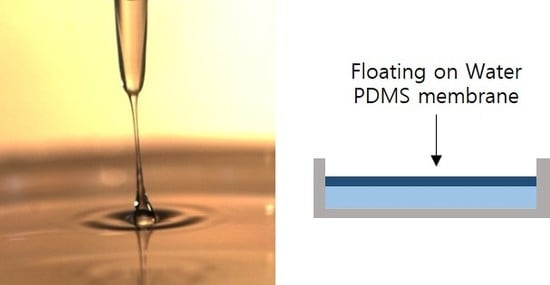
Floating-on-water Fabrication Method for Thin Polydimethylsiloxane Membranes
Abstract
1. Introduction
2. Materials and Methods
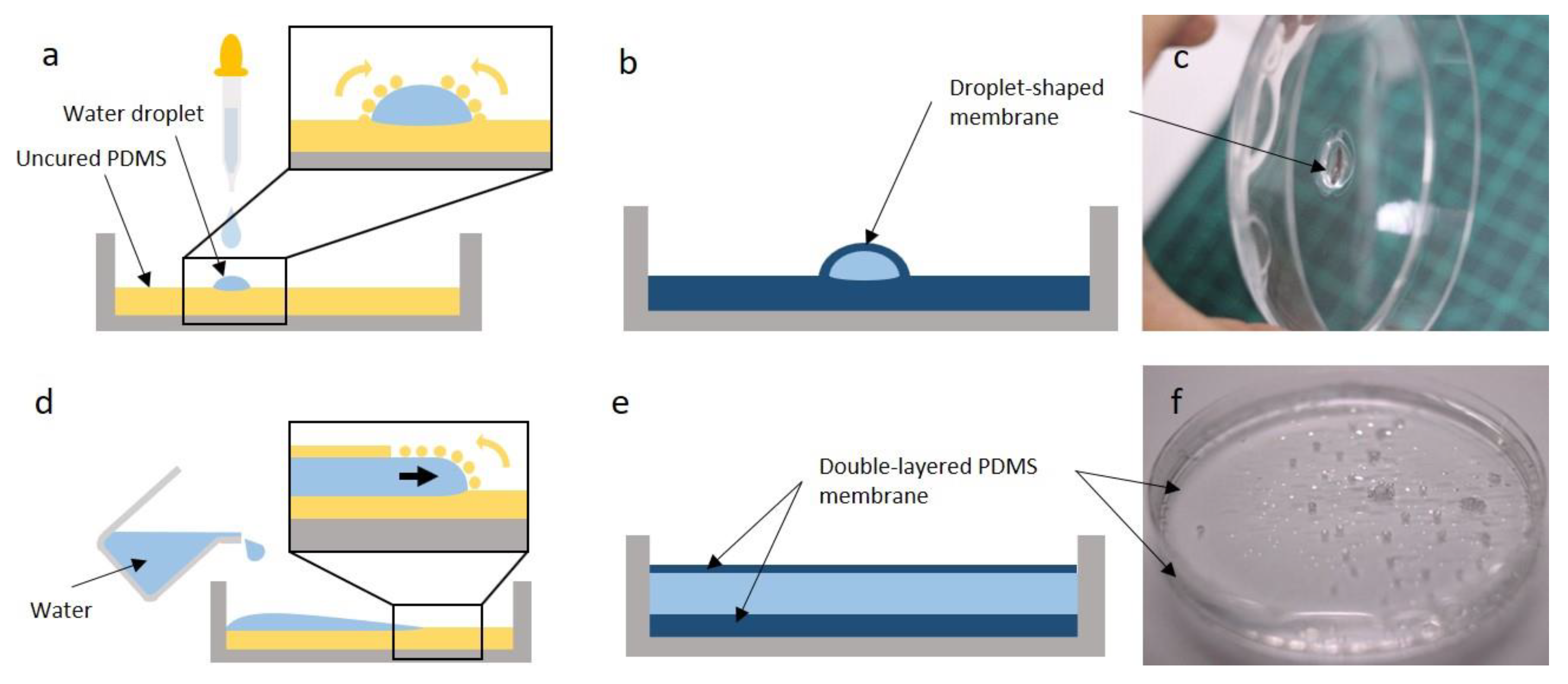
2.1. Fabrication
2.2. Membrane Thickness Control
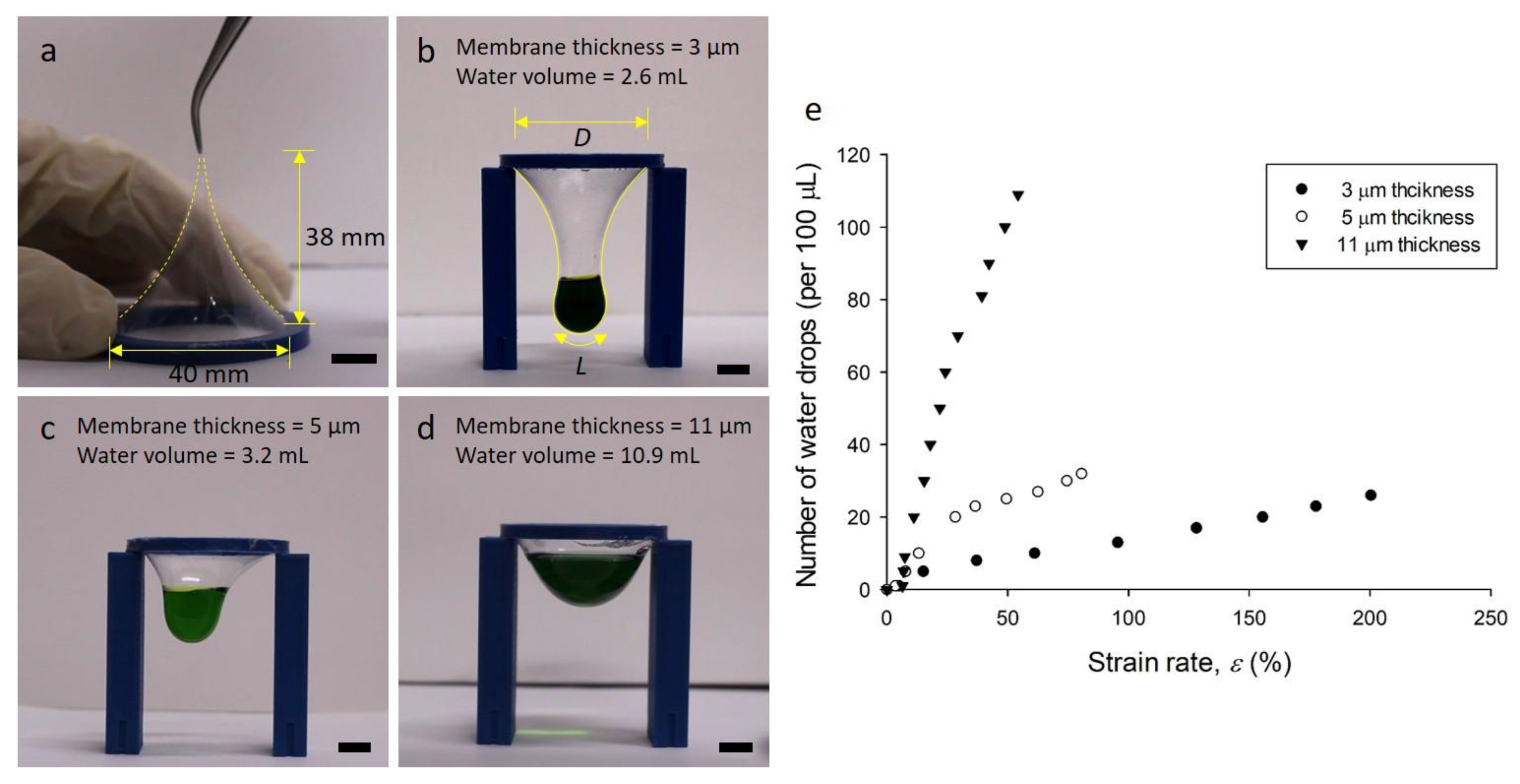
2.3. Membrane Strain Rate Test
2.4. Statistical Analysis
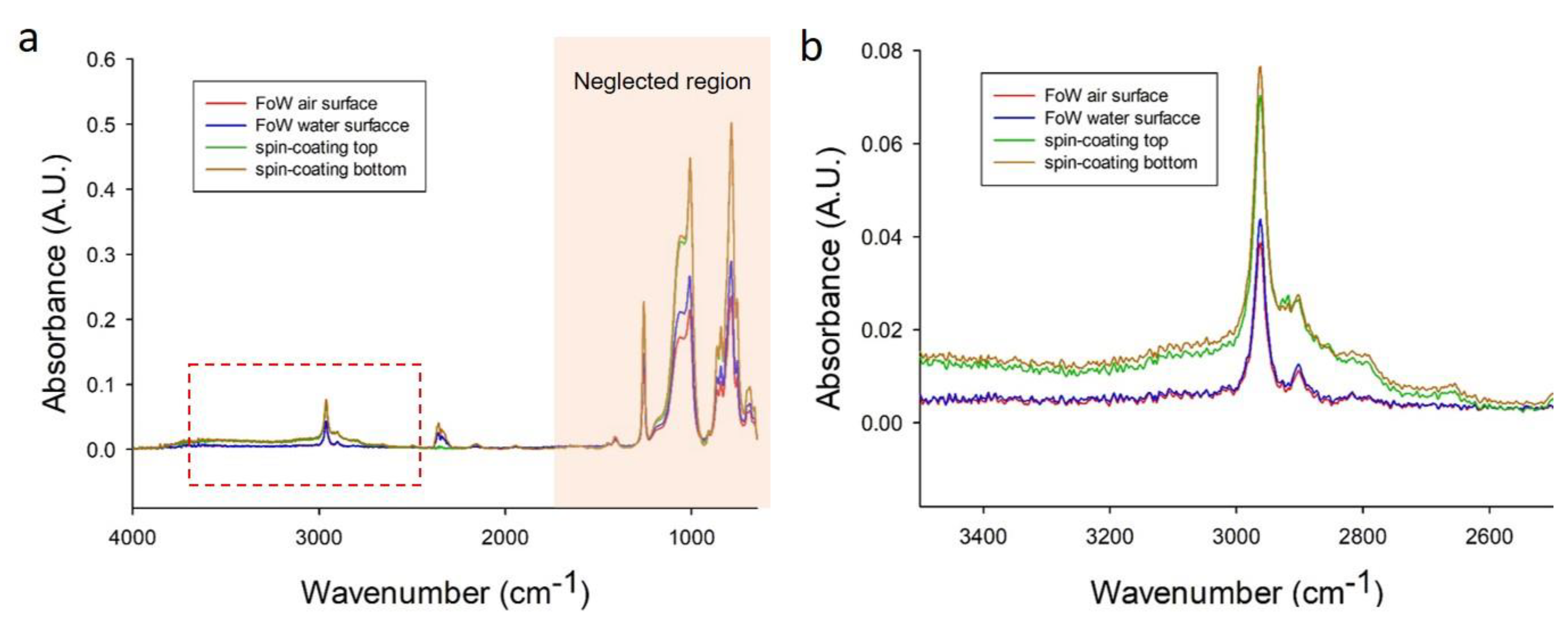
2.5. ATR-FTIR Surface Chemical Analysis
2.6. Topography Analysis by AFM
3. Results and Discussion
3.1. Membrane Fabrication
3.2. Membrane Strain Rate
3.3. Alternative Membrane Structures
3.4. Large-Area Membrane
3.5. Membrane Handling
3.6. Surface Analysis of FoW Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mcdonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T. Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- de Jong, J.; Lammertink, R.G.H.; Wessling, M. Membranes and microfluidics: a review. Lab Chip 2006, 6, 1125–1139. [Google Scholar] [CrossRef] [PubMed]
- Go, J.S.; Shoji, S. A disposable, dead volume-free and leak-free in-plane PDMS microvalve. Sens. Actuators Phys. 2004, 114, 438–444. [Google Scholar] [CrossRef]
- Kaigala, G.V.; Hoang, V.N.; Backhouse, C.J. Electrically controlled microvalves to integrate microchip polymerase chain reaction and capillary electrophoresis. Lab Chip 2008, 8, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, K.; Maeda, R. A pneumatically-actuated three-way microvalve fabricated with polydimethylsiloxane using the. J. Micromech. Microeng. 2000, 10, 415–420. [Google Scholar] [CrossRef]
