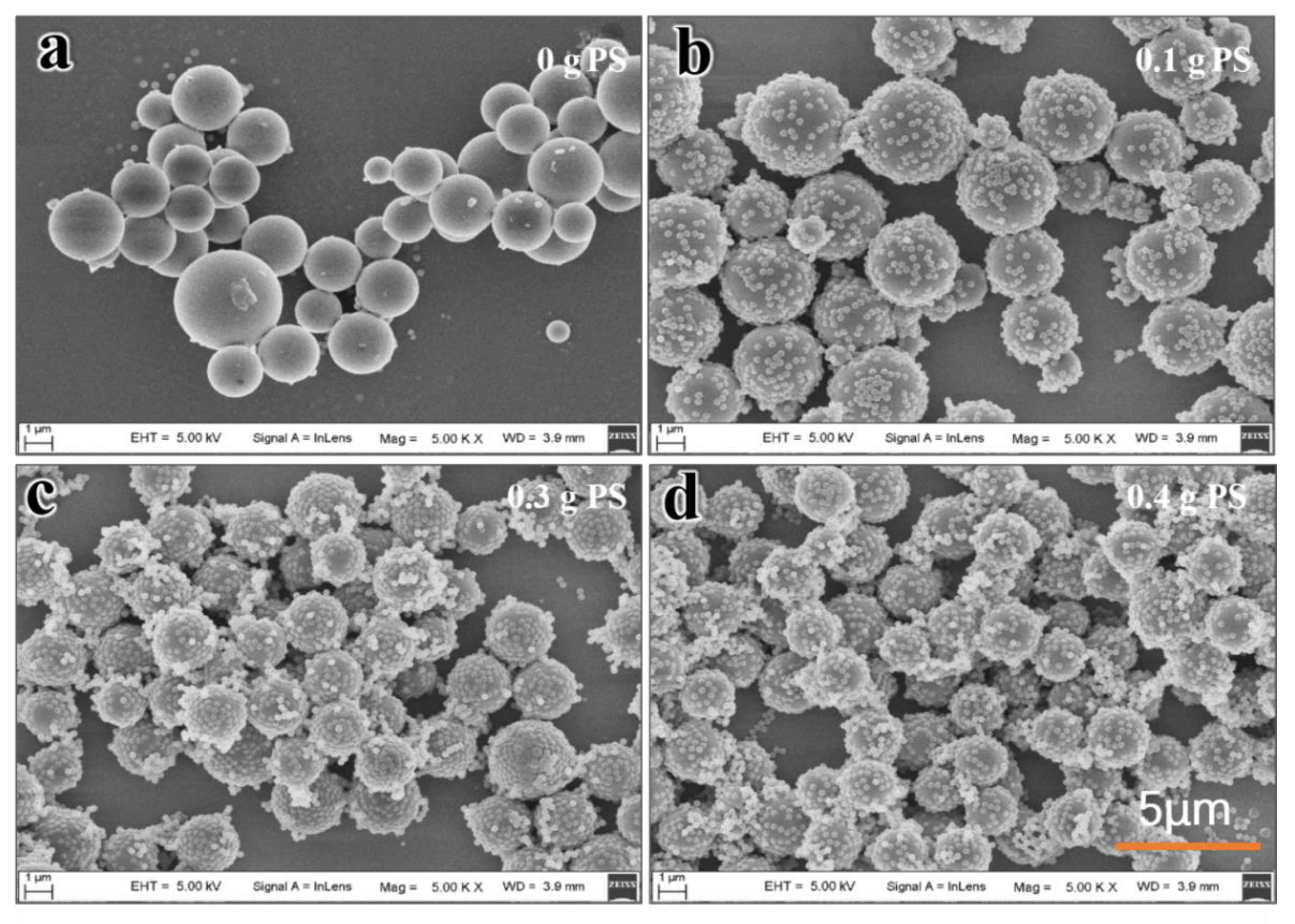
3.5. Effect of PS Latex
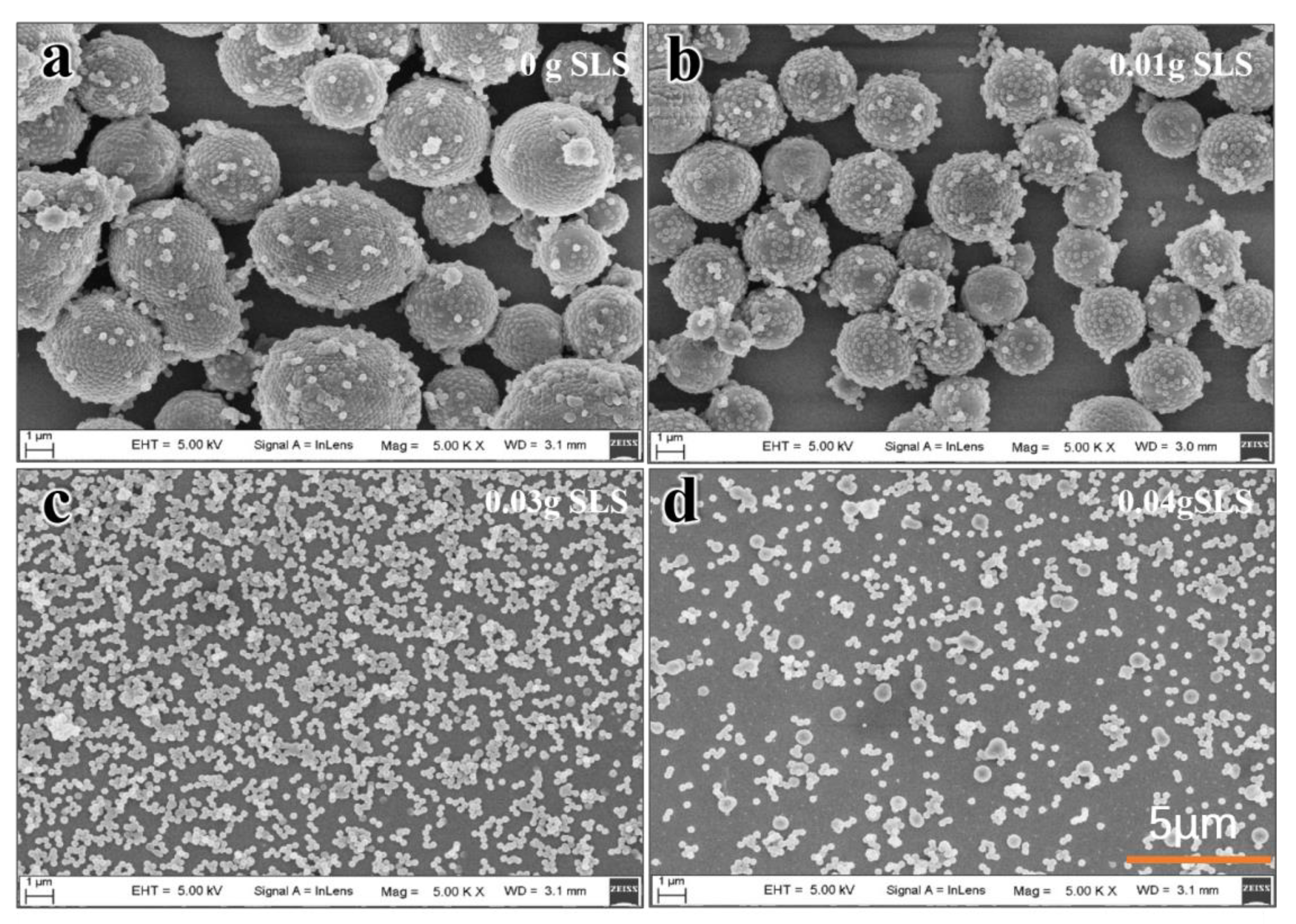
3.6. Effect of PVP and SLS
3.7. Au@HSOSs for Catalyst Reduction of 4-NP
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578–12591. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Skinley, K.; Herodotou, S.; Zhang, H. Core-shell microspheres with porous nanostructured shells for liquid chromatography. J. Separat. Sci. 2018, 41, 99–124. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.; Xuan, H.; Zhang, K.; Chen, X.; Sun, S.; Ding, Y.; Feng, S.; Xu, Q. Rods-on-sphere silica particles for high performance liquid chromatography. J. Chromatogr. A 2017, 1497, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chi, K.; Duan, A.; Zhao, Z.; Dong, H.; Gong, Y.; Song, S.; Wang, X.; Xu, C. Hierarchically structured porous silica spheres by microemulsion/vesicle templating for hydrodesulfurization of fluid catalytic cracking diesel. Particle Particle Syst. Character. 2016, 33, 190–203. [Google Scholar] [CrossRef]
- Guo, R.; Chen, X.; Zhu, X.; Dong, A.; Zhang, J. A facile strategy to fabricate covalently linked raspberry-like nanocomposites with ph and thermo tunable structures. RSC Adv. 2016, 6, 40991–41001. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Valle-Vigon, P.; Sevilla, M. One-step synthesis of silica@resorcinol-formaldehyde spheres and their application for the fabrication of polymer and carbon capsules. Chem. Commun. 2012, 48, 6124–6126. [Google Scholar] [CrossRef]
- Dong, F.; Lu, L.; Ha, C.-S. Silsesquioxane-containing hybrid nanomaterials: Fascinating platforms for advanced applications. Macromol. Chem. Phys. 2019, 220. [Google Scholar] [CrossRef]
- Jung, J.-S.; Ko, S.-J.; Lee, H.-B.; Lee, S.-B.; Kim, H.-J.; Oh, J.-M. Hierarchical ag nanostructures fabricated from silver coordination polymers for antibacterial surface. Polymers 2019, 11. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, J. Organosilica: Chemistry of mesoporous organosilica in nanotechnology: Molecularly organic-inorganic hybridization into frameworks (adv. Mater. 17/2016). Adv. Mater. 2016, 28, 3234. [Google Scholar] [CrossRef]
- Dong, F.; Ha, C.-S. Multifunctional materials based on polysilsesquioxanes. Macromol. Res. 2012, 20, 335–343. [Google Scholar] [CrossRef]
- Saxena, S.; Lyon, L.A. Influence of microgel packing on raspberry-like heteroaggregate assembly. J. Colloid Interf. Sci. 2015, 442, 39–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acharyya, S.S.; Ghosh, S.; Bal, R. Fabrication of three-dimensional (3d) raspberry-like copper chromite spinel catalyst in a facile hydrothermal route and its activity in selective hydroxylation of benzene to phenol. ACS Appl. Mater. Interf. 2014, 6, 14451–14459. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Jin, S.H.; Jeong, S.-G.; Lee, B.; Kang, K.-K.; Lee, C.-S. Microfluidic preparation of monodisperse polymeric microspheres coated with silica nanoparticles. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.; Voogt, S.; Keul, H.; Moeller, M.; Verheijen, M.; Buskens, P. Synthesis of polystyrene-polyphenylsiloxane janus particles through colloidal assembly with unexpected high selectivity: Mechanistic insights and their application in the design of polystyrene particles with multiple polyphenylsiloxane patches. Polymers 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Collinson, M.M. Well-defined hierarchical templates for multimodal porous material fabrication. Chem. Mater. 2010, 22, 4312–4319. [Google Scholar] [CrossRef]
- Yao, T.; Wang, C.; Wu, J.; Lin, Q.; Lv, H.; Zhang, K.; Yu, K.; Yang, B. Preparation of raspberry-like polypyrrole composites with applications in catalysis. J. Colloid Interf. Sci. 2009, 338, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Ming, W.; Wu, D.; van Benthem, R.; de With, G. Superhydrophobic films from raspberry-like particles. Nano Lett. 2005, 5, 2298–2301. [Google Scholar] [CrossRef]
- Cano, M.; de la Cueva-Mendez, G. Self-assembly of a superparamagnetic raspberry-like silica/iron oxide nanocomposite using epoxy-amine coupling chemistry. Chem. Commun. 2015, 51, 3620–3622. [Google Scholar] [CrossRef]
- Dong, F.; Xie, H.; Zheng, Q.; Ha, C.-S. Superhydrophobic polysilsesquioxane/polystyrene microspheres with controllable morphology: From raspberry-like to flower-like structure. RSC Adv. 2017, 7, 6685–6690. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Zhang, M.; Deng, Q.; Chen, D. Facile fabrication of raspberry-like composite microspheres for the construction of superhydrophobic films and applications in highly efficient oil-water separation. RSC Adv. 2017, 7, 39471–39479. [Google Scholar] [CrossRef]