- Yamahata, C.; Lotto, C.; Al-Assaf, E.; Gijs, M.A.M. Glass valveless micropump using electromagnetic actuation. Microelectron. Eng. 2005, 78–79, 132–137. [Google Scholar] [CrossRef]
- Lai, H.; Folch, A. Design and dynamic characterization of “single-stroke” peristaltic PDMS micropumps. Lab Chip 2011, 11, 336–342. [Google Scholar] [CrossRef]
- Zeng, X.; Jiang, H. Liquid tunable microlenses based on MEMS techniques. J. Phys. D. Appl. Phys. 2013, 46, 1–20. [Google Scholar] [CrossRef]
- Schneider, F.; Draheim, J.; Müller, C.; Wallrabe, U. Optimization of an adaptive PDMS-membrane lens with an integrated actuator. Sens. Actuators Phys. 2009, 154, 316–321. [Google Scholar] [CrossRef]
- Chronis, N.; Liu, G.; Jeong, K.-H.; Lee, L. Tunable liquid-filled microlens array integrated with microfluidic network. Opt. Express 2010, 11, 2370–2378. [Google Scholar] [CrossRef]
- Vespini, V.; Coppola, S.; Todino, M.; Paturzo, M.; Bianco, V.; Grilli, S.; Ferraro, P. Forward electrohydrodynamic inkjet printing of optical microlenses on microfluidic devices. Lab Chip 2016, 16, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.S.; Chang, S.F.; Shih, M.C.; Tseng, S.H.; Lai, C.H. Scaffold-Free Liver-On-A-Chip with Multiscale Organotypic Cultures. Adv. Mater. 