- Caruso, F.; Caruso, R.A.; Moehwald, H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 1998, 282, 1111–1114. [Google Scholar] [CrossRef]
- Schmid, A.; Tonnar, J.; Armes, S.P. A new highly efficient route to polymer-silica colloidal nanocomposite particles. Adv. Mater. 2008, 20, 3331–3336. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.; You, B.; Wu, L. A novel preparation method of raspberry-like pmma/sio2 hybrid microspheres. Macromolecules 2005, 38, 6411–6417. [Google Scholar] [CrossRef]
- Lu, L.; Li, J.; Li, H.; Gao, C.; Xie, H.; Xiong, Y.; Luo, Z.; Sun, Q.; Dong, F. Controllable synthesis of hierarchical polysilsesquioxane surfaces: From spheres-on-sphere to bowls-on-sphere structure. Appl. Surf. Sci. 2019, 481, 75–82. [Google Scholar] [CrossRef]
- Ahmed, A.; Ritchie, H.; Myers, P.; Zhang, H. One-pot synthesis of spheres-on-sphere silica particles from a single precursor for fast hplc with low back pressure. Adv. Mater. 2012, 24, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Forster, M.; Clowes, R.; Bradshaw, D.; Myers, P.; Zhang, H. Silica sos@hkust-1 composite microspheres as easily packed stationary phases for fast separation. J. Mater. Chem. A 2013, 1, 3276–3286. [Google Scholar] [CrossRef]
- Ahmed, A.; Forster, M.; Jin, J.; Myers, P.; Zhang, H. Tuning morphology of nanostructured zif-8 on silica microspheres and applications in liquid chromatography and dye degradation. ACS Appl. Mater. Interf. 2015, 7, 18054–18063. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Zhang, W. Yolk-shell catalyst of single au nanoparticle encapsulated within hollow mesoporous silica microspheres. ACS Catal. 2011, 1, 207–211. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, S.; Zhai, B.; Xiao, Z.; An, Q. Preparation and catalytic properties of nano-au catalytic materials based on the reduction of 4-nitrophenol. Prog. Chem. 2014, 26, 234–247. [Google Scholar]
- Dong, F.; Guo, W.; Park, S.-K.; Ha, C.-S. Controlled synthesis of novel cyanopropyl polysilsesquioxane hollow spheres loaded with highly dispersed au nanoparticles for catalytic applications. Chem. Commun. 2012, 48, 1108–1110. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, G.-M.; Bae, J.G.; Kim, Y.H.; Bae, B.-S. High-performance and simply-synthesized ladder-like structured methacrylate siloxane hybrid material for flexible hard coating. Polymers 2018, 10, 449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, F.; Chen, G.X.; Li, Q.F. Synthesis and characterization of mercaptopropyl polyhedral oligomeric silsesquioxane (POSS). Beijing Huagong Daxue Xuebao (Ziran Kexueban) 2009, 36, 33–37. [Google Scholar]
- Dong, F.; Guo, W.; Park, S.-S.; Ha, C.-S. Uniform and monodisperse polysilsesquioxane hollow spheres: Synthesis from aqueous solution and use in pollutant removal. J. Mater. Chem. 2011, 21, 10744–10749. [Google Scholar] [CrossRef]
- Guo, W.; Wang, J.; Lee, S.-J.; Dong, F.; Park, S.S.; Ha, C.-S. A general ph-responsive supramolecular nanovalve based on mesoporous organosilica hollow nanospheres. Chem. Euro. J. 2010, 16, 8641–8646. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Guo, W.; Chu, S.-W.; Ha, C.-S. Novel fluorinated polysilsesquioxane hollow spheres: Synthesis and application in drug release. Chem. Commun. 2010, 46, 7498–7500. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Dong, F.; Lu, L.; Li, H.; Xiong, Y.; Ha, C.-S. Raspberry-Like Polysilsesquioxane Particles with Hollow-Spheres-on-Sphere Structure: Rational Design, Controllable Synthesis, and Catalytic Application. Polymers 2019, 11, 1350. https://doi.org/10.3390/polym11081350
Li J, Dong F, Lu L, Li H, Xiong Y, Ha C-S. Raspberry-Like Polysilsesquioxane Particles with Hollow-Spheres-on-Sphere Structure: Rational Design, Controllable Synthesis, and Catalytic Application. Polymers. 2019; 11(8):1350. https://doi.org/10.3390/polym11081350
Chicago/Turabian StyleLi, Jian, Fuping Dong, Liangyu Lu, Hongwei Li, Yuzhu Xiong, and Chang-Sik Ha. 2019. "Raspberry-Like Polysilsesquioxane Particles with Hollow-Spheres-on-Sphere Structure: Rational Design, Controllable Synthesis, and Catalytic Application" Polymers 11, no. 8: 1350. https://doi.org/10.3390/polym11081350
APA StyleLi, J., Dong, F., Lu, L., Li, H., Xiong, Y., & Ha, C.-S. (2019). Raspberry-Like Polysilsesquioxane Particles with Hollow-Spheres-on-Sphere Structure: Rational Design, Controllable Synthesis, and Catalytic Application. Polymers, 11(8), 1350. https://doi.org/10.3390/polym11081350