2017, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Ryoo, J.; Jeong, G.S.; Choi, Y.Y.; Jeong, S.M.; Ju, J.; Chung, S.; Takayama, S.; Lee, S.H. Large-scale, ultrapliable, and free-standing nanomembranes. Adv. Mater. 2013, 25, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Thangawng, A.L.; Ruoff, R.S.; Swartz, M.A.; Glucksberg, M.R. An ultra-thin PDMS membrane as a bio/micro-nano interface: Fabrication and characterization. Biomed. Microdevices 2007, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Jiang, Z.; Hu, C.; Wang, Y.; Xu, H.; Liu, J. Removing benzene from aqueous solution using CMS-filled PDMS pervaporation membranes. Sep. Purif. Technol. 2006, 48, 229–234. [Google Scholar] [CrossRef]
- El Haitami, A.; Backus, E.H.G.; Cantin, S. Synthesis at the Air-Water Interface of a Two-Dimensional Semi-Interpenetrating Network Based on Poly(dimethylsiloxane) and Cellulose Acetate Butyrate. Langmuir 2014, 30, 11919–11927. [Google Scholar] [CrossRef]
- Kim, C.; Gurau, M.C.; Cremer, P.S.; Yu, H. Chain conformation of poly(dimethyl siloxane) at the air/water interface by sum frequency generation. Langmuir 2008, 24, 10155–10160. [Google Scholar] [CrossRef]
- Okahata, Y.; Tsuruta, T.; Ijiro, K.; Ariga, K. Preparations of Langmuir-Blodgett films of enzyme-lipid complexes: A glucose sensor membrane. Thin Solid Films 1989, 180, 65–72. [Google Scholar] [CrossRef]
- Park, J.Y.; Hwang, C.M.; Lee, S.H. Ice-lithographic fabrication of concave microwells and a microfluidic network. Biomed. Microdevices 2009, 11, 129–133. [Google Scholar] [CrossRef]
- Kuo, A.C.M. Poly(dimethylsiloxane). Polym. Data Handb. 1999, 411–435. [Google Scholar]
- Gerhardt, L.J.; Manke, C.W.; Gulari, E. Rheology of polydimethylsiloxane swollen with supercritical carbon dioxide. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 523–534. [Google Scholar] [CrossRef]
- Liu, M.; Sun, J.; Sun, Y.; Bock, C.; Chen, Q. Thickness-dependent mechanical properties of polydimethylsiloxane membranes. J. Micromech. Microeng. 2009, 19. [Google Scholar] [CrossRef]
- Firpo, G.; Angeli, E.; Repetto, L.; Valbusa, U. Permeability thickness dependence of polydimethylsiloxane (PDMS) membranes. J. Membr. Sci. 2015, 481, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Han, D.; Zhao, Y.P. Kinetic behaviour of the cells touching substrate: The interfacial stiffness guides cell spreading. Sci. Rep. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, A.; Marasso, S.L.; Cocuzza, M. PDMS membranes with tunable gas permeability for microfluidic applications. RSC Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Keranov, I.; Vladkova, T.G.; Minchev, M.; Kostadinova, A.; Altankov, G.; Dineff, P. Topography characterization and initial cellular interaction of plasma-based ar1 beam-treated PDMS surfaces. J. Appl. Polym. Sci. 2009, 111, 2637–2646. [Google Scholar] [CrossRef]
- Zhang, W.; Choi, D.S.; Nguyen, Y.H.; Chang, J.; Qin, L. Studying cancer stem cell dynamics on PDMS surfaces for microfluidics device design. Sci. Rep. 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]





| Experimental Conditions | Amount of PDMS Solution (μg) | Water Temperature (°C) | Initial PDMS Solution Temperature (°C) | Remarks |
|---|---|---|---|---|
| Condition 1 | 10 | 80 | 20 | Control |
| Condition 2 | 20 | 80 | 20 | Amount of PDMS solution is changed |
| Condition 3 | 10 | 40 | 20 | Water temperature is changed |
| Condition 4 | 10 | 80 | −15 | Initial PDMS solution temperature is changed |
| Condition 5 | 30 | 80 | 20 | Condition 5–7; extra experimental conditions with various amount of PDMS solution |
| Condition 6 | 40 | 80 | 20 | |
| Condition 7 | 50 | 80 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, S.-H.; Park, J.Y. Floating-on-water Fabrication Method for Thin Polydimethylsiloxane Membranes. Polymers 2019, 11, 1264. https://doi.org/10.3390/polym11081264
Kim D, Kim S-H, Park JY. Floating-on-water Fabrication Method for Thin Polydimethylsiloxane Membranes. Polymers. 2019; 11(8):1264. https://doi.org/10.3390/polym11081264
Chicago/Turabian StyleKim, Daehan, Sung-Hwan Kim, and Joong Yull Park. 2019. "Floating-on-water Fabrication Method for Thin Polydimethylsiloxane Membranes" Polymers 11, no. 8: 1264. https://doi.org/10.3390/polym11081264
APA StyleKim, D., Kim, S.-H., & Park, J. Y. (2019). Floating-on-water Fabrication Method for Thin Polydimethylsiloxane Membranes. Polymers, 11(8), 1264. https://doi.org/10.3390/polym11081